Abstract
Monoliths that set rapidly and harden at ambient temperature have been prepared by exploiting sol–gel type condensation reactions between sodium silicate, formed in-situ by alkaline dissolution of silica fume, and a solution of sodium aluminate. Structural characterisation of the product was carried out by XRD, 27Al and 29Si MAS NMR spectroscopy) and the samples showed reproducibly good compressive strengths, indicating that the products display all the characteristics of typical aluminosilicate geopolymers.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
The term geopolymer was originally applied to three-dimensional aluminosilicate materials formed by condensation of a solid aluminosilicate source such as dehydroxylated kaolinite (metakaolin) with an alkali silicate solution under highly alkaline conditions [1]. Industrial waste products such as fly ash and blast furnace slag have received much attention as geopolymer raw materials because of their lower cost and reported superior mechanical strength and durability relative to products from other starting materials such as metakaolin [2–4]. However, the chemical and mineralogical composition of fly ash and slag is far more complex than metakaolin and can change depending on the conditions of the manufacturing processes from which they are derived. In addition, competing reaction mechanisms operating in fly ash and slag-based systems result in the co-precipitation of products such as calcium and magnesium aluminium silicate hydrates. As a result, elucidation of the chemistry of geopolymer formation from fly ash and slag-based materials remains a challenge.
The use of simpler precursors such as clay or other aluminosilicate minerals renders the chemical system less complex by reducing the number of competing mechanisms or removing them altogether. Nevertheless, differences in the structural and chemical characteristics of aluminosilicate minerals give rise to variations in their reactivities. While thermal dehydroxylation will render some aluminosilicate minerals suitable as geopolymer starting materials, this is not always effective. Some minerals will not form a viable geopolymer by simple addition of alkali silicate. For example, pyrophyllite exhibits little geopolymer reactivity after heating to 800 °C. This has been provisionally attributed to the shielding effect of the silicate layers, which hinder access of the alkaline solution to the inner aluminate layer [5]. Aluminosilicate minerals, while more homogeneous than fly ashes or slags, still retain some chemical and structural complexity even after pre-treatment.
These considerations have led to the present investigation of an even simpler chemical system than that involving solid aluminosilicates. Monolithic samples have been synthesised using silica fume as the silicate source. To provide a fully labile source of aluminium, sodium aluminate solution was added to the alkaline solution of silica fume. Selected structural properties of the silica fume/sodium aluminate-based monolith are reported and compared with a number of structural criteria that have been suggested [6] to constitute the definitive characteristics of geopolymer materials formed from other aluminosilicate systems.
Experimental
Stock solutions of sodium hydroxide (Scharlau, Australia) and sodium aluminate (Fisher Scientific, Australia) were prepared using de-gassed, distilled water and allowed to cool to ambient temperature. The slight brown colour of the sodium aluminate solution was attributed to the trace iron noted in the product specification sheet. The appropriate mass of silica fume (SF98, Australian Fine Minerals) was added to the calculated volume of sodium hydroxide stock solution and mixed with a hand-held blender until it formed a consistent slurry. The opaque appearance of the slurry was attributed to the presence of partially dissolved or undissolved silica fume particles.
The silica fume slurry was added to the sodium aluminate solution with stirring. When addition was complete, the mixture was stirred for a further 5 min, then cast into a plastic mould with a lid and allowed to stand at 40 °C for approximately 48 h.
The cured monoliths were demoulded and characterised by X-ray diffraction (XRD) and solid-state magic angle spinning nuclear magnetic resonance spectroscopy (MAS NMR). Compressive strength tests were carried out on three monolith cubes using an Instron Model TTKM 25 tonne Universal Tester with a crosshead speed of 10 mm min−1. A fragment of monolith from the crushing test was retained, powdered and dried overnight at 65 °C for the remaining analyses. X-ray diffraction (XRD) was performed using a Phillips PW 1700 computer-controlled goniometer with graphite monochromator and CoKα radiation. The 27Al and 29Si MAS NMR spectra were acquired at 11.7T using a Varian Unity 500 spectrometer with 4 and 5 mm Doty MAS probes spun at 10–12 kHz, under the following conditions:
-
27Al: spectrometer frequency 130.244 MHz with a 1 μs (π/10 pulse for solution) and a 1 s delay, spectra referenced to Al(H2O) 3+6 .
