Abstract
Beta-cyclodextrin polymers (pbCD) cross-linked by epichlorohydrin (pbCDE) and citric acid (pbCDC) were prepared in this work. The inclusion complexes of pbCDE and pbCDC with curcumin and two commercial UV filters, 2-ethylhexyl-p-methoxycinnamate (EHMC) and 4-tert-butyl-4′-methoxydibenzoylmethane (DBM) were investigated. The UV absorption of these three compounds observed in water indicated that the water solubility of these three hydrophobic compounds increased. The amount of EHMC was higher in both pbCDE and pbCDC than curcumin and DBM, respectively. The photostability of these three compound inclusion complexes with pbCDE and pbCDC were also studied in water and ethylene glycol. It was found that the photostability of the three compounds improved upon formation of the inclusion complex with pbCDE in an aqueous and ethylene glycol solution. The acidity of the crosslink moiety effects to the inclusion complex formation and the photostability of the guests suggesting that more acidity of citric acid decreased the formation and stability of all guest compounds.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Cyclodextrins (CDs) are biocompatible cyclic oligosaccharides, widely used in food, cosmetics, pharmaceutical excipients and as solubilizing and stabilizing agents for hydrophobic compounds in aqueous formulations [1,2,3,4]. CD derivatives have been reported as solubilizing and stabilizing for water insoluble UV filters, 2-ethylhexyl-p-methoxycinnamate (EHMC) UVB absorber and 4-tert-butyl-4′-methoxydibenzoylmethane (DBM) UVA absorber [5] (Fig. 1). The degradation of trans-EHMC-βCD complexes in emulsion vehicles was reduced to 26.1% compared to 35.8% for free trans-EHMC [6, 7]. In the case of DBM, it was found that the photodegradation was reduced by complexation with hydroxypropyl-βCD (27.6% for the complex compared to 63.1% for free DBM) [6, 8]. Moreover, the solubility of DBM and 3-(4-methylbenzylidene)-camphor has been further improved by complexation with random methylated-βCD than the unsubstituted βCD [5]. Curcumin, a natural compound, has been reported to have a number of biological activities including anti-inflammatory, anti-cancer, anti-oxidant, etc. [9, 10]. The pharmaceutical potential of curcumin is limited due to its poor water solubility. To increase the stability and solubility of curcumin, the inclusion complexes with CDs have been reported on numerous occasions. The effect of curcumin with alpha (α), beta (β) and gamma (γ)-cyclodextrin complexes on its solubility and bioavailability have been investigated [11,12,13,14]. It was reported that the solubility of curcumin-αCD (0.364 mg/mL) > βCD (0.186 mg/mL) > γCD (0.068 mg/mL) compared to free curcumin (0.002 mg/mL) [15].
Crosslinked βCD polymers have been extensively studied and used to form nanoparticles in aqueous solutions. The solubility of insoluble substances has been dramatically improved compared to the parent CD or CD-derivatives [16, 17]. The most frequently used crosslinkers for CD are epichlorohydrin (Ep) as well as citric acid (Fig. 2) [18,19,20,21,22,23]. However, to the best of our knowledge there are no reports on the inclusion of UV filters and curcumin on CD-polymers. Most of the literature studies are on inclusion complexes of drugs or essential oils [24,25,26,27,28]. One of the goals of this study was to examine the ability of pbCD to increase the photostability and aqueous solubility of non-polar active compounds used in cosmetics. Moreover, cyclodextrin can be modified to form ionic derivatives. An anionic crosslinked water-soluble cyclodextrin polymer can be interacted with cationic polymers or nanofibers to form the nanoparticles, particularly used for biomedical applications [29, 30].
In this study, we prepared a βCD polymer (pbCD) crosslinked with epicholrohydrin and citric acid. We have focused on the complexation behavior and photostability of two UV filters, EHMC and DBM, as well as curcumin, a natural compound used in food and pharmaceutical excipients. The effect of crosslink agents, Ep and citric acid, were investigated in aqueous and ethylene glycol media.
Materials and methods
Materials
β-Cyclodextrin (βCD), curcumin, citric acid and ethylene glycol were purchased from Sigma-Aldrich (Steinheim, Germany). Epichlorohