Abstract
The genus Lantana is widely used in folk medicine because its essential oil has antibacterial, antifungal and repellent activity. However, its thermal instability and low solubility in water reduce its technological application. In this work, an inclusion complex was prepared consisting of the essential oil of Latana camara L. (LCEO) and β-cyclodextrin (β-CD). The complex was characterizated by vibrational spectroscopy (Raman and FTIR spectroscopy), differential scanning calorimetry (DSC) and X-ray diffraction (XRD). The optimization of the ratio of the LCEO and β-CD in the inclusion complex was determined by gas chromatography coupled to mass spectrometry (GC–MS); it was inferred that the ratio of guest–host = 6:94 (m:m) was the optimal ratio. Shifts in some peak positions of the vibrations modes in Raman spectra of L. camara and β-CD provided clearer and better evidence of inclusion complex formation than infrared spectroscopy did. Results of DSC and XRD characterization of inclusion complex are in good agreement with Raman results.
Graphical Abstract
Formation of the inclusion complex between β-CD and LCEO as well as its characterization by different analytical techniques such as infrared spectroscopy and Raman spectroscopy, DSC, XRD and GC–MS.

Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Lantana camara is representative of the family Verbenaceae, a native of America and South America. It is grown as an ornamental species and has been adapted to various regions of the planet [1]. It is used in folk medicine to treat itching, ulcers, hepatitis and rheumatism [2]. In Brazil it was used in traditional medicine in combination with other species to treat lung diseases [3]. However, the introduction of synthetic drugs and the lack of pharmacological and toxicological testing has led to the disuse of this species in Brazil [4]. Recent studies have shown that the essential oil obtained from the leaves of L. camara (LCEO) has a wide spectrum of performance as an antibacterial, antifungal, anti-inflammatory, antioxidant [5,6,7,8,9,10,11], and larvicide against the larvae of the mosquitoes Aedes aegypti and Culex pipiens, which are vectors of serious endemic diseases in the southeast of tropical countries [10, 12]. It also has insecticidal activity against insects of the genus Sitophilus [2, 13] and acaricide activity [5].
Among the main active constituents of LCEO are β-caryophyllene and germacrene-d, wich exhibit anti-inflammatory and antibacterial activity [10]. β-Caryophyllene and γ-elemene have demonstrated cytotoxic activity against certain cell types [14, 15], and the β-caryophyllene has local anesthetic activity [16]. However, LCEO is insoluble in aqueous media and is unstable to light, heat, and oxygen. Its applications are restricted to narrow fields. To expand the application fields of the representative essential oil in the food, medication, and cosmetic industries, it is necessary to study the inclusion interactions and molecular microcapsule of β-cyclodextrin (β-CD) with LCEO [17].
Natural CD are formed by six, seven or eitght glucopyranose units, known as α, β and γ-CD, respectively. Cyclic oligosaccharides are capable of forming inclusion complexes (ICs) with a variety of organic compounds [18] through weak interactions such as hydrogen bonding and Van der Waals forces [19,20,21]. From a structural point of view, the spatial conformation of CDs is a truncated cone. The hydrophilic character of the surface is due to the presence of primary and secondary hydroxyls facing the outer surface. Since oxygen atoms are involved in glycosidic linkages between units of glucopyranose and hydrogen atoms, this confers a hydrophobic character to the interior of its cavity [20,21,22].
Some authors have studied the associations of CD with several essential oils [20, 23,24,25,26,27,28,29]. The use of inclusion compounds of CD (β-CD in particular) with several guest molecules has become common in the food industry, and this application has generated a large number of reports in the literature concerned with natural products [30].
One of the significant problems in the structural chemistry of such complexes is the final product characterization. In this respect, Raman spectroscopy emerges an important technique for verifying the presence of the different substances inside the CD cavity giving rise to an inclusion compound, since the guest molecule is a good Raman scatterer [30]. In this context, verification of the signal from a control substance β-CD in the present study in the vibrational profile obtained for standard LCEO will be useful for further identification and characterization of such molecules in complex matrices.
The present study describes the formation of the IC between β-CD and LCEO as well as its characterization using various analytical techniques such as infrared spectroscopy (IR), Raman spectroscopy, differential scanning calorimetry (DSC), X-ray diffraction (XRD) and gas chromatography coupled to mass spectrometry (GC–MS).
Experimental
Plant material
Leaves of L. camara were collected in the city of Simões (S = 7°35′900″, W = 40°40′404″; 786 m), Piauí, Brazil, in February of 2011. The specimen was deposited at the Herbarium of the Federal University of Piauí under reference number 27183.
Essential oil extraction
Samples of fresh leaves (300 g) were submitted to a hydrodistillation process for 3 h, in a Clevenger-type apparatus [31]. The essential oils collected were subsequently dried over anhydrous sodium sulfate (Na2SO4) and kept refrigerated at 4 °C until they could be analyzed.