-
29Si: spectrometer frequency 99.926 MHz with a 6 μs (π/10 pulse for solution) and a 100 s delay, spectra referenced to tetramethylsilane (TMS).
Results
Figure 1a shows the XRD trace obtained from a cured monolith in which the H2O/Na2O ratio is 12. This consists of amorphous material, with superimposed peaks corresponding to synthetic zeolite LTA (ICDD file No. 89-8015). As the H2O/Na2O ratio increases to 15, zeolite LTA is almost exclusively present. When the water content is decreased to <10, the material is X-ray amorphous, and contains no crystalline zeolite LTA (Fig. 1b). This sample shows a broad XRD peak maximum located near 30–35°2θ, with slight indication of minor peaks between 20–40°2θ, possibly arising from gibbsite, Al(OH)3. Further studies were confined to the samples of lower water content, which show the typical XRD-amorphous characteristics of geopolymers.
Each monolith showed a slight brown surface discolouration, attributable to the presence of iron in the sodium aluminate solution. After curing, the monoliths achieved sufficient strength to be demoulded and handled, and showed no visual evidence of cracking. The compressive strengths obtained for triplicate samples were 26.3, 26.1 and 26.3 MPa, this small variation being due to slight inhomogeneity in starting mixture. The average compressive strength of 26 MPa indicates that significant microstructural development has occurred despite the relatively mild curing conditions.
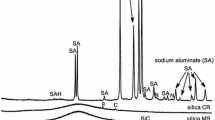
Figure 2 shows the 27Al MAS NMR spectra of the aluminate starting material and the monolithic product. The spectrum of sodium aluminate shows a sharp resonance at 78.3 ppm indicative of crystalline material containing aluminium in tetrahedral co-ordination. The corresponding 27Al MAS NMR spectrum of the monolith shows that the aluminium is still in tetrahedral co-ordination, but the increased broadness of the NMR resonance indicates a greater range of tetrahedral environments, consistent with the X-ray-amorphous nature of the product. The upfield shift in the NMR peak also indicated that the Al environment of the product has changed by the inclusion of Si nearest neighbours. There is also evidence of a very small, broad octahedral Al resonance, which is also present in the sodium aluminate starting material.
Figure 3 shows the 29Si MAS NMR spectra of starting material, silica fume, and the monolithic product. The upper spectrum of silica fume contains a single broad Q4 resonance at −109 ppm, indicative of amorphous SiO2. The lower 29Si MAS NMR spectrum of the monolithic product exhibits three features, a Q3 (4Al) resonance at −87 ppm, a Q3 (0Al) resonance at −97 ppm and a Q4 resonance at −109 ppm similar to the original silica fume reactant.
Discussion
Although there is no definitive set of criteria that define a geopolymer, the literature suggests that a geopolymer should demonstrate the following structural characteristics [6–8]:
-
(1)
the material sets and hardens at ambient temperature;
-
(2)
the material is X-ray amorphous;
-
(3)
the 27Al MAS NMR spectrum indicates tetrahedral aluminium co-ordination;
-
(4)
the 29Si MAS NMR spectrum indicates a range of Q3 environments corresponding to cross-linked silicate bonds.
The present monolithic samples are in good agreement with these criteria. In the first instance, significant strength is developed reproducibly within the monolith, such that it will bear a compressive load of 26 MPa after curing under relatively mild conditions. This strength does not appear to be due solely to the drying of excess sodium silicate between the sol–gel particles, since the monolith did not fragment when immersed in fresh distilled water overnight at room temperature.
