Abstract
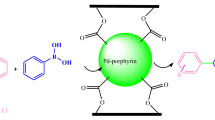
In the current study, a Pd–porphyrin catalyst was anchored covalently onto functionalized polymeric spine based on a calix[4]resorcinarene. The achieved catalytic system was characterized, and its catalytic activity was investigated in the reduction of nitroarenes. The main advantages of the present work are: using water as green solvent, catalyst reusability, easy refinement of the products, excellent yields, and relatively short reaction times.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
The reduction of nitroarene is a common and simple way to produce amino-aromatics (AAs), which are versatile intermediates and precursors in the preparation of pharmaceuticals, pigments, dyes, agrochemicals, and polymers [1, 2]. The most typical way to generate aromatic amines is reduction of corresponding nitroarenes. One of the customary and interesting methods to reduce of the nitro group is catalytic transfer hydrogenation. Recently, noble metal nanoparticles are known for their modern applications in the field of catalysis, biotechnology, bio-engineering, and environmental remediation [3, 4]. Hence, the synthesis of noble metal nanoparticles and also their applications, particularly in catalysis, are of major interest for further studies, among which, palladium is the most effective catalyst in the surface hydrogenation reaction due to the simple availability of valence electrons. Therefore, many research groups have focused their interest on the expansion of efficient and active catalytic system. Porphyrins and metalloporphyrins are found widely in nature. They play important roles in the basic mechanisms of vital organisms [5,6,7,8]. These systems have been extensively studied due to the catalytic application of organic synthesis, electrical light applications, and therapeutic properties. Although, they are known as homogeneous catalysts and their role can be highlighted by selectivity [9,10,11], their industrial applications have been limited due to the difficulty in the separation of catalysts from products. To take the advantages of both homogeneous and heterogeneous catalysts, immobilizing porphyrin onto high surface solids would be a powerful approach. A large number of materials were selected to be the supports of metal species in hydrogenation reactions, such as silica, zeolite, carbonaceous materials, metal oxides, and etc. Unlike the above compound, polymers are applicable and attractive support materials to synthesize of heterogeneous catalysts.
Today, porous organic polymers (POPs) with various potential applications attract a great deal of attention by incorporating porous properties with organic functionalization features. In this procedure, meso porous organic polymers (mesoPOP)s have been known incredibly, having to their structural pattern which equip suitable situations for immobilizing homogeneous catalysts and making them heterogenized [12, 13]. In fact, these extremely cross-linked amorphous polymers’ porosity fortifies the catalytic activity of these materials by raising the number of accessible catalytic sites, increasing their concentration, adsorbing reactant molecules, and therewith growing the reaction rates. 3D-Network polymer based on calix[4]resorcinarene due to easy preparation and its unique properties, such as high thermal and chemical stability coupled with its susceptibility for various chemical modifications (acylation, alkylation, or silylation of hydroxyl groups and also nucleophilic substitution of the aromatic rings) under mild reaction conditions encouraged us to serve it. Under this light, as part of our continuing efforts to develop high performance and environmentally friendly procedures for various important reactions and transformations and according to our new interest in the application of the 3D-network porous polymer based on calix[4]resorcinarene for the preparation of biologically important molecules [14,15,16,17,18,19,20,21], herein, for the first time, we report the functionalization of polymeric calix[4]resorcinarene via a covalently anchored metalloporphyrin and the investigation of its efficiency as a heterogeneous catalyst for the synthesis of arylamines through the reduction of nitroarenes in water.
