Abstract
Cyclohexene vapor, instead of air, is applied to cure polycarbosilane (PCS) fibers. The cured fibers are characterized by infrared (IR), electron spin resonance (ESR), elements analysis (EA) and simulated through the HyperChemTM program for comparison. The curing process is investigated by thermoanalysis. The results indicate that the Si–H and Si–CH3 bonds in PCS are induced by cyclohexene to cleavage and form Si-central radicals. A fully developed cross-linking fibers come into being through the combination of these radicals, and the byproducts, some cyclohexyls bonded onto PCS derived from cyclohexene, introduce the variations in IR spectra, weight gain and carbon contents increase of PCS. On the basis of investigation and simulation, a likely mechanism of curing reaction is presented.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Silicon carbide (SiC) fibers derived from polycarbosilane (PCS) precursor are flexible, continuous and quite stable at elevated-temperatures performing high mechanical properties. Earlier work has shown SiC fibers’ thermal stability is dependent on their oxygen content [1], and as for the precursor deriving process, the oxygen is mostly introduced at the oxidation-cure stage of the PCS green fibers. Therefore, several non-oxidation curing methods have been applied to improve the fibers’ performance at high temperatures, such as electron beam (EB)/γ-ray radiation fabricated Hi-NicalonTM fiber (oxygen content <0.5 wt.%, enduring 1500 °C with acceptable strength residual), chemical vapor curing (CVC) fabricated fibers (oxygen content <0.5 wt.%, enduring 1400 °C [2, 3]), and dry spinning of high molecular weight PCS fabricated UF-SiC fibers (oxygen content <2 wt.%, enduring 1400 °C) and so on. Compared with others, CVC method selects non-oxygen active reactants (cyclohexene, 1-octylene etc.) vapors to cure green fibers, which is feasible and suit for a batch of production. Although has successfully fabricated low oxygen content SiC fibers, the mechanism of CVC is still unclear and the research is insufficient except for the speculation of Yoshio Hasegawa, the method inventor, presented in his papers [2, 3] as following:
 , space
, space
 , space
, space
 , space
, space

This speculation has given the elementary instruction to understand the CVC course, but there are still some important problems, as the evolution of radicals, the formation of cross-linking structures and the role of cyclohexene in reactions etc., to explore, besides, further experimental evidence is required to support the concept presented above.
In this work, the reaction of PCS fibers and cyclohexene vapor is investigated through thermal gravimetric and differential thermal analysis (TG-DTA), Fourier Infra-red (FT-IR), electron spin resonance (ESR) spectra, elements analysis (EA), and simulated by the HyperChemTM. On the basis of the experimental results and former speculation, we present a new and detailed mechanism of CVC process.
Experiments
Experimental details
The PCS green fibers (melt points ranging from 190–230 °C) were heated in the cyclohexene atmosphere to specific temperatures ranging from 100 to 400 °C, held for 0.5 h, then cooled naturally to the ambient temperature. The cured PCS fibers were ground with KBr powder and compressed to tablets for the IR analysis with Nicolet-360 FT-IR spectrometer. The thermoanalysis (TG-DTA) was carried out via heating the PCS powder from 130 to 250 °C in the cyclohexene/N2 (carrying gas) mixing flow and 250–350 °C in pure N2 flow, both at the heating rate of 5 K/min.The ESR spectra were recorded on the ER200D-SRC spectrometer using as-cured PCS fibers. The spin concentrations of the samples were calculated on the assumption that the number of the spins is proportional to product of (peak-to-peak width)2 × (peak-to-peak height) in the ESR spectra. The Si, C atoms contents in the samples were measured by the CS-444 Carbon /Sulfur analyzer and GB 4333.1-84 method.
Computer simulation
To simulate the reaction between PCS and cyclohexene vapor, the molecular simulation software HyperChemTM 7.1 was applied. Selecting the semi-experiential method PM3, the molecule of PCS and all possible reaction products were constructed and optimized through the Polak–Ribiere process. The vibration spectra of all the molecules above were calculated and compared with the experimental ones to give more clues of the real reaction mechanism.