GC–MS measurements
The analysis of the LCEO was performed in a gas chromatograph (Thermo Scientific) coupled to an ISQ® mass spectrometer (Thermo Scientific) under the following conditions: HP-5MS capillary column (J & W Scientific) using helium as a drag gas under a flow of 1 mL min−1. The injector and detector temperatures were maintained at 270 and 290 °C, respectively. The column temperature was kept at 50 °C for 5 min and then programmed to 180 °C at a rate of 4 °C min−1, and finally increased to 260 °C at a rate of 10 °C min−1, with a final hold for 10 min at this temperature. Samples of 1 µL of essential oil diluted in 5% n-hexane were injected, and n-alkanes were used as reference points for the calculation of relative retention indices. The percentage compositions were obtained from electronic integration of peak area measurements. The mass spectra were recorded on Electron Impact mode at 70 eV and the scanned mass ranged from 43 to 500 Da. Identification of individual components of the essential oil was performed by computerized matching of the acquired mass spectra with those stored in the NIST mass spectral libraries of the GC–MS data system. Retention indices (RI) for all compounds were compared to those published in the literature [7, 31,32,33,34]. The GC–MS (full scan) chromatogram was used to determine the relative concentrations using peak areas.
Preparation of LCEO/β-CD
The IC between LCEO and β-CD was prepared in duplicate according to the method proposed by Bhandari et al. [35], with some modifications. Briefly, the IC was prepared in the proportions of LCEO:β-CD (mass:mass) of 3:97, 6:94, 9:91, 12:88, and 15:85. To this end, an adequate mass of LCEO was solubilized in ethanol PA. while the β-CD was solubilized in an ethanol: water (1:2) mixture heated to 55 °C. The LCEO was added under stirring to the solution of β-CD, removed from the heat, and stirred continuously for 4 h at 150 rpm at an average temperature of 25 °C. After this period, the mixture was cooled without stirring to an average temperature of 4 °C over 12 h. The precipitate was collected, dried by lyophilization, weighed and stored in a desiccator until analysis.
Preparation of the physical mixture
The physical mixture (PM) between the LCEO and β-CD was prepared by simple grinding of LCEO and the β-CD with the aid of a mortar with pestle.
Characterization of the inclusion complex
The total amount of oil in the IC was determined by the method of extraction with solvent proposed by Harangi and Nánási [36], with some modifications. Briefly, samples equivalent to 10 mg of LCEO of ICs were solubilized with 8 mL of distilled water in a test tube sealed with a Bakelite screw cap and extracted with 4 mL of n-hexane. For this, the mixture was heated in a water bath at a temperature of 80 ± 2 °C for 15 min with intermittent shaking. Then it was cooled to room temperature. The n-hexane phase containing the essential oil was collected with the help of a pipette, and the aqueous phase was further subjected to two successive extractions with n-hexane (2 × 4 mL). The combined extracts were concentrated and transferred to a volumetric flask of 5 mL and analyzed by GC–MS. Aiming to reduce experimental errors, the LCEO was subjected to the same process of extraction as for the complexed oil. Therefore, 10 mg of essential oil was weighed with 200 mg of β-CD, and then submitted to the method of extraction as for the complexed oil.
Evaluation of the chemical profiles of the LCEO versus the complexed oil
Evaluation of the chemical profiles of the LCEO and complexed LCEO was performed by comparison of their chromatographic content [36]. The complexation efficiency (CE) was calculated according to the following equation:
where AIC is the sum of the areas of the peaks identified in the IC, and ALCEO is the sum of the areas of the peaks identified in the LCEO.
Fourier transform-infrared spectroscopy (FT-IR)
The FT-IR transmittance spectra of LCEO, β-CD, PM and IC were obtained in the mid-IR region of the spectrum (4000–400 cm−1) using a Vertex 70 spectrometer (Bruker). Approximately 10 µL of LCEO was placed directly between two KBr plates. The β-CD, PM, and the IC were crushed with about 100 mg of potassium bromide (KBr), then pressed into pellets of 1 mm under a pressure at about 7 tons. The spectra were obtained with 64 scans and a resolution of 4 cm−1.
Attenuated total reflectance (ATR)
The ATR spectra of LCEO, β-CD, PM and IC were also obtained in the mid IR (4000–600 cm−1) using the same spectrometer with an accessory for ATR. Approximately 20 µL of LCEO and 5 mg of β-CD, of the PM and IC, were placed directly on the surface of the crystal (Ge) of the ATR accessory, with light pressure applied to promote contact between the solid samples and the crystal surface. The spectra were obtained from 64 scans and a resolution of 4 cm−1.
Raman spectroscopy
The Raman spectra were recorded using a confocal Raman microscope NTEGRA (NT-MDT). A solid-state laser (Cobalt) of wavelength 473 nm was used as the excitation source, at an output power of about 25 mW. A × 100 objective was used to focus the beam onto the samples. The measurements were calibrated with crystalline silicon and obtained from ten acquisitions of 60 s each, with a spectral resolution of 4 cm−1.
