Abstract
Amorphous indium oxide (In2O3) and 10-wt% SnO2 doped In2O3 (ITO) thin films were prepared by pulsed-laser deposition. These films were crystallized upon heating in vacuum at an effective heating rate of 0.00847 °C/s, while the evolution of the structure was observed by in situ X-ray diffraction measurements. Fast crystallization of the films is observed in the temperature ranges 165–210 °C and 185–230 °C for the In2O3 and ITO films, respectively. The crystallization kinetics is described by a reaction equation, with activation energies of 2.31 ± 0.06 eV and 2.41 eV and order of reactions of 0.75 ± 0.07 and 0.75 for the In2O3 and ITO films, respectively. The structures of the films observed here during heating are compared with those obtained upon film growth at different temperatures. The resistivity of the films depends on the evolution of the structure, the oxygen content and the activation of tin dopants in the films. A low resistivity of 5.5 × 10−4 Ω cm was obtained for the In2O3 and ITO films at room temperature, after annealing to 250 °C the resistivity of the ITO film reduces to 1.2 × 10−4 Ω cm.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Indium oxide or indium-tin-oxide (In2O3 or ITO) film is a transparent conductor. It is found in many technological applications, including flat panel displays, solar energy and other optoelectronic devices because of its excellent combination of electrical and optical properties [1, 2]. In2O3 or ITO film exists in amorphous or crystalline phase. Crystalline films, which display better electro-optical properties, are acquired when the films are deposited on heated substrates or after annealing of the amorphous films. It has a complex cubic bixbyte Mn2O3 (I) structure emanating from a range of vacant tetrahedral oxygen anion sites. The structure of In2O3 film is affected by the growth conditions such as substrate temperature, oxygen pressure and deposition rate [1, 2].
The crystallization processes and dynamics of nucleation of In2O3 and ITO films have been reported [2–10]. The results show a disparity between the two materials and are sometimes dependent on the processing techniques. In the case of sputtering and pulsed laser deposition (PLD), the substrate temperature and the energy of the particles are found to have profound effect on the properties of the growing films [3]. During growths, the ratio of the amorphous to crystalline phase reduces with increasing temperature, the film still remaining partially amorphous at (150–165) °C, the so-called crystallization temperature [5, 11, 12]. Since the structures of the In2O3 and ITO films are directly related to their electrical and optical properties, a clear understanding of the crystallization kinetics is useful for applications where high-quality films have to be deposited at a low substrate temperature.
Studies of the crystallization of ITO or In2O3 films by X-ray diffraction (XRD) is commonly done on samples that have been cooled to room temperature after crystallization [4, 7, 9]. This method is uncertain because; (i) cooled samples may not represent the conditions at high temperature; (ii) the structure of the samples may alter on cooling; (iii) different samples are used which often makes reproduction of the sample conditions difficult. Indirect methods involving time dependent changes in the resistivity, reflectivity, or examination of the microstructure have been used to explain the crystallization of In2O3 and ITO films [5, 7]. These methods only presume that the change in the resistivity or reflectivity of the films during annealing translates into the amorphous–crystalline transition [5, 10]. Based on the different analytical techniques and differently prepared samples, crystallization temperatures ranging from 100 °C to 250 °C have been reported [3–10]. Thin film crystallization using real-time in situ XRD removes these doubts and has been rarely used for In2O3 or ITO films [3, 6].
We have followed the evolution of the structure of In2O3 and ITO by in situ XRD measurements at temperatures from 100 °C to 300 °C. The structures of the films observed here after heating are compared with those obtained upon film growth at different temperatures. The electrical and optical properties of the as-deposited films at different oxygen pressures and temperatures are also reported.
Experimental
In2O3 and ITO (In2O3 doped with 10 wt.% SnO2) films were deposited using a Lambda Physik, COMPex 102 KrF (λ = 248 nm) excimer laser. Details on the PLD apparatus have been reported elsewhere [2]. Sintered ceramic In2O3 and ITO targets with a purity of 99.997% were used. The laser system was operated at an energy of ∼230 mJ and a pulse frequency of 20 Hz. Amorphous and polycrystalline films were deposited on single crystal Si(100) substrates at oxygen pressures of 10−3 to 5 × 10−2 Torr and substrate temperatures of 23 °C (room temperature) to 300 °C. The nominal deposition rate and film thickness were 2 nm min−1 and 100 ± 20 nm, respectively.
