Abstract
In this study, nylon microcapsules were prepared on a paper surface using an interfacial polymerization technique for the preparation of smart paper. Filter paper impregnated with ethylenediamine solution and NaOH solution was left in a beaker containing cyclohexane or chloroform. Subsequently, the organic solvent terephthaloyl chloride was added to the beaker and interfacial polymerization occurred on the paper surface. When cyclohexane was used as the organic solvent, formation of nylon microcapsules on the paper surface was confirmed. Fixation of nylon microcapsules on the paper surface was successfully performed using interfacial polymerization. On the other hand, it was not possible to form nylon microcapsules on the paper surface when interfacial polymerization with chloroform was carried out. These results are due to differences in the spreading coefficient of the organic solvent on the ethylenediamine solution adsorbed on the paper surface.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Intelligent materials responsive to temperature [1–3], pH [2, 4], chemicals [5–7], electric fields [8] and light [9] have been actively studied for potential new technical applications in drug delivery systems [3–7], self-repairing materials [10] and an intelligent window [9]. In our study, we tried to apply the concept of intelligent material to a functional paper [11]. Functional paper, which is paper made with functional material such as adsorbent, antimicrobial material or conductive material [12–14], utilizes the native properties of the materials present in the paper. Smart paper, which is defined as a functional sheet having the intelligent functions in the paper itself, can perceive outside environmental changes and select the best countermeasure using functions within the paper, thus operating the actuating functions according to the signals.
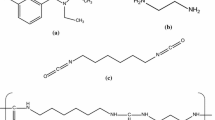
In this study, the preparation of a smart paper, which could, for instance, release an agricultural chemical near a temperature for the incidence of harmful insects, was attempted. Microcapsules composed of a semipermeable nylon-capsule membrane coated with dialkyldimethylammonium amphiphiles [1] were used. These microcapsules have a thermal response near the phase transition temperature of dialkyldimethylammonium amphiphiles and can reversibly control permeation of the functional material, for example, the agricultural chemical, included in the microcapsule. Therefore, the smart paper prepared in this study was composed of the paper and the thermally responsive microcapsules. Paper containing microcapsule, such as pressure-sensitive and thermally sensitive papers, has usually been prepared by coating the microcapsules with a binder [15]. However, it was supposed that smart paper prepared by the above coating procedure would not be sufficiently functional, as the microcapsule surface would be covered with the binder. Thus, it was important to solve the above problem for the preparation of smart paper that could maintain the intelligent function of the microcapsules. In other words, a new technology for the fixation of intelligent microcapsules onto paper without the need for a binder was required.
In this work, an interfacial polymerization technique, polymerization performed at an oil–water interface, which is one of the methods used for microcapsule preparation [1, 11, 16, 17], was used to prepare nylon microcapsules directly on a paper surface. By utilizing the interfacial polymerization technique, the nylon microcapsules would be expected to be formed at the oil–water interface formed on the paper surface and to be fixed on a paper surface without using a binder that would inhibit the intelligent function. Suitable conditions for the fixation and formation of the nylon microcapsules on the paper surface were sought. This report describes the effects of the concentration of ethylenediamine (ED) as an aqueous monomer and the kinds of organic solvents on the fixation and formation of nylon microcapsules on a paper surface. The nylon microcapsules on the paper surface were characterized using Fourier transform infrared (FT-IR) spectrometry and scanning electron microscopy (SEM).
Experimental section
Preparation of nylon microcapsules on the paper surface using interfacial polymerization
Filter paper (Toyo Roshi Kaisha, Ltd., NO. 2) impregnated with an aqueous mixed solution of 2.5–25% (w/w) anhydrous ED (3.0 × 10−6 m3) and 1 N NaOH (3.0 × 10−6 m3) was immersed in cyclohexane or chloroform solution (1.0 × 10−5 m3) for 10 min. Subsequently, cyclohexane or chloroform solution (1.0 × 10−5 m3) of terephthaloyl chloride (TC) (0.1 g) was added to the above beaker. The beaker containing the filter paper was then left in a refrigerator at 4 °C for 12 h, after which the filter paper was washed with cyclohexane and air-dried at room temperature. Chloroform, NaOH and TC were purchased from Wako Pure Chemical Industries, Ltd., Japan, and anhydrous ED and cyclohexane were obtained from Kanto Chemical Co., Inc., Japan.