X-ray diffraction (XRD)
The XRD diffractograms of the samples were obtained in a Shimadzu XRD-6000 X-ray diffractometer. The diffractograms were measured at an angle of 2θ, ranging from 5° to 75° at a rate of 2° min−1.
Differential scanning calorimetry (DSC)
The DSC curves were obtained on a TA Instruments apparatus (DSC-2920) with a nitrogen atmosphere of 50 mL min−1 and a heating rate of 10 °C min−1 in the temperature range 30–300 °C, using a port sample of hermetically sealed aluminum containing samples of 5.0 mg ± 0.2 of the LCEO, β-CD, PM, and IC.
Results and discussion
GC–MS analysis of the essential oil
The identification of the essential oils’ components was accomplished by comparison of their GC–MS retention indices. Spectra were considered coincident if the similarity index was higher than 95%. The yield of essential oils obtained was 0.35%. Table 1 gives the chemical composition and retention indices of the compounds identified: in total, 14 compounds were identified. The most representative compounds of the oil were mono and sesquiterpenes, such as β-caryophyllene (23.91%), γ-elemene (32.81%), and germacrene-d (17.77%), similar to the oils of L. camara obtained from other regions [5, 7, 9, 34, 37,38,39,40,41,42]. It was also observed that the molecular mass of the constituents identified is compatible with the capacity for complexation of the β-CD, which in according to Veiga et al. [43], should be around 200 below 800 Da.
Complexing efficiency
Several methods can be used in the preparation of the IC, with co-precipitation being the method of most used on laboratory scale, because of the ease of execution and high efficiency [20]; it was used here for the preparation of the IC of LCEO/β-CD. The CE varied between 20.6 and 38.0% and the mass recovered ranged from 57.9 to 75.9%, as shown in Table 2.
Wang et al. [20], in their study of the IC with garlic oil, reported a complexed oil content of 10.5% and a recovery of 78.2%. On the other hand, Hadaruga et al. [44] reported from contents of 7.1–11.5% with yields of 55–84%. As can be seen, the efficiency of complexation of several essential oils is variable due to very diverse compositions: each constituent of the oil can form a complex with higher or lower affinity with the CD [36]. Analyzing the data in Table 2, it can be observed that IC 6:94 was the one that showed the highest efficiency of complexation, and that an increase in concentration of LCEO does not increase the efficiency of complexation. For IC formation to occur very efficiently, one should observe the stoichiometric ratio between LCEO and the β-CD [30].
Figure 1 shows the relative abundance of the main constituents of LCEO and ICs. It can be seen that the quantitative composition of the complexed oil differs from the composition of LCEO. However, the qualitative profile of the main constituents of IC 3:97, IC 6:94, and the LCEO oil are similar, probably due to the equimolar ratio between LCEO and the β-CD present in the complexes. According to Harangi and Nánási [36], there is a difference in affinity between the different components of the essential oils to the β-CD, such that there is a qualitative and quantitative difference in the composition of the complexed oil and the uncomplexed oil. This explains the change in the qualitative profile of the IC complexes in the proportions 9:91, 12:88 and 15:85, since in these cases the LCEO is in excess and those components with greater affinity can displace those with lower affinity.
Infrared spectroscopy results
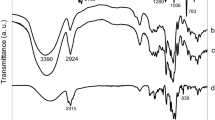
Figure 2 shows the FT-IR spectra of β-CD, LCEO, PM, and IC obtained in transmittance mode. The IR spectrum of the LCEO is characterized by several main peaks, which are listed and assigned in Table 3. We can observe in the LCEO the presence of mainly vibrational modes related to sesquiterpenes and monoterpenes carbons, hydrogenated and oxygenated, such as alkyl groups, and C=C, C–O, and C–O–C groups that are characteristic of terpenoids [45, 46]. The FT-IR spectrum of β-CD obtained is typical and similar to that obtained in previous work [25, 47,48,49].
On analyzing Fig. 2, a reduction in intensity of the absorption bands of LCEO located at approximately 2953, 2924, 2858, 1452 and 1372 cm−1 is observed when compared to the spectra of PM and IC. Nevertheless these intensity changes may be caused by the effect of dilution of the PM and IC samples in KBr, thus justifying the higher intensity of the bands of LCEO when compared to the other spectra. Furthermore, there was no shift or appearance of new bands in the IR spectrum of the IC, unlike changes seen by Zhan et al. [26], who observed a shift and change in intensity of bands of vibration of the IC, eugenol, and β-CD. Therefore, we cannot use this spectrum to characterize the formation of the IC. To reduce the effect of dilution of the samples, we performed an IR spectroscopy analysis by ATR (Fig. 3) since this technique does not require prior preparation of the sample. However, in this technique, there is no good correlation between the sample concentration and the intensity of absorption, in this way the characterization of the IC is more complex. On the other hand, this technique is very efficient in detecting displacements, enlargements, and reductions in intensity of the bands [11, 52].