Experimental
Materials and methods
All the chemicals were purchased from Merck, Fluka and Aldrich companies. The progress of the reactions were monitored by TLC and the reported yields referred to isolated products. Products were characterized by comparison of their spectroscopic data (1H and 13C NMR, IR) with those reported in the literature. The IR spectra were recorded on a Perkin Elmer Spectrum Version 10.4.4. FT-IR spectrophotometer via KBr pellets for the samples and catalyst in the range of 4000–400 cm−1. The SEM analyses were carried out using a LEO 1455VP scanning electron microscope, operating at 1–30 kV. The polymeric texture and its chemical composition was investigated using a Zeiss-EM10C transmission electron microscope (TEM) operated at 80 kV. Ultraviolet–Visible (UV–Vis) spectra were obtained from a Shimadzu UV-2600 UV–Vis spectrophotometer. Analyses for liquid samples were accomplished using a quartz cell with 1 cm path length. 1H and 13C NMR spectra were recorded in CDCl3 on a Bruker Advanced DPX 400 MHz spectrometer using TMS as internal standard. The heterogeneous catalysts were identified by X-ray powder diffraction (XRD) using a STOE diffractometer with Cu-Ka radiation (l = 0.15418 nm) at 40 kV and 40 mA and amount of Pd in the prepared catalyst was specified by a Perkin-Elmer ICP analysis.
Preparation of catalyst
Synthesis of calix[4]resorcinarene (1)
Calix[4]resorcinarene was made based on the already mentioned procedure [22, 23].
Synthesis of the 3D‑network polymer based on calix[4]resorcinarene 2
In a round-bottomed flask, 42 mmol of formaldehyde was added to 14 mmol of the prepared calix[4]resorcinarene, and the mixture was dissolved in 40 mL NaOH solution (10%) at room temperature. The resulted mixture was heated to 90 °C and maintained at this temperature for 20 h. The surplus alkali was washed out from the resultant gel with cold water. Then, the gel was allowed to stand at 100 °C for 1 h. Then, the gel became acidic via treatment with the 0.1 M HCl solution. The produced solid was dried at 100 °C for 10 h [24].
Preparation of meso-tetrakis[4-(methoxycarbonyl)phenyl]porphyrin 3
Meso-tetrakis[4-(methoxycarbonyl)phenyl]porphyrin was made through modifying of A. D. Adler-F. R. Longo method [25]. Briefly, in a typical reaction, 2.33 mmol of freshly distilled pyrrole and 2.33 mmol of p-methoxycarbonylbenzaldehyde were added into 200 mL acetic acid. The mixture was refluxed for 4 h and cooled based on the room temperature. After cooling, the mixture was added to 100 ml methanol in an ice bath. The purple crystals were filtrated and washed with methanol and hot distilled water. Then, crude product was purified by column chromatography (silica gel, ethyl acetate /n-hexane = 20:1 as an eluent), and a desired purple solid of meso-tetrakis[4-(methoxycarbonyl)phenyl]porphyrin was obtained (37%).
Synthesis of Pd-porphyrin 4
In a round-bottomed flask, PdCl2 (0.135 g) was added into a solution of meso-tetrakis[4-(methoxycarbonyl)phenyl]porphyrin (H2TMCPP) (0.3 g) in 100 ml of DMF and refluxed for 12 h. Then, Pd-TMCPP was purified by recrystallizing frequently and precipitating from DMF–H2O solutions [26].
Synthesis of Pd-porphyrin@ polymer 5
According to the method reported by Fareghi-Alamdari, first, 5gr of polymer(2) and 0.5 gr Pd-porphyrin[4] was added in 20 ml DMF after heating at 130 °C for 36 h. Then, the reaction mixture was cooled and filtered. Afterwards, it was washed exhaustively with DMF and deionized water. Finally, The produced catalyst was dried and stored under vacuum (Scheme 1) [27].
Typical method for the reduction of nitroarenes catalyzed by Pd-porphyrin@polymer
In a round bottom flask, nitrobenzene (1 mmol), NaBH4 (4 mmol), catalyst (15 mg), and water were added and heated at 55 °C, then, reacted for the required time. The completion of the reaction was monitored by TLC, using ethyl acetate/ n-hexane (1:10) as eluent. After the finalization of the reaction, the Pd-porphyrin@polymer was separated from the reaction mixture by filtration. Then, the product was extracted from the liquid with Et2O and purified by column chromatography using n-hexane/ ethyl acetate as solvent to obtain the pure compound.