While the X-ray diffractogram (Fig. 1b) shows the monolith is essentially X-ray amorphous, there is slight evidence of a gibbsite-like product, which may also be responsible for the minor amounts of octahedral Al in the 27Al MAS NMR spectrum. It should be noted that the monolith preparations were not performed in an inert atmosphere and in air aluminate is known to react with carbon dioxide to yield gibbsite as one of the reaction products [9].
It is probable that if gibbsite is formed in this system, it would result from this reaction pathway or be present as a contaminant rather than form as a co-precipitate. The SiO2/Al2O3 ratio used here does not provide the excess aluminate favouring gibbsite formation conditions. The 27Al MAS NMR spectra indicate that, apart from a small amount of intensity in the octahedral region of the spectra which may arise from the presence of a small amount of poorly ordered gibbsite, the vast majority of the aluminium is incorporated into the tetrahedral geopolymer framework.
The 29Si MAS NMR spectra show that in addition to the resonance at −109 ppm denoting uncombined amorphous silica, two Q3 silicon environments are present, namely Q3 (4Al) (−88 ppm) and Q3 (0Al) (−97 ppm). The Q3 (0Al) shift is more negative compared to the Q3 shifts assigned as cross-linked geopolymer units reported in other works (typically a broad resonance centred at about –92 ppm [6–8, 10]). The broadness of this envelope encompasses a range of shifts, including the present Q3 (0Al), but the greatest concentration of silicate units in more conventional geopolymers are those which contain more nearest-neighbour aluminium atoms, as evidenced by the centre-of-gravity of this broad spectral feature. The present Q3 (0Al) peak may be evidence of the presence of a significant proportion of partially dissolved silica fume particles. The less negative Q3 (4Al) peak indicates the presence of cross-linking silicate units of silicate saturated with aluminium which might form the strength-providing framework of the monolith. Further work is required to investigate this hypothesis. The Q4 shift suggests that some silica fume particles have persisted within the structure, and may act as fillers, lowering the overall porosity of the structure and contributing to the compressive strength.
The monoliths reported here have been made by sol–gel condensation reactions between sodium aluminate and sodium silicate formed in-situ by dissolution of silica fume particles in an alkaline solution. Often in sol–gel preparations, water is very much in excess and the silicate and aluminate species react together to yield a voluminous precipitate that is stabilised by an alkali counter ion, commonly Na+ (aq); these systems do not, however, develop significant strength. The present synthesis does not involve excess water, since the water content appears to influence the product outcome; too much water facilitates the formation of a zeolitic phase, in some cases to the exclusion of the geopolymer. Another point of difference between the present monolith and a standard sol–gel product is the presence of partially dissolved or undissolved particles in the former. These factors combine to produce a material with reasonable compressive strength, which also meets the other basic structural criteria for a geopolymer. Further structural characterisation of these monoliths is underway and will be reported later.
References
Davidovits J (1991) J Thermal Anal 37:1633
Swanepoel JC, Strydom CA (2002) Appl Geochem 17:1143
van Jaarsveld JGS, van Deventer JSJ, Lukey GC (2003) Mater Lett 57:1272
Cheng TW, Chiu JP (2003) Minerals Eng 16:205
MacKenzie KJD, Brew DRM, Fletcher RA, Vagana R, J Mater Sci (submitted) (2005)
MacKenzie KJD (2003) Ceram Trans 153:175
Barbosa VFF, MacKenzie KJD, Thaumaturgo C (2000) Int J Inorg Mater 2:309
Fletcher RA, MacKenzie KJD, Nicholson CL, Shimada S (2005) J Eur Ceram Soc 25(9):1471
Taylor HFW (1997) Cement chemistry, 2nd ed. Thomas Telford, London, UK
Puyam SS, Trigg M, Burgar I, Bastow T (2005) Mater Sci Eng A 396(1–2):392
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Brew, D.R.M., MacKenzie, K.J.D. Geopolymer synthesis using silica fume and sodium aluminate. J Mater Sci 42, 3990–3993 (2007). https://doi.org/10.1007/s10853-006-0376-1
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10853-006-0376-1