In general, we can observe that the FTIR spectra of ICs and the free CDs are very similar. Bands of LCEO were almost completely obscured by very intense and broad CD bands. In particular, looking at the Fig. 3 we observed a reduction in intensity of the bands characteristic of LCEO, as in Fig. 2: we assume that this decrease is an effect of dilution of LCEO in the β-CD. However, we did not observe significant changes or displacements (beyond the resolution of the IR equipment) of bands in the IR spectrum of the IC or PM with respect to LCEO or β-CD. This may allow us to assume formation of the IC, showing that spectroscopy in the IR was not sufficient to prove the formation of the same with LCEO.
These results are in agreement with those found by other researchers, indicating that the IR absorption spectroscopy is not a very sensitive technique with which to characterize the IC with CDs [52].
Raman spectroscopy results
Raman spectroscopy can be used to monitor vibration of C=C bands with vibration wave number around 1646 cm−1, which are present in the various components of LCEO. These bands are highly sensitive to the formation of the IC with β-CD, since it does not have spectral bands in this region which may hide changes [18, 30, 49, 53]. In Fig. 4 the Raman spectrum of LCEO presents two very pronounced bands around 1444 cm−1 (strain CH or CH2) and 1646 cm−1 (C=C stretch) characteristic of the presence of sesquiterpenes [45, 54,55,56,57]. These peaks represent the fingerprint of the LCEO, given that they exhibit the characteristic profile of its main constituents [54, 56, 57]. These bands are quite pronounced in the PM (Fig. 4), suggesting that this spectrum is the simple sum of the isolated spectra of LCEO with the characteristic bands associated with β-CD around 1340, 932, 856, 584, and 480 cm−1. These are related to the vibrations of hydroxyls, primary and secondary alcohols, bands of linked C–O–C, breathing modes, glucose, and vibration of C–C–C bonds, all suggesting that the sample is just a mixture of the two substances without any significant interactions.
When analyzing the spectrum of the IC we observed a slight reduction in intensity and a shift to lower wave number, by around 20 cm−1 (well above of the resolution limit of the spectrometer), of the band around 1646 cm−1 when compared with the LCEO spectrum. This suggests that the C=C double bonds are possibly involved in the formation of these complexes, since the insertion of a guest molecule within the cavity of β-CD causes a conformational constraint on it, reducing the free movement of encapsulated molecules. This action, besides the β-CD serve as barrier against interaction of the laser beam with the guest molecule, which helps to reduce the intensity of its Raman signal [58]. We also observed significant shifts (to lower wavenumber) in the bands around 480 and 1117 cm−1 of β-CD, which prove the interaction of LCEO and β-CD. These effects were also observed by Oliveira et al. [30], Fini et al. [58], and Lamcharfi et al. [59], whith prepared ICs between β-CD and other compounds.
We did not observe the appearance of new vibrational bands in the spectrum of the IC in Fig. 4, indicating that no chemical bonds (of the covalent type) were formed between the LCEO and β-CD. This confirm the weak interaction between the β-CD and the guest molecules. However, for the Raman spectrum of the PM we observed small displacements in the same bands mentioned above, which can characterize the formation of the IC in a very few amount.
XRD results
In trying to assess whether the change in the pattern of XRD of the IC compared to the standard of β-CD was caused by the preparation technique, the β-CD was subjected to the same treatment as the IC. The diffraction pattern of β-CD in natura and β-CD after this treatment showed no significant differences (data not shown). Figure 5 shows the diffraction pattern typical of β-CD with peaks at angles of diffraction 2θ around 9°, 10°, 12°, 23°, 24°, 25°, and 27°, suggesting that the β-CD appears as a crystalline material, as seen in previous studies [21]. In the PM we observed a small change in the diffraction pattern, suggesting the existence of a small interaction between the LCEO and the β-CD as seen in the Raman spectrum. In the IC, an alteration of the diffraction patterns was observed, with the disappearance of peaks and the appearance of new peaks around 5°, 6°, 11°, and 18° suggesting the formation of a new crystalline structure, reinforcing the evidence for formation of the IC by Raman spectroscopy.
DSC results
The DSC curve of β-CD (Fig. 6) shows a sharp endothermic peak at 118 °C caused by the loss of water present in its cavity [20]. The DSC curve characteristic of LCEO, constitutes an exothermic peak at 177 °C, followed by an endothermic peak around at 229 °C. The DSC curve of the PM seems to be the simple superposition of the DSC curves of LCEO and β-CD. However, a different pattern was observed in the DSC curve of the IC. The endothermic peak at 118 °C had its intensity reduced, indicating that part of the water that was initially present in β-CD was displaced by LCEO, due to its greater affinity for the hydrophobic cavity of β-CD [20], again suggesting the formation of the IC shown by Raman spectroscopy. Moreover, the exothermic peak disappeared, indicating a greater stability of complexed LCEO when compared to LCEO which was non-complexed.