Results and discussion
Thermoanalysis
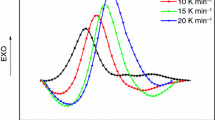
Figure 1 indicates the thermal behavior of PCS reacting with cyclohexene at different temperatures. As the curve shows, there was no obvious exothermic peaks appeared until the temperature reached 180 °C. Above 180 °C, a drastic exothermal status occurred with the temperature increasing, at about 250 °C, when the cyclohexene shut down, the exotherm reached maximum level and then decreased rapidly till 350 °C. Correspondingly, the weight gain did not happen until 180 °C, and increased continuously and steadily even beyond 250 °C. These results exhibit that the reaction between PCS and cyclohexene is a typical exothermic one and causes weight gain of PCS, just the same as the oxidation, the beginning temperature of the reaction is 180 °C that is higher than the one of oxidation, which indicates that it is much harder to occur and needs more energies to induce. The further weight gain of PCS without cyclohexene flow is probably due to some cyclohexene adsorbed in the PCS keeping on reacting at higher temperature.
FT-IR
Figure 2 overlaps the representative IR spectra of the PCS cured by cyclohexene with the ones of green fibers and air cured fibers to give a clear comparison. As the spectrum shown, cyclohexene mainly consumed the Si–H bonds of the PCS, which lead to the apparent degradation of the peak at 2100 cm−1 assigned to their stretching vibrations. This is the same as that of the air cured PCS.
To show the structure variation of the PCS cured by cyclohexene quantitatively, Table 1 lists the ratios of the absorption peaks’ strength of main PCS functional groups to the strength of the C–H bonds’ peak at 2950 cm−1.
As the ratios show, besides the Si–H bonds, the Si–CH3 structures in cured PCS also decreased while the Si–CH2–Si increased, which was independent of either the curing conditions or PCS variety. This trend is noticeable for it is similar with the one of the EB cured PCS fibers in which the radicals produced by radiation recombined and formed the Si–CH2–Si bridges [4]. Therefore, it is quite reasonable to believe that the newly formed Si–CH2–Si in cured PCS, shown as increasing strength ratio in Table 1, originated from the Si–H and Si–CH3.
Furthermore, some new absorbing peaks not belonging to the green fibers appeared on the more detailed spectra of cured PCS, as the regions marked I and II in Fig. 3 show. Magnifying the Region I, II and presenting them in the Figs. 4 and 5, respectively. In Fig. 4, there is a small shoulder peak and a side peak at 2880 and 2838 cm−1, respectively, blue shifting from the C–H stretching vibration peak. In Fig. 5, the situation is more complicated: besides the C–H bending vibration peaks of Si–CH3 (1400 cm−1) and Si–CH2–Si (1360 cm−1) in PCS, two weak peaks not belonging to PCS appear at 1376 and 1456 cm−1, respectively.
To assign these peaks, the cyclohexene IR spectrum was referred to, as Fig. 6 presented.
According to the cyclohexene IR spectrum, there should be apparent cyclic C=C double bonds peak at 3020 cm−1, if cyclohexene had not reacted with PCS during cure process but only been adsorbed on the surface of fibers or infiltrated in the bundles. However, the blank of peaks in the region above 3000 cm−1, as shown in Fig. 4, proves this assumed situation did not actually occur, the four new peaks (at 2880, 2838, 1376 and 1456 cm−1, respectively) be assigned to the new structures formed during the reaction of PCS and cyclohexene. As speculated by Yoshio Hasegawa, these structures were possibly cyclohexyls bonded with the PCS molecules. This speculation also explains reasonably the weight gain of fibers indicated in Fig. 1 and the increasing carbon contents shown in Table 2.