Results and discussion
Growing interest in applying heterogeneous catalysis for developing useful and green synthetic methodologies in chemical transformations, prompted us to report an efficient, convenient, and practical procedure for synthesizing a series of aromatic amines using the new developed metalloporphyrin immobilized on network polymer based on calix[4]resorcinarene as an eco-friendly solid supported catalyst with high catalytic activity under mild conditions. Scheme 1 shows the synthetic strategy for the mentioned catalyst preparation. To achieve this purpose, first, the 3D-network polymer2 was prepared via the reaction of resorcinol and acetaldehyde, followed by the polycondensation of calix[4]resorcinarene1 with formaldehyde [22, 24] as outlined in Scheme 1. The formation of compounds 1 and 2 was confirmed through a series of spectroscopic analysis (available in the ESI) [14]. In the second step, the porphyrin ligand was synthesized based on the results of the previous reports [25]. Then, the porphyrin ligand was metalated [26] (compound 4). Subsequently, metalloporphyrin immobilized on polymer 2 was produced as a result of the reaction between compound 2 and compound 4 (Scheme 1). To verify the covalent interactions between metalloporphyrin and polymer in metallporphyrin@polymer, FTIR spectra were recorded (Fig. 1). As shown in Fig. 1a, d different IR spectra were observed for porphyrin and Pd-porphyrin complex. Moreover, the results indicated the stretching and bending frequencies of the N–H bond related to free base porphyrin placed at ~ 3310 and 960 cm−1. However, the palladium ion was entered into the porphyrin ring, the N–H bond vibration frequency of porphyrin ligand was vanished, and the functional groups of Pd–N bond were established at ~ 1000 cm−1, which resulted in forming Pd–porphyrin complex [28]. Additionally, the bands as 1722 and 1604 cm−1 were assigned to carbonyl of ester and C=N, respectively. In addition, a broad band at 3200–3600 cm−1 revealed that all phenolic hydroxyl groups of polymer 2 were not completely functionalized due to steric hindrance (compared with the IR spectrum of polymer 2 in the ESI†). So, the FTIR data qualitatively confirmed the successful grafting of Pd–porphyrin onto the polymer surface.
In addition to FTIR spectrum, other techniques were considered for better catalyst verification (UV–VIS, SEM, XRD, and EDS). Furthermore, UV–Vis spectroscopy was also used to evaluate the Pd-porphyrin@polymer. As shown in Fig. 2, the absorptions peaks at 417, 525, 560, 600, and 658 nm relayed on H2TMCPP, whereas the sample gives new absorption peaks at 413, 535, and 580 nm after metalation with the palladium cation (solvent: CHCl3).
The morphology and size of the Pd–porphyrin@polymer were observed by scanning electron microscopy (SEM). The image shows that the Pd–porphyrin is placed on the polymer (Fig. 3a). The energy dispersive spectroscopy (EDS) analysis was used to determine the Pd–porphyrin@polymer, confirming the presence of nitrogen and palladium in the mentioned catalyst evaluate (Fig. 3b).
The TEM image of the fresh and used catalyst after 5thruns was provided to study the feasible deactivation mechanism of the Pd–porphyrin@Polymer catalysts following the recycling tests. The comparison among TEM images of primary catalyst and reused Pd–porphyrin@polymer(Fig. 4) displayed slight degree of agglomeration of in reused catalyst. This result shows that the mechanism of deactivation is likely to involve the formation of agglomerated Pd black.
The wide-angle X-ray diffraction was conducted to trace the crystalline structure and size of the supported Pd. Figure 5 depicts that four prominent peaks at 2q values of about 40°, 46°, 68°, and 81° corresponding to the characteristic of crystalline Pd. (111), (200), (220), and (311) confirmed the success of Pd ions’ reduction [29].