In summary, DSC, Raman spectra, and FT-IR analyses proved that CD/LCEO IC by the co-precipitation method had different physicochemical characteristics than free relative CD and LCEO, and their PM.
Conclusions
Raman spectroscopy results clearly show formation of the IC between LCEO and β-CD by the co-precipitation method. The IC 6:94 was the complex which showed the highest CE according to GC–MS measurements. The results of XRD and DSC are not so conclusive in detecting differences in the physicochemical properties of complexed and uncomplexed LCEO, but do corroborate the results of Raman spectroscopy. The results also show the difference in sensitivity between the vibrational spectroscopic techniques, with Raman spectroscopy clearly the only one sensitive enough to monitor the formation of the IC with the essential oil consisting predominantly of monoterpenes and sesquiterpene hydrocarbons, such as LCEO.
Thus, this work showed that complexation or encapsulation with CDs might be a useful and promising way to improve the application of LCEO in, for example, the food and pharmaceutical industries.
References
Patel, S.: A weed with multiple utility: Lantana camara. Rev. Environ. Sci. Biotechnol. 10, 341–351 (2011). https://doi.org/10.1007/s11157-011-9254-7
Bouda, H., Tapondjou, L.A., Fontem, D.A., Gumedzoe, M.Y.D.: Effect of essential oils from leaves of Ageratum conyzoides, Lantana camara and Chromolaena odorata on the mortality of Sitophilus zeamais (Coleoptera, Curculionidae). J. Stored Prod. Res. 37, 103–109 (2001). https://doi.org/10.1016/S0022-474X(00)00011-4
Silva, R.A.D.: Pharmacopeia Brasileira. Companhia Editora Nacional, Rio de Janeiro (1929)
Brandão, M.G.L., Zanetti, N.N.S., Oliveira, G.R.R., Goulart, L.O., Monte-Mor, R.L.M.: Other medicinal plants and botanical products from the first edition of the Brazilian Official Pharmacopoeia. Rev. Bras. Farmacogn. 18, 127–134 (2008). https://doi.org/10.1590/S0102-695X2008000100022
Alitonou, G., Avlessi, F., Bokossa, I., Ahoussi, E., Dangou, J., Sohounhloué, D.C.K.: Composition chimique et activités biologiques de l’huile essentielle de Lantana camara Linn. Comptes Rendus Chim. 7, 1101–1105 (2004). https://doi.org/10.1016/j.crci.2003.11.017
Deena, M.J., Thoppil, J.E.: Antimicrobial activity of the essential oil of Lantana camara. Fitoterapia 71, 453–455 (2000). https://doi.org/10.1016/S0367-326X(00)00140-4
Sousa, E.O., Silva, N.F., Rodrigues, F.F.G., Campos, A.R., Lima, S.G., Costa, J.G.M.: Chemical composition and resistance-modifying effect of the essential oil of Lantana camara Linn. Pharmacogn. Mag. 6, 79–82 (2010). https://doi.org/10.4103/0973-1296.62890
Benites, J., Moiteiro, C., Miguel, G., Rojo, L., López, J., Venâncio, F., Ramalho, L., Feio, S., Dandlen, S., Casanova, H., Torres, I.: Composition and biological activity of the essential oil of peruvian Lantana camara. J. Chil. Chem. Soc. 54, 379–384 (2009). https://doi.org/10.4067/S0717-97072009000400012
Costa, J.G.M., Sousa, E.O., Rodrigues, F.F.G., De Lima, S.G., Braz-Filho, R.: Composição química e avaliação das atividades antibacteriana e de toxicidade dos óleos essenciais de Lantana camara L. e Lantana sp. Braz. J. Pharmacogn. 19, 710–714 (2009). https://doi.org/10.1590/S0102-695X2009000500010
Costa, J.G.M., Rodrigues, F.F.G., Sousa, E.O., Junior, D.M.S., Campos, A.R., Coutinho, H.D.M., De Lima, S.G.: Composition and larvicidal activity of the essential oils of lantana camara and lantana montevidensis. Chem. Nat. Compd. 46, 313–315 (2010). https://doi.org/10.1007/s10600-010-9601-x
Heise, H.M., Kuckuk, R., Bereck, A., Riegel, D.: Infrared spectroscopy and Raman spectroscopy of cyclodextrin derivatives and their ferrocene inclusion complexes. Vib. Spectrosc. 53, 19–23 (2010). https://doi.org/10.1016/j.vibspec.2010.01.012