Characterization of nylon microcapsules on the paper surface
Fourier transform infrared (FT-IR) spectra were measured on an FT-IR-480 (JASCO, Inc.) spectrometer using attenuated total reflection (ATR) at a resolution of 4.000 × 10−2 m−1 throughout the spectral range (40.00–7.000 m−1), and accumulation of 100 scans. The paper surface treated by the interfacial polymerization technique was analyzed by scanning electron microscopy (SEM, JSM-5510LV, JEOL Inc.) with an accelerating voltage of 15 kV after Pt coating. The sizes of the nylon microcapsules formed on the paper surface were measured by analyzing SEM photographs using Smile View (Ver.2.05, JEOL Inc.). The thickness of the nylon film formed on the paper surface was determined by subtracting the thickness of the original paper from that of the resultant paper using a thickness micrometer (TM-600, Kumagai Riki Kogyo Co., Ltd.).
Evaluation of spreading coefficient
The spreading coefficient (S) of cyclohexane or chloroform on the ED solution was calculated using:
where γa is the surface tension of the ED solution, γb is the surface tension of cyclohexane or chloroform and γa/b is the interfacial force of ED-cyclohexane and ED-chloroform. The surface tension (γa and γb) and the interfacial force (γa/b) were measured on a DM 300 (Kyowa Interface Science Co., Ltd.) using a pendant drop method and the Young–Laplace equation. Relative humidity and temperature were about 50% and 23 °C, respectively.
Results and discussion
Characteristics and fixation of nylon film formed on the paper surface
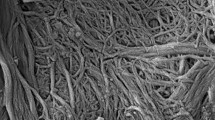
Figures 1 and 2 show FT-IR spectra and SEM images of the filter paper treated with the interfacial polymerization technique. Cellulose peaks (C–O and C–O–C stretching vibrations) attributed to pulp of the filter paper were confirmed at 12.00–9.000 m−1 as shown in Fig. 1a. For the filter paper treated by the interfacial polymerization technique, three characteristic peaks due to a C=O stretching vibration at around 16.33 m−1, an N–H bending vibration at around 15.45 m−1 and a C–N stretching vibration at around 12.92 m−1, attributed to a C–N–H bond, were observed as shown in Fig. 1b. These were attributed to polyamide and, thus, nylon film on the paper surface was prepared by utilizing the interfacial polymerization technique. The formation of the nylon film on the paper surface was also confirmed by SEM (Fig. 2). The nylon film on the paper surface was formed by the reaction between the amino group from the ED adsorbed on the paper by the physical bond, and the carbonyl group of TC. Therefore, the fixation of the nylon film on the paper was thought to be caused by both the physical bond between the paper and the ED, and the chemical bond between the ED and TC.
When chloroform was used as the organic solvent and 25% ED was used as the aqueous monomer for the interfacial polymerization, cracks in the nylon film formed on the paper surface were observed and formation of nylon film on the paper surface was difficult, as is shown in Fig. 3c and Table 1. In contrast, the nylon film prepared using cyclohexane as the organic solvent and 25% ED as the monomer could form on the paper surface, as shown in Fig. 2b and Table 1.
The influences of the ED concentration and the type of organic solvent on the thickness of the nylon film formed on the paper surface are shown in Fig. 4. When chloroform was used as the organic solvent with 25% ED (Fig. 3c), the thickness of the nylon film formed on the paper surface was evaluated from the thickness of the nylon film remaining on the paper surface, as shown in Fig. 3c.
The thickness of the nylon film prepared on the paper surface increased with an increasing ED concentration. When an ED concentration of 25% was used, the nylon film on the paper surface prepared using chloroform was thicker than that prepared using cyclohexane. The thickness of the nylon film on the paper surface formed using chloroform was 24.7 μm. Fixation of the nylon film on the paper surface was found to be difficult when the thickness of the nylon film formed on the paper surface by interfacial polymerization was reached about 25 μm. Thus, the nylon film prepared using the interfacial polymerization technique with chloroform and ED concentration of 25% could not fix onto the paper surface.
Preparation conditions for nylon microcapsules formed on the paper surface using interfacial polymerization
The SEM images shown in Figs. 3 and 5 show the effect of chloroform and cyclohexane, respectively, on the morphology of the nylon film.