ESR
Table 3 summarizes the obtained ESR data of cured PCS by cyclohexene and air. For all samples, the same g factor of 2.0035 reveals that the radicals produced by these two procedures are identical. In several previous reports [5–8], ESR signals with g factor of about 2.003 were attributed to the so-called carbon dangling bonds or the free carbon defects, especially in the pyrolysed residues of PCS, and it was therefore believed that the ESR intensity was in direct proportion to the excess carbon amounts. Although the signals’ g factors in this study are quite close to 2.003, it is not reasonable to assign them to the carbon dangling bonds definitely, for the following reasons: first, no matter CVC nor oxidation processing temperatures exceeded 400 °C which was much lower than the pyrolysis value and not able to make some specific transformations and rearrangements occur; second, as shown in Table 3, the intensity of oxidation cured PCS is 2–3 times higher than that of CVC, so the oxidized PCS should contain more excess carbon than the CVC ones, if the ESR peaks had been assign to carbon dangling bonds, which is obviously opposite from the results of EA in Table 2; furthermore, M. Sato et al. reported the radiation cured PCS ESR signals’ g value was 2.004 ± 0.0005 and assigned them to Si/C-central radicals [9], and M. Sugimoto et al. drew the same conclusion at the similar situation in their work [10]. Based on reasoning and evidences above, it is deduced that all of the samples’ ESR signals, depicted in Fig. 7, are produced by the Si/C-central radicals, most of which are Si-central ones considering the apparent degradation of mass Si–H peaks in the IR spectra.
Besides the g factors, these four samples have nearly the same peak-to-peak width, for (Hpps are all about 5G. Since the ESR peak width depends on the contribution of H-atom hyperfine splitting with the radicals dwelling at the Si/C atoms, the same width can be interpreted as the similar chemical environments where the radicals reside. However, there is a clear difference between the profiles of the oxidized PCS and CVC cured ones as shown in Fig. 7, which is due to the different specific intensities of them as discussed earlier. Moreover, the intensities of oxidized samples are relatively independent to the oxidation conditions that cause the variant cross-linking levels, whereas the ones of CVC cured PCS are more sensitive to the conditions and increase with the cross-linking levels rising, as the data of PCS-C, D indicate. Another phenomenon that cause great interests is the peculiar stabilities of the radicals in all samples even in air. Also reported by M. Sugimoto, the radicals in the radiation cured PCS were rather stable under vacuum at room temperature, but reacted quickly with oxygen in air forming a peroxy radicals and then decayed rapidly. However, all the samples in this work were exposed to air for a period of time before the ESR measuring and still showed ESR activities associated with the residue of radicals. A possible explanation is that the radicals induced by heat in air and cyclohexene are less active than those by radiation, when curing ends and temperature drops, they are trapped or frozen in the PCS and not able to react with oxygen at room temperature, by which they are protected and retained. At present, there are still no further experimental evidences to support this assumption, however.
Computer simulation
To simulate the PCS molecular structural features accurately and avoiding the mass calculations, 2,4,4-trimethyl-2,4-disila-pentane (TMDSP) was selected as the simplified model of PCS, whose structural formula is:

As marked on the formula, there are three potential active points in TMDSP which are able to form the four radicals below correspondingly, possibly taking part in the cross-linking reactions: space

Considering the bonding energies trend that Si–H (312 kJ/mol) < Si–C (314 kJ/mol) < C–H (414 kJ/mol), it is easy to know the forming difficulties trend of the four radicals by bonds cleavages is (a) < (b) < (c), (d), i.e. radicals (a) and (b) are dominant during reactions, while (c), (d) can be ignored, since the cleavage of C–H occurs at much higher temperatures. Bond the cyclohexyl as mentioned onto these two intermediates, radicals (a) and (b), we got another two new models A and B which illustrated the new structures displayed in the IR spectra of cured PCS. space

The simulated vibration spectra of TMDSP, model molecules A and B are shown as Figs. 8 and 9, which mainly indicated the distribution of the characteristic vibration peaks.
As shown in Fig. 8, the calculated vibration spectrum of TMDSP matches the PCS experimental IR spectrum (see Fig. 3) accurately, which demonstrates the feasibility of making TMDSP model PCS. Additionally, in comparison with TMDSP spectrum, there are apparent variations in those of model A and B as indicated in Fig. 9: first, some peaks (marked ➀) attributed to stretching vibration of C–H bonds in cyclohexyl appear in the high-frequency fields (2900–3040 cm−1) of methylene (2920 cm−1) in the main chain of TMDSP. These peaks correspond well with the ones at 2880, 2800 cm−1 in Fig. 4; second, interfered by the cyclohexyl methylene, there exist some new peaks (marked ➁) near the TMDSP C–H bending vibration regions, 1360 and 1400 cm−1, which is in agreement with the status at 1376 and 1456 cm−1 in Fig. 5. Moreover, the difference between the wavenumbers of these new peaks in model A, B is attributed to the diverse chemical circumstances of radical’s β atoms. Whereas, it has not been able to determine the exact attribution of these new peaks between 1300–1500 cm−1 yet, because the cyclohexyl and TMDSP vibration peaks appeared alternately in the calculated spectra and are hard to separate from each other.