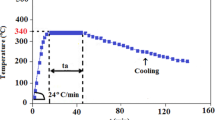
The amorphous to crystalline transitions of the In2O3 and ITO film were monitored by XRD in vacuum during real-time annealing over temperatures ranging from 100 °C to 300 °C. The temperature was raised in steps of 5 °C at an effective heating rate of 0.00847 °C/s. The XRD analyses were performed using a Rigaku Rint-2000 and a custom built X-ray diffractometers with CuKα radiation (λ = 1.542 Å). The custom built diffractometer operating in the θ–θ mode equipped with pyrolytic graphite monochromator and analyzer crystals, and a scintillation counter was used. The sample stage was mounted with a Boraelectric Heater capped with an aluminum plate. The sample was mounted on the aluminum plate with thermal paste to allow good thermal contact. The temperature difference between the heater and the sample was ±2 °C. To avoid air scattering, samples were measured in a vacuum chamber with a Kapton window at a pressure of 10−2 Torr. The scattered XRD intensity was measured as a function of the scattering angle, 2θ. Each measurement during the annealing is a composite of two 10 min interlaced scans. One set of points was measured while increasing 2θ from 28o to 32°, while a second set of points is obtained subsequently at decreasing 2θ values. The XRD peaks were fitted by Gaussian profile to obtain peak parameters such as the peak position, peak width, peak area and correction for resolution. The crystal sizes of the films were calculated from the peak parameters.
The resistivity of the annealed films was measured using a four-point probe. The thickness and the real part of the optical refractive index were determined by an ellipsometer (Mizojiri DVA-36L3), with He–Ne laser beam operating at a wavelength of 632.8 nm.
Results and discussion
Structural properties
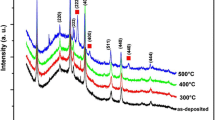
Figure 1 shows the XRD spectra of the (222) peak for the In2O3 and ITO films annealed in situ at 100 °C to 300 °C. The diffraction patterns represent the real-time phase changes during the crystallization process. During the annealing of the amorphous films, In2O3 is found to crystallize at a lower temperature of 165 °C compared to ITO film at a temperature of 220 °C. The In2O3 film also displays stronger peak intensity. The ‘zigzag’ seen in the XRD spectra result from interlacing of the upward (28–32°) and downward (32–28°) 2θ XRD scans during the annealing cycle. Thus the unavoidable change of the sample state while measuring becomes apparent, rather than giving rise to an artificial shift of the peak in 2θ. Plots of the XRD peak intensity and crystal sizes versus annealing temperature are shown in Fig. 2. Below 165 °C, In2O3 film is amorphous as shown in Fig. 2a. The (222) peak appears at 165 °C, indicating the outset of crystallization. The intensity of the peak increases rapidly as the annealing temperature increases to 210 °C. A further increase in the annealing temperature produces no peak growth.
In contrast, a higher crystallization temperature of around 220 °C is observed for the ITO films as shown in Fig. 1b. At 230 °C a fully crystalline ITO film is attained. Because of the weak (222) reflection, evidence of crystallization could only be observed at around 220 °C in Fig. 2. But from the plots of the peak intensity versus annealing temperature in Fig. 2b, it appears that film crystallization starts at around 200 °C. The evolution of the (222) peak for In2O3 and ITO at annealing temperatures of (165–210 °C) and (200–230 °C), respectively, shows a rapid crystallization process, which is completed within 45 °C from the outset temperature. That is a total time of 88 min for temperatures between 165 °C and 210 °C (including measuring times of 10 min per measurement under steady set temperatures). A transition in the crystallization mechanism is suspected to occur from pure grain nucleation to grain growth according to the structure zone model of thin films at temperatures of (165–210 °C) and (200–230 °C) for the In2O3 and ITO film, respectively [13]. A crystallization temperature of 165 °C agrees with that reported for In2O3 films [14–19]. However, different crystallization temperatures ranging from 150 °C to 250 °C have been reported for ITO films, including a recent work on real-time annealing of ITO films where a crystallization temperature of 250 °C was observed [6, 14–19]. Our findings are close to temperatures of 180 °C and 230 °C reported for sputter-annealed In2O3 and 10 wt. % SnO2 doped In2O3 films, respectively [7]. The different crystallization temperatures may be due to the inclusion of Sn in the ITO crystal lattice.