Zoubiri, S., Baaliouamer, A.: GC and GC/MS analyses of the Algerian Lantana camara leaf essential oil: effect against Sitophilus granarius adults. J. Saudi Chem. Soc. 16, 291–297 (2012). https://doi.org/10.1016/j.jscs.2011.01.013
Zoubiri, S., Baaliouamer, A.: Larvicidal activity of two Algerian Verbenaceae essential oils against Culex pipiens. Vet. Parasitol. 181, 370–373 (2011). https://doi.org/10.1016/j.vetpar.2011.04.033
Kubo, A., Lunde, C.S., Kubo, I.: Antimicrobial activity of the olive oil flavor compounds. J. Agric. Food Chem. 43, 1629–1633 (1995). https://doi.org/10.1021/jf00054a040
Lu, J.J., Dang, Y.Y., Huang, M., Xu, W.S., Chen, X.P., Wang, Y.T.: Anti-cancer properties of terpenoids isolated from Rhizoma Curcumae: a review. J. Ethnopharmacol. 143, 406–411 (2012). https://doi.org/10.1016/j.jep.2012.07.009
Ghelardini, C., Galeotti, N., Di Cesare Mannelli, L., Mazzanti, G., Bartolini, A.: Local anaesthetic activity of β-caryophyllene. Farmaco 56, 387–389 (2001). https://doi.org/10.1016/S0014-827X(01)01092-8
Aguiara, U.N., De Lima, S.G., Rocha, M.S., De Freitas, R.M., Oliveira, T.M., Silva, R.M., Moura, L.C.B., De Almeidab, L.T.G.: Preparação e caracterização do complexo de inclusão do óleo essencial de croton zehntneri com b-ciclodextrina. Quim. Nova. 37, 50–55 (2014). https://doi.org/10.1590/S0100-40422014000100010
Lyra, M.A.M., Alves, L.D.S., Fontes, D.A.F., Soares-Sobrinho, J.L., Rolim-Neto, P.J.: Ferramentas analíticas aplicadas à caracterizaçã o de complexos de inclusão fármaco-ciclodextrina. Rev. Ciencias Farm. Basica Apl. 31, 117–124 (2010)
Salústio, P.J., Feio, G., Figueirinhas, J.L., Pinto, J.F., Cabral Marques, H.M.: The influence of the preparation methods on the inclusion of model drugs in a β-cyclodextrin cavity. Eur. J. Pharm. Biopharm. 71, 377–386 (2009). https://doi.org/10.1016/j.ejpb.2008.09.027
Wang, J., Cao, Y., Sun, B., Wang, C.: Physicochemical and release characterisation of garlic oil-β-cyclodextrin inclusion complexes. Food Chem. 127, 1680–1685 (2011). https://doi.org/10.1016/j.foodchem.2011.02.036
Abbehausen, C., Formiga, A.L.B., Sabadini, E., Yoshida, I.V.P.: A-βcyclodextrin/siloxane hybrid polymer: synthesis, characterization and inclusion complexes. J. Braz. Chem. Soc. 21, 1867–1876 (2010). https://doi.org/10.1590/S0103-50532010001000011
Britto, M.A.F.O., Nascimento, C.S., Dos Santos, H.F.: Análise estrutural de ciclodextrinas: Um estudo comparativo entre métodos teóricos clássicos e quânticos. Quim. Nova. 27, 882–888 (2004). https://doi.org/10.1590/S0100-40422004000600008
Fernandes, L.P., Oliveira, W.P., Sztatisz, J., Szilágyi, I.M., Novák, C.: Solid state studies on molecular inclusions of Lippia sidoides essential oil obtained by spray drying. J. Therm. Anal. Calorim. 95, 855–863 (2009). https://doi.org/10.1007/s10973-008-9149-1
Waleczek, K.J., Cabral Marques, H.M., Hempel, B., Schmidt, P.C.: Phase solubility studies of pure (−)-α-bisabolol and camomile essential oil with β-cyclodextrin. Eur. J. Pharm. Biopharm. 55, 247–251 (2003). https://doi.org/10.1016/S0939-6411(02)00166-2
Wang, Y., Jiang, Z.-T., Li, R.: Complexation and molecular microcapsules of Litsea cubeba essential oil with β-cyclodextrin and its derivatives. Eur. Food Res. Technol. 228, 865–873 (2009). https://doi.org/10.1007/s00217-008-0999-3
Zhan, H., Jiang, Z.-T., Wang, Y., Li, R., Dong, T.-S.: Molecular microcapsules and inclusion interactions of eugenol with β-cyclodextrin and its derivatives. Eur. Food Res. Technol. 227, 1507–1513 (2008). https://doi.org/10.1007/s00217-008-0873-3
Jiang, S., Li, J.-N., Jiang, Z.-T.: Inclusion reactions of β-cyclodextrin and its derivatives with cinnamaldehyde in Cinnamomum loureirii essential oil. Eur. Food Res. Technol. 230, 543–550 (2010). https://doi.org/10.1007/s00217-009-1192-z