Mechanism of the reaction between PCS and cyclohexene
The experimental results of CVC reflect that the free radicals are generated during the curing process, indicating the reaction between PCS and cyclohexene be a typical radical style and proceed through following mechanisms possibly:
At the initial stage, the radicals are produced by the cleavage of PCS when heated under cyclohexene atmosphere, demonstrated as following. The presence of cyclohexene here lowers the active energies of the cleavage of PCS and facilitates the radicals formation, though it was less active in comparison with oxygen
 .
.
Then, owing to the predominant amount of cyclohexene, parts of newly formed radicals at the beginning attack the unsaturated carbons of it, bonding with it and introducing the ring of cyclohexyl into PCS
 .
.
With the reaction going on, more and more radicals are produced including the Si-central ones and the cyclocarbon central ones; whereas, due to the steric repulsive effects, the latter radicals could not combine with others easily but incline to terminate by capturing else protons, transmitting radicals simultaneously at some situation. These terminated cycloradicals come into being the cyclohexyl bonded on PCS finally, as observed in the IR spectra and EA results
 .
.
Investigating both the stage (2) and (3), it can be known that the integral function of cyclohexene during the curing is to transmit the radicals, therefore, the reaction proceeded continuously.
With the amount increasing, the radicals, mainly Si-central ones, combine with each other and the cross-linking bridges (e.g. Si–CH2–Si) formed among single PCS molecules; when the cross-linking reached some level, a three-dimension network was accomplished, resulting in cured PCS finally.space

Conclusions
The reaction between PCS and cyclohexene is an exothermic one, accompanied by weight gain, and proceed through free radical evolution route based on ESR measurements. Although less active than oxygen and requires higher temperature to react, cyclohexene induces the cleavage of Si–H and Si–CH3 in PCS to form Si-central radicals, similar with those in oxidation process, transmits radicals and makes the reaction proceed successively. With the combination of these free radicals, cross-linking level of PCS molecules increases steadily, three-dimension network comes into being and PCS becomes infusible and insoluble finally. Owing to the radicals transmitting and terminating, some cyclohexyls derived from cyclohexene bond onto PCS and become side groups, which brings the variations in IR spectra, weight gain and carbon contents increase of PCS. Obviously, the addition of cyclohexyl will affect the composition of the pyrolyzed fibers and their properties consequently, which deserves further research.
Reference
Dong SM, Chollon G, Labrugere C et al (2001) J Mater Sci 36:2371
Hasegawa Y (1992) J Inorg Org Polym 2:161
Hasegawa Y (1994) Comp Sci Tech 51:161
Chu ZY, Song YC (2000) J Mater Sci Lett 19:1771
MR Mucalo, Mcgavin DG, Milestone NB (1997) J Mater Sci 32:3271
Martin HP, Műller E, Richter R et al (1997) J Mater Sci 32:1381
Soraru GD, Babonneau F, Mackenzie JD (1990) J Mater Sci 25:3886
Ting SJ, Chu CJ, Mackenzie JD (1992) J Mater Res 7:164
Sato M, Yamamura T, Seguchi T et al (1990) J Chem Soc Jpn 5:554
Sugimoto M, Shimoo T, Okamura K et al (1995) J Am Ceram Soc 78:1849
Acknowledgement
We are grateful to Professor Shiming Chen (Fudan University) for performing ESR-measurements and also for useful discussions and advices.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Li, W., Song, Y.C. & Mao, X.H. Mechanism of cyclohexene vapor curing polycarbosilane fibers. J Mater Sci 41, 7011–7018 (2006). https://doi.org/10.1007/s10853-006-0807-z
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10853-006-0807-z