Figure 2b shows the development of the crystal sizes as a function of the annealing temperature. The mean crystal sizes are obtained from the full width at half maximum of the (222) peak based on the Debye-Scherrer method [20]. The crystal sizes of the emerging crystallites could only be resolved at temperatures above 170 °C and 220 °C for the In2O3 and ITO films, respectively. For In2O3 film, the crystal size increases rapidly between 170 °C and 200 °C and then stabilizes with a further increase in the annealing temperature, suggesting growth and impingements of the crystallized regions of the film. For a fully crystallized film, average crystal sizes of 32 and 26 nm are obtained for the In2O3 and ITO films, respectively.
The crystallization kinetics is based on the following reaction rate equation:
where E is the activation energy, and s is the order of reaction. T is temperature and A is frequency factor
The integral form of (1) gives.
Fitting Eq. (2) to the growth profiles in Fig. 2a, produce activation energies of 2.31 ± 0.06 eV and 2.41 eV for In2O3 and ITO film, respectively. Reaction rate factor, log(A/φ), gives 21.7 ± 0.7 and 21.7 (fixed), while the order of reaction gives 0.75 ± 0.07 and 0.75 (fixed) for the In2O3 and ITO films, respectively. In2O3 film yields better estimates of the reaction parameters while fitting the reaction equation. The activation energies of the In2O3 and ITO materials are similar; however these values are higher than 0.67 ± 0.18 eV, 0.77 eV or 1.3 ± 0.2 eV reported in the literature [ 5 , 8 , 10 ]. The low reaction order of ∼0.75 suggests that the crystallization kinetics is dominated by grain boundary nucleation/growth.
Figure 3 shows the full scan XRD spectra of the In2O3 and ITO films measured during annealing at 300 °C and at room temperature after the samples are cooled. The In2O3 films exhibit a strong (222) preferred orientation, but no preferred orientation is observed in the ITO films, Fig. 3a and b. The observation of peak at 33.1o (forbidden peak) belonging to Si(200) is due to the effect of the Si(100) substrate and it only occurs when the substrate is distorted [21]. The striking difference in the intensity of the Si(200) reflection contrasts the crystallilnity of In2O3 and ITO films. However, the suppression of the Si(200) peak intensity for the ITO sample (Fig. 3a) compared to Si(200) reflections for In2O3 sample (Fig. 3b) could originate from a mis-alignment of the Φ-axis which would move the scattered intensity originating from {k00} reflections of Si substrate out of coverage of the detector during the θ/2θ scans. In general, better crystallinity is obtained in the In2O3 compared to ITO films. The calculated lattice constants for the In2O3 films at 300 °C and room temperature are 10.23 ± 0.20 Å and 10.14 ± 0.01 Å, respectively. For the ITO films, the lattice constant is 10.12 ± 0.01 Å at 300 °C and 10.16 ± 0.01 Å at room temperature. No major change in the lattice constant is observed between the heated and cooled samples and the values are close to the theoretical value of 10.12 Å for cubic In2O3.
Figure 4 shows the XRD spectra of In2O3 and ITO films deposited at different substrate temperatures at a fixed oxygen pressure of 10−2 Torr. The In2O3 film is amorphous below 150 °C. At 150 °C and above, polycrystalline cubic bixbyite In2O3 appears. The film shows a preferred orientation along the (222) axis with increasing substrate temperature during the deposition. In the case of the ITO films, reflections are evident at room temperature. However, the (222) peak only appears at 200 °C and the film shows no preferential orientation.
In comparison, the crystallization temperature of In2O3 and ITO films deposited on heated substrates is lower than that of in situ annealed films, as shown in Figs. 1 and 4. It is known that energetic particle bombardment of the film surface during growths lowers the crystallization temperature [2, 6]. Therefore, the reduced crystallization temperature in the films deposited on heated substrate is caused by a rise of the chemical driving force due to energetic particles impinging on the film surface during growths [10, 14].