Rosa, R.-D.L., Cevallos-Ferriz, R.A., Silva-Pineda, S.R.S.A.: Paleobiological implications of Campanian coprolites. Palaeogeogr. Palaeoclimatol. Palaeoecol. 142, 231–254 (1998). https://doi.org/10.1016/S0031-0182(98)00052-2
Reineccius, T.A., Reineccius, G.A., Peppard, T.L.: The effect of solvent interactions on α-, β-, and γ-cyclodextrin/flavor molecular inclusion complexes. J. Agric. Food Chem. 53, 388–392 (2005). https://doi.org/10.1021/jf0488716
de Oliveira, V.E., Almeida, E.W.C., Castro, H.V., Edwards, H.G.M., Dos Santos, H.F., de Oliveira, L.F.C.: Carotenoids and β-cyclodextrin inclusion complexes: Raman spectroscopy and theoretical investigation. J. Phys. Chem. A 115, 8511–8519 (2011). https://doi.org/10.1021/jp2028142
de Lima, S.G., Neto, J.M.M., Lopes Citó, A.M.G., da Costa, J.G.M., Reis, F.A.M.: Monoterpenes, sesquiterpenes and fatty acids from julocroton triqueter (Euphorbiaceae) from Ceara-Brazil. J. Chil. Chem. Soc. 54, 55–57 (2009). https://doi.org/10.4067/S0717-97072009000100013
Van Den Dool, H., Dec. Kratz, P.: A generalization of the retention index system including linear temperature programmed gas–liquid partition chromatography. J. Chromatogr. A 11, 463–471 (1963). https://doi.org/10.1016/S0021-9673(01)80947-X
Adams, R.P.: Identification of essential oil components by gas chromatography/massspectrometry. Allured Pub. Corp, Carol Stream (2007)
Medeiros, L.B.P., Rocha, MdosS., de Lima, S.G., de Sousa Júnior, G.R., Lopes Citó, A.M.G., da Silva, D., Lopes, J.A.D., Moura, D.J., Saffi, J., Mobin, M., da Costa, J.G.M.: Chemical constituents and evaluation of cytotoxic and antifungal activity of Lantana camara essential oils. Rev. Bras. Farmacogn. 22, 1259–1267 (2012). https://doi.org/10.1590/S0102-695X2012005000098
Bhandari, B.R., Arcy, B.R.D., Le, L., Bich, T.: Lemon oil to β-cyclodextrin ratio effect on the inclusion efficiency of β-cyclodextrin and the retention of oil volatiles in the complex. Analysis 8561, 1494–1499 (1998). https://doi.org/10.1021/jf970605n
Harangi, J., Nánási, P.: Measurement of the essential oil in inclusion complexes with cyclodextrin by means of capillary gas chromatography. Anal. Chim. Acta. 156, 103–109 (1984). https://doi.org/10.1016/S0003-2670(00)85541-5
Sonibare, O., Effiong, I.: African journal of biotechnology. Academic Journals (2002)
Hernández, T., Canales, M., Avila, J.G., García, A.M., Martínez, A., Caballero, J., De Vivar, R., Lira, A.R.: Composition and antibacterial activity of essential oil of Lantana achyranthifolia Desf. (Verbenaceae). J. Ethnopharmacol. 96, 551–554 (2005). https://doi.org/10.1016/j.jep.2004.09.044
Randrianalijaona, J.A., Ramanoelina, P.A.R., Rasoarahona, J.R.E., Gaydou, E.M.: Seasonal and chemotype influences on the chemical composition of Lantana camara L.: essential oils from Madagascar. Anal. Chim. Acta. 545, 46–52 (2005). https://doi.org/10.1016/j.aca.2005.04.028
Baranska, M., Schulz, H., Walter, A., Rösch, P., Quilitzsch, R., Lösing, G., Popp, J.: Investigation of eucalyptus essential oil by using vibrational spectroscopy methods. Vib. Spectrosc. 42, 341–345 (2006). https://doi.org/10.1016/j.vibspec.2006.08.004
Misra, L., Laatsch, H.: Triterpenoids, essential oil and photo-oxidative 28→13-lactonization of oleanolic acid from Lantana camara. Phytochemistry 54, 969–974 (2000)
Ngassoum, M.B., Yonkeu, S., Jirovetz, L., Buchbauer, G., Schmaus, G., Hammerschmidt, F.J.: Chemical composition of essential oils of Lantana camara leaves and flowers from Cameroon and Madagascar. Flavour Fragr. J. 14, 245–250 (1999)
Veiga, F., Pecorelli, C., Ribeiro, L.: As ciclodextrinas em tecnologia farmacêutica. MinervaCoimbra, Coimbra (2006)
Hǎdǎrugǎ, N.G., Hǎdǎrugǎ, D.I., Pǎunescu, V., Tatu, C., Ordodi, V.L., Bandur, G., Lupea, A.X.: Thermal stability of the linoleic acid/α- and β-cyclodextrin complexes. Food Chem. 99, 500–508 (2006). https://doi.org/10.1016/j.foodchem.2005.08.012