After the catalyst verification, we examined the catalytic activity and application of Pd–porphyrin@polymer in the aqueous reduction of nitroarenes in the presence of NaBH4. The reduction of nitrobenzene was selected as a model compound over Pd–porpyrin@polymer catalyst to optimize the reaction conditions (Scheme 2). We studied various conditions, including solvents, molar ratio of hydrogen source, temperature, and amount of catalyst, the results of which are summarized in Table 1. Match up with Table 1, the best yield and the shortest time for this catalytic system were observed in the presence of 15 mg catalyst which is equal to 0.36 mol% of Pd, 4 mmol of NaBH4 in water at 55 °C for 1 mmol of nitrobenzene. To verify the catalyst efficiency, the model compound was probed in the absence of catalyst. In this case, only a trace amount of product was detected (Table 1, entry 1).
To determine the catalytic activity of Pd-porphyrin@polymer, a wide variety of nitroarenes compounds were investigated. As displayed in Table 2 that either electron withdrawing or electron donating substituents in all positions of the phenyl ring (ortho, meta, or para) participated well in this reaction to give the corresponding products in high to excellent yields. Also, in entries (8, 9), both nitro and aldehyde functional groups were reduced by the present protocol.
A brief comparison between the efficiency of the present catalyst and some of those catalysts reported in the literature is listed in Table 3. It is clearly displayed that the presented methodology in this paper is superior to the most of the previously reported methods in terms of catalyst amount, reaction time or product yield.
The catalyst reusability was also examined for the model reaction under optimum conditions. Upon the completion of the reaction, the catalyst was recycled via simple filtration, washed with methanol and water, dried, and reused for five another successive runs without remarkable loss in activity. The results are displayed in Table4. Furthermore, its amount in the product was determined by ICP-OES, and no leaching of palladium was discovered.
Conclusion
In summary, we successfully proposed a new type of Pd–porphyrin modified organic polymer-based catalyst combining the catalytic power of transition metal complexes with the architecture of 3D-network polymer and shows particularly high efficiency for the reduction of nitroarenes. Water as a green solvent, catalyst reusability, high product yields, short reaction times, relatively mild reaction conditions, and simple work-up are possibly the most favorable advantages of the reported method. The described catalytic system provides a highly promising material through coupling the advantages of homogeneous and heterogeneous catalysts to be applied extensively in different aspects of science, industry, and technology.
References
Zhang, K., Suh, J.M., Choi, J.-W., Jang, H.W., Shokouhimehr, M., Varma, R.S.: Recent advances in the nanocatalyst-assisted NaBH4 reduction of nitroaromatics in water. ACS Omega 4, 483–495 (2019)
Goswami, A., Rathi, A.K., Aparicio, C., Tomanec, O., Petr, M., Pocklanova, R., Gawande, M.B., Varma, R.S., Zboril, R.: In situ generation of Pd–Pt core–shell nanoparticles on reduced graphene oxide (Pd@Pt/rGO) using microwaves: applications in dehalogenation reactions and reduction of olefins. ACS Appl. Mater. Interfaces 9, 2815–2824 (2017)
Ghosh Chaudhuri, R., Paria, S.: Core/shell nanoparticles: classes, properties, synthesis mechanisms, characterization, and applications. Chem. Rev. 112, 2373–2433 (2012)
Doria, G., Conde, J., Veigas, B., Giestas, L., Almeida, C., Assunção, M., Rosa, J., Baptista, P.V.: Noble metal nanoparticles for biosensing applications. Sensors 12, 1657–1687 (2012)
Goldberg, I.: Metalloporphyrin molecular sieves. Chem. Eur. J. 6, 3863–3870 (2000)
Barona-Castaño, J.C., Carmona-Vargas, C.C., Brocksom, T.J., De Oliveira, K.T.: Porphyrins as catalysts in scalable organic reactions. Molecules 21, 310 (2016)
Betoni Momo, P., Pavani, C., Baptista, M.S., Brocksom, T.J., Thiago de Oliveira, K.: Chemical transformations and photophysical properties of meso-tetrathienyl-substituted porphyrin derivatives. Eur. J. Org. Chem. 2014, 4536–4547 (2014)
Carvalho, C.M.B., Fujita, M.A., Brocksom, T.J., de Oliveira, K.T.: Synthesis and photophysical evaluations of β-fused uracil-porphyrin derivatives. Tetrahedron 69, 9986–9993 (2013)
Cornils, B., Herrmann, W.A.: Concepts in homogeneous catalysis: the industrial view. J. Catal. 216, 23–31 (2003)
Dioos, B.M., Vankelecom, I.F., Jacobs, P.A.: Aspects of immobilisation of catalysts on polymeric supports. Adv. Synth. Catal. 348, 1413–1446 (2006)
Wan, Q.-X., Liu, Y.: The ionic palladium porphyrin as a highly efficient and recyclable catalyst for Heck reaction in ionic liquid solution under aerobic conditions. Catal. Lett. 128, 487–492 (2009)
Dinari, M., Mohammadnezhad, G., Nabiyan, A.: Preparation and characterization of nanocomposite materials based on polyamide-6 and modified ordered mesoporous silica KIT-6. J. Appl. Polym. 133, 43098 (2016)
Totten, R.K., Kim, Y.-S., Weston, M.H., Farha, O.K., Hupp, J.T., Nguyen, S.T.: Enhanced catalytic activity through the tuning of micropore environment and supercritical CO2 processing: Al (Porphyrin)-based porous organic polymers for the degradation of a nerve agent simulant. J. Am. Chem. Soc. 135, 11720–11723 (2013)
Mouradzadegun, A., Kiasat, A.R., Fard, P.K.: 3D-network porous polymer based on calix[4]resorcinarenes as an efficient phase transfer catalyst in regioselective conversion of epoxides to azidohydrins. Catal. Commun. 29, 1–5 (2012)
Mouradzadegun, A., Elahi, S., Abadast, F.: One-pot synthesis of tweezer-like calix[4]resorcinarene decorated with pendant heterocyclic moieties: an efficient and recyclable heterogeneous PTC for the preparation of azidohydrins in water. Catal. Lett. 144, 1636–1641 (2014)
Mouradzadegun, A., Abadast, F.: An improved organic/inorganic solid receptor for colorimetric cyanide-chemosensing in water: towards new mechanism aspects, simplistic use and portability. Chem. Commun. 50, 15983–15986 (2014)
Mouradzadegun, A., Mostafavi, M.A.: Copper-loaded hypercrosslinked polymer decorated with pendant amine groups: a green and retrievable catalytic system for quick [3+ 2] Huisgen cycloaddition in water. RSC Adv. 6, 42522–42531 (2016)
Mouradzadegun, A., Dianat, S.: Facile and selective solvent-free synthesis of 2-isoxazolines under microwave irradiation. J. Heterocycl. Chem. 46, 778–781 (2009)
Mouradzadegun, A., Gheitasvand, N.: Efficient reduction of thiopyrylium salts to corresponding 2H-and 4H-thiopyrans under solvent-free condition: regioselectivity and mechanism. Phosphorus Sulfur Silicon Relat. Elem. 180, 1385–1388 (2005)
Mouradzadegun, A., Mostafavi, M.A.: Design and synthesis of a new porous organic polymer equipped with N-propyl sulfamic acid functionalities: As an efficient heterogeneous catalyst for the synthesis of 1,8-dioxo-octahydroxanthene derivatives. Polym. Eng. Sci. 58, 1362–1370 (2018)