Electrical and optical properties
Figure 5 shows the dependence of the resistivity on the oxygen pressure for In2O3 and ITO films deposited at room temperature and 200 °C. At room temperature, comparable resistivities are achieved for the In2O3 and ITO films. However, the resistivity of the In2O3 film is slightly lower at 1 × 10−3 Torr. A minimum resistivity of 5.5 × 10−4 Ω cm is obtained for the In2O3 and ITO films at an oxygen pressure of 1 × 10−2 Torr. At higher oxygen pressures sharp increase in the resistivity of the films is noticed. For ITO films deposited at 200 °C, lower resistivities are obtained; however, the resistivity curves follow a similar trend with changes in the oxygen pressure. The resistivity of In2O3 is known to depend on the oxidation state of indium; hence the creation of oxygen vacancies dominated the free electron generation mechanism that lowered the resistivity of the In2O3 film [2, 16, 22–28].
Figure 6 shows the resistivity and the real part of the refractive index versus the substrate temperature for In2O3 and ITO films deposited at an oxygen pressure of 10−2 Torr. The resistivity of the films decreases with increasing substrate temperature up to around 150 °C (crystallization temperature) as shown in Fig. 6a. Above 150 °C, a marked rise in the resistivity of the In2O3 is noticed while that of ITO films continues to reduce exponentially with increasing substrate temperature. A minimum resistivity of 1.4 × 10−4 Ω cm is obtained for the ITO film above 250 °C. The sudden increase in the resistivity of the In2O3 films from 150 °C is associated with the depletion of oxygen vacancies upon crystallization [2, 7]. In ITO films, the presence of Sn lowers the resistivity due to thermal activation of Sn into Sn4+: as Sn finds substitutional sites during crystallization, it adds extra free electrons to the conduction band [22, 25, 28]. In amorphous ITO film, Sn is not efficiently activated, hence the higher resistivity at lower substrate temperatures.
The real part of the refractive indices is shown in Fig. 6b. Due to limitations in our ellipsometry equipment, only the real part of the refractive index is measured, the imaginary part could not be provided. Moreover, since the samples were deposited on silicon substrates, other information such as transmittance, reflectance and absorption coefficients of the films could not be determined. Other researchers have used only the real-part of the refractive index to describe the optical properties of their ITO films [6, 29–31]. From Fig. 6b, it is observed that between room temperature and 150 °C, the refractive indices are comparable. Above 150 °C, the refractive index of the In2O3 films increases, while that of the ITO decreases rapidly with increasing substrate temperature. The crossover region of the two curves at around 165 °C coincides with the crystallization temperature of In2O3. The observed refractive indices of 1.87 to 2.24 fall within the limits reported for In2O3 and ITO films [6, 29–31]. Note that the behavior of the refractive index and the resistivity with deposition temperatures are closely related, indicating the deposition conditions and the presence of Sn in the films affect both the electrical and optical properties of the In2O3 and ITO films. It has been reported that a change in the refractive index with changes in the ratio of In2O3 and SnO2 are associated with the carrier concentration of ITO films [32]. The changes in the refractive index can most reasonably be explained by the contribution of Sn or oxygen vacancies to the available carriers in the material, which affects the electro-optical properties of the films [33].
Conclusion
Amorphous indium oxide (In2O3) and 10-wt% SnO2 doped In2O3 (ITO) thin films prepared by PLD were crystallized upon heating in vacuum at an effective heating rate of 0.00847 °C/s, while the evolution of the structure was observed by in situ XRD measurements. The films crystallized in the temperature ranges 165–210°C and 200–230 °C for the In2O3 and ITO, respectively. The crystallization kinetics was described by activation energies of 2.31 ± 0.06 eV and 2.41 eV and reaction order of 0.75 ± 0.07 and 0.75 for the In2O3 and ITO films, respectively. The structures of the films observed here during heating were compared with those obtained upon film growth at different temperatures and the results show a lower crystallization temperature for films grown on heated substrates. The reduced crystallization temperature in the films deposited on heated substrates is ascribed to impinging energetic particles on the substrate surface during growths. The resistivity of the films depended on the evolution of the structure, the oxygen content and the activation of Sn dopants in the films. A low resistivity of 5 × 10−4 Ω cm was obtained for the In2O3 and ITO films at room temperature, while a value of 1.2 × 10−4 Ω cm was achieved for an ITO film at 250 °C.