Figure 5 shows the nylon microcapsules on the paper surface prepared using interfacial polymerization with cyclohexane as the organic solvent. The average particle size tended to increase with increasing ED concentration, as shown in Table 2. However, the formation of nylon microcapsules on the paper surface was not confirmed when chloroform was used as the organic solvent (Fig. 3). The different results obtained for the different kinds of organic solvents with respect to the formation of nylon microcapsules on a paper surface were thought to be caused by the spreading coefficient (S) of the organic solvent on the ED solution. S was calculated from the values of the surface tension of the ED solution, cyclohexane and chloroform, and the interfacial force of ED solution–cyclohexane and ED solution–chloroform, as shown in Table 3. It is well known that S is utilized for the evaluating the wetting of oil drops on water; S < 0 or S > 0 cause oil drops on water to form a spherical morphology or to spread over water like a membrane, respectively [18]. Ss of cyclohexane on 2.5% ED, 10% ED and 25% ED solutions were −3.30, −1.30 and −0.30, respectively. Therefore, when cyclohexane was used as the organic solvent, the TC solution of cyclohexane was though to have a spherical morphology on the paper surface, and the microcapsules on the paper surface obtained by the reaction between ED and TC at the oil–water interface. While, Ss of chloroform on 2.5% ED, 10% ED and 25% ED solutions were 17.0, 18.5 and 19.3, respectively. In the case of chloroform, the TC solution of chloroform spread over the filter paper like a membrane, and so nylon microcapsules could not be formed on the paper surface whereas a nylon membrane was. Our results show that the spreading coefficient of the organic solvent on the ED solution adsorbed on the paper surface is an important factor in the preparation of nylon microcapsules on a paper surface by interfacial polymerization. In conclusion, nylon microcapsules can be formed on a paper surface by utilizing interfacial polymerization with cyclohexane as the organic solvent. This interfacial polymerization technique, the method used to form nylon microcapsules at the oil–water interface formed on the paper surface, is effective for the preparation of smart paper that has intelligent microcapsules, without requiring the use of a binder.
Conclusions
Nylon microcapsules were formed on a paper surface using interfacial polymerization with cyclohexane as the organic solvent. When chloroform was used as the organic solvent instead, nylon microcapsules could not be obtained. These results are due to the spreading coefficient of the organic solvent on the ED solution. Ss of cyclohexane and chloroform on an ED solution were S < 0 and S > 0, respectively. As the TC solution formed a spherical structure at the oil–water interface on the paper surface when cyclohexane, which resulted in S < 0, was used, it was thought that the microcapsules were prepared on the paper surface by a reaction between the ED and the TC at the oil–water interface on the paper surface. Therefore, interfacial polymerization is useful for the preparation of nylon microcapsules directly on paper surfaces, and hence for smart paper, when microcapsules with intelligent function are used. The interfacial polymerization technique would be expected to be a useful method for the fixation of intelligent material onto paper.
References
Okahata Y, Lim H-J, Nakamura G, Hachiya S (1983) J Am Chem Soc 105(15):4855
Chen C-W, Arai K, Yamamoto K, Serizawa T, Akashi M (2000) Macromol Chem Phys 201(18):2811
Kim I-S, Jeong Y-I, Cho C-S, Kim S-H (2000) Int J Pharm 205:165
Ramkissoon-Ganorkar C, Liu F, Baudyš M, Kim SW (1999) J Controll Rel 59:287
Shino D, Murata Y, Kubo A, Kim YJ, Kataoka K, Koyama Y, Kikuchi A, Yokoyama M, Sakurai Y, Okano T (1995) J Controll Rel 37:269
Kataoka K, Miyazaki H, Bunya M, Okano T, Sakurai Y (1998) J Am Chem Soc 120:12694
Hisamitsu I, Kataoka K, Okano T, Sakurai Y (1997) Pharm Res 14(3):289
Zrínyi M (2000) Colloid Polym Sci 278:98
Watanabe H (1998) Sol Energy Mater Sol Cells 54:203
White SR, Sottos NR, Geubelle PH, Moore JS, Kessler MR, Sriram SR, Brown EN, Viswanathan S (2001) Nature 409:794
Ichiura H, Morikawa M, Fujiwara K (2005) J Mater Sci 40:1987
Matsubara H, Takada M, Koyama S, Hashimoto K, Fujishima A (1995) Chem Lett 767
Iguchi Y, Ichiura H, Kitaoka T, Tanaka H (2003) Chemosphere 53:1193
Ichiura H, Kitaoka T, Tanaka H (2002) J Mater Sci 37(14):2937
Ichimura K (2000) In: Development of chromic materials. CMC Publishing Co., Ltd., Japan, pp 253–261 (in Japanese)
Park S-J, Shin Y-S, Lee J-R (2001) J Colloid Inter Sci 241:502
Makino K, Arakawa M, Kondo T (1981) J Colloid Inter Sci 83(2):652
Kawaguchi M (1999) In: Interface and colloid science of polymer. Corona Publishing Co., Ltd., Japan, pp 15–16
Acknowledgements
This work has been supported in part by a Grant-in-Aid for Cooperation of Innovative Technology and Advanced Research in Evolutional Areas (CITY AREA) from the Ministry of Education, Science and Culture, Japan. Dr. Biance Kuipers and Dr. Kerry Greer kindly made a linguistic revision of the manuscript.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Ichiura, H., Morikawa, M. & Ninomiya, J. Preparation of smart paper part I—formation of nylon microcapsules on paper surface using interfacial polymerization. J Mater Sci 41, 7019–7024 (2006). https://doi.org/10.1007/s10853-006-0789-x
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10853-006-0789-x