Mouradzadegun, A., Elahi, S., Abadast, F.: Synthesis of a 3D-network polymer supported Bronsted acid ionic liquid based on calix[4]resorcinarene via two post-functionalization steps: a highly efficient and recyclable acid catalyst for the preparation of symmetrical bisamides. RSC Adv. 4, 31239–31248 (2014)
Tunstad, L.M., Tucker, J.A., Dalcanale, E., Weiser, J., Bryant, J.A., Sherman, J.C., Helgeson, R.C., Knobler, C.B., Cram, D.J.: Host-guest complexation. 48. Octol building blocks for cavitands and carcerands. J. Org. Chem. 54, 1305–1312 (1989)
Guo, D.-S., Liu, Y.: Calixarene-based supramolecular polymerization in solution. Chem. Soc. Rev. 41, 5907–5921 (2012)
Altshuler, H., Ostapova, E., Fedyaeva, O., Sapozhnikova, L., Altshuler, O.: Novel Network Polymers Based on Calixresorcinarenes, Macromolecular Symposia, pp. 1–4. Wiley Online Library, Hoboken (2002)
Jeong, E.-Y., Burri, A., Lee, S.-Y., Park, S.-E.: Synthesis and catalytic behavior of tetrakis (4-carboxyphenyl) porphyrin-periodic mesoporous organosilica. J. Mater. Chem. 20, 10869–10875 (2010)
Amao, Y., Asai, K., Okura, I.: Novel optical oxygen sensing by phosphorescence quenching of palladium porphyrin self-assembled film on alumina plate. J. Porphyr. Phthalocyanines 4, 179–184 (2000)
Fareghi-Alamdari, R., Golestanzadeh, M., Bagheri, O.: meso-Tetrakis [4-(methoxycarbonyl)phenyl] porphyrinatopalladium(II) supported on graphene oxide nanosheets (Pd(II)-TMCPP-GO): synthesis and catalytic activity. RSC Adv. 6, 108755–108767 (2016)
Prendergast, K., Spiro, T.G.: Core expansion, ruffling, and doming effects on metalloporphyrin vibrational frequencies. J. Am. Chem. Soc. 114, 3793–3801 (1992)
Li, N., Wang, Z., Zhao, K., Shi, Z., Xu, S., Gu, Z.: Graphene-Pd composite as highly active catalyst for the Suzuki-Miyaura coupling reaction. J. Nanosci. Nanotechnol. 10, 6748–6751 (2010)
Huang, H., Wang, X., Li, X., Chen, C., Zou, X., Ding, W., Lu, X.: Highly chemoselective reduction of nitroarenes over non-noble metal nickel-molybdenum oxide catalysts. Green Chem. 19, 809–815 (2017)
Zhang, H.-Y., Feng, C., Shang, N.-Z., Gao, S.-T., Wang, C., Wang, Z.: Efficient reduction of nitroarenes catalyzed by graphene-based magnetic nanocomposite. Lett. Org. Chem. 10, 17–21 (2013)
Dell’Anna, M.M., Intini, S., Romanazzi, G., Rizzuti, A., Leonelli, C., Piccinni, F., Mastrorilli, P.: Polymer supported palladium nanocrystals as efficient and recyclable catalyst for the reduction of nitroarenes to anilines under mild conditions in water. J. Mol. Catal. A 395, 307–314 (2014)
Nandi, D., Siwal, S., Choudhary, M., Mallick, K.: Carbon nitride supported palladium nanoparticles: an active system for the reduction of aromatic nitro-compounds. Appl. Catal. A 523, 31–38 (2016)
Genc, H.: Efficient reductions of various nitroarenes with scrap automobile catalyst and NaBH4. Catal. Commun. 67, 64–67 (2015)
Vernekar, A.A., Patil, S., Bhat, C., Tilve, S.G.: Magnetically recoverable catalytic Co–Co2B nanocomposites for the chemoselective reduction of aromatic nitro compounds. RSC Adv. 3, 13243–13250 (2013)
Acknowledgements
This work was supported by the Research Council at the University of Shahid Chamran.
Author information
Authors and Affiliations
Corresponding author
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Hamid, S., Mouradzadegun, A. Metalloporphyrin supported on hyper cross-linked polymer: green protocol for reduction of nitroarenes. J Incl Phenom Macrocycl Chem 98, 213–221 (2020). https://doi.org/10.1007/s10847-020-01021-x
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10847-020-01021-x