References
Granqvist CG, (2000) Solar Energy Mater Solar Cells 60:201
Adurodija FO (2001) In: Nalwa HS (ed) Handbook of thin films: deposition and processing of films, vol 1. Academic Press, New York, p 161
Ellmer K, Mientus R, Weiß V, Rossner H (2003) Meas Sci Technol 14:336
Diniz ASAC, Keily CJ (2004) Renewable Energy 29:2037
Ow-Yang CW, Springer D, Shigesato Y, Paine DC (1998) J Appl Phys 88:145
Rogozin A, Shevchenko N, Vinnichenko M, Prokert F, Cantelli V (2004) Appl Phys Lett 85:212
Morikawa H, Fujita M (2000) Thin Solid Films 359:61
Wulff H, Quaas M, Steffen H, Hippler R (2000) Thin Solid Films 377–378:418
Morikawa H, Sumi H, Kohyama M (1996) Thin Solid Films 281–282:202
Paine DC, Whitson T, Janiac D, Beresford R, Ow-Yang CW (1999) J Appl Phys 85:8445
Muranaka S, Bando Y, Tanaka T (1987) Thin Solid Films 25:35
Muranaka S (1991) Jpn J Appl Phys 30:L2062
De Beardemaeker J, Dauwe C, Deduytsche D, Detavernier C, Egger W, Sperr P (2004) Mater Sci Forum 445–446:69
Song PK, Shigesato Y, Yasui I, Ow-Yang CW, Paine DC (1998) Jpn J Appl Phys 37:1870
Oyama T, Hashimoto N, Shimazu J, Akao Y, Kojima H, Aikawa K, Suzuki K (1992) J Vac Sci Technol A 10:1682
Sun XW, Huang HC, Kwok HS (1996) Appl Phys Lett 68:2663
Thornton JA, Hoffman WD (1989) Thin Solid Films 171:5
Bardos L, Libra M (1989) Vacuum 39:33
Bender M, Katsarakis N, Gagaoudakis E, Hourdakis E, Douloufakis E, Cimalla V, Kiriakidis G (2001) J Appl Phys 90:5382
Bertaut E (1968) In: International tables for x-ray crystallography, vol 3. Kynoch Press, Birmingham, p 318
Zhao L, Steinhart M, Yosef M, Lee SK, Geppert T, Pippel E, Scholz R, Gosele U, Schecht S (2005) Chem Mater 17:3
Tahar RBH, Ban T, Ohya Y, Takahashi Y (1998) J Appl Phys 83:2631
Adurodija FO, Bruning R, Asia IO, H Izumi, Ishihara T, Yoshioka H (2005) Appl Phys A 81:953
Izumi H, Adurodija FO, Kaneyoshi H, Ishihara T, Yoshoika Y, Motoyama M (2002) J Appl Phys 91:1213
Kim H, Gilmore C, Pique A, Horwitz JS, Matoussi H, Murata H, Kafifi ZH, Chrisey DB (1999) J Appl Phys 86:6451
Kwok HS, Sun XW, Kim DH (1998) Thin Solid Films 335:229
Adurodija FO, Izumi H, Ishihara T, Yoshioka Y, Matsui H, Motoyama M (1999) Appl Phys Lett 74:3059
Yu Y, Maree CHM, Haglund RF Jr, Hamilton JD, Morales Paliza MA, Huang MB, Felman LC, Weller RA (1999) J Appl Phys 86:991
Jung YS (2004) Thin Solid Films 467:36
Martino M, Luches A, Fernandez M, Anobile P, Petruzzelli V (2001) J Phys D: Appl Phys 34:2606
Losurdo M, Griangregorio M, Capezzuto P, Bruno G, De Rosa R, Roca F, Summonte C, Pla J, Rizzoli R (2002) J Vac Sci Tech A 20:37
Chen RT, Robinson D (1992) Appl Phys Lett 60:1541
Yeom HY, Popovich N, Chason E, Paine DC (2002) Thin Solid Films 411:17
Acknowledgments
This project is partly sponsored by Atlantic Innovation Fund (AIF) and Natural Science and Engineering Research Council (NSERC).
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Adurodija, F.O., Semple, L. & Brüning, R. Crystallization process and electro-optical properties of In2O3 and ITO thin films. J Mater Sci 41, 7096–7102 (2006). https://doi.org/10.1007/s10853-006-0038-3
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10853-006-0038-3