Schulz, H., Baranska, M.: Identification and quantification of valuable plant substances by IR and Raman spectroscopy. Vib. Spectrosc. 43, 13–25 (2007). https://doi.org/10.1016/j.vibspec.2006.06.001
Schulz, H., Quilitzsch, R., Krüger, H.: Rapid evaluation and quantitative analysis of thyme, origano and chamomile essential oils by ATR-IR and NIR spectroscopy. J. Mol. Struct. 661–662, 299–306 (2003). https://doi.org/10.1016/S0022-2860(03)00517-9
Li, W., Lu, B., Chen, F., Yang, F., Wang, Z.: Host–guest complex of cypermethrin with β-cyclodextrin: a spectroscopy and theoretical investigation. J. Mol. Struct. 990, 244–252 (2011). https://doi.org/10.1016/j.molstruc.2011.01.053
Li, W., Lu, B., Sheng, A., Yang, F., Wang, Z.: Spectroscopic and theoretical study on inclusion complexation of beta-cyclodextrin with permethrin. J. Mol. Struct. 981, 194–203 (2010). https://doi.org/10.1016/j.molstruc.2010.08.008
Egyed, O.: Spectroscopic studies on β-cyclodextrin. Anal. Chim. Acta. 240, 225–227 (1990). https://doi.org/10.1016/0924-2031(90)80041-2
Schulz, H., Özkan, G., Baranska, M., Krüger, H., Özcan, M.: Characterisation of essential oil plants from Turkey by IR and Raman spectroscopy. Vib. Spectrosc. 39, 249–256 (2005). https://doi.org/10.1016/j.vibspec.2005.04.009
Larkin, P.: Infrared and Raman spectroscopy: principles and spectral interpretation. Elsevier, Amsterdam (2011)
Stancanelli, R., Ficarra, R., Cannavà, C., Guardo, M., Calabrò, M.L., Ficarra, P., Ottanà, R., Maccari, R., Crupi, V., Majolino, D., Venuti, V.: UV-VIS and FTIR-ATR characterization of 9-fluorenon-2-carboxyester/(2-hydroxypropyl)-β-cyclodextrin inclusion complex. J. Pharm. Biomed. Anal. 47, 704–709 (2008). https://doi.org/10.1016/j.jpba.2008.02.018
Iliescu, T., Baia, M., Miclăuş, V.: A Raman spectroscopic study of the diclofenac sodium–β-cyclodextrin interaction. Eur. J. Pharm. Sci. 22, 487–495 (2004). https://doi.org/10.1016/j.ejps.2004.05.003
Seidler-Lozykowska, K., Baranska, M., Baranski, R., Krol, D.: Raman analysis of caraway (Carum carvi L.) single fruits. Evaluation of essential oil content and its composition. J. Agric. Food Chem. 58, 5271–5275 (2010). https://doi.org/10.1021/jf100298z
Siatis, N.G., Kimbaris, A.C., Pappas, C.S., Tarantilis, P.A., Daferera, D.J., Polissiou, M.G.: Rapid method for simultaneous quantitative determination of four major essential oil components from Oregano (Oreganum sp.) and Thyme (Thymus sp.) using FT-Raman spectroscopy. J. Agric. Food Chem. 53, 202–206 (2005). https://doi.org/10.1021/jf048930f
Daferera, D.J., Tarantilis, P.A., Polissiou, M.G.: Characterization of essential oils from lamiaceae species by fourier transform Raman spectroscopy. J. Agric. Food Chem. 50, 5503–5507 (2002)
Daferera, D., Pappas, C., Tarantilis, P.A., Polissiou, M.: Quantitative analysis of α-pinene and β-myrcene in mastic gum oil using FT-Raman spectroscopy. Food Chem. 77, 511–515 (2002). https://doi.org/10.1016/S0308-8146(01)00382-X
Fini, A., Ospitali, F., Zoppetti, G., Puppini, N.: ATR/Raman and fractal characterization of HPBCD/progesterone complex solid particles. Pharm. Res. 25, 2030–2040 (2008). https://doi.org/10.1007/s11095-008-9593-4
Lamcharfi, E., Kunesch, G., Meyer, C., Robert, B.: Using FT-IR and Raman spectroscopy. Spectroscopy 51, 1861–1870 (1995)
Acknowledgements
The authors would like to acknowledge the UFPI and CNPq for financial support and FISMAT and LAPETRO for the analyzes.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Rocha, M.d.S., de Lima, S.G., Viana, B.C. et al. Characterization of the inclusion complex of the essential oil of Lantana camara L. and β-cyclodextrin by vibrational spectroscopy, GC–MS, and X-ray diffraction. J Incl Phenom Macrocycl Chem 91, 95–104 (2018). https://doi.org/10.1007/s10847-018-0799-8
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10847-018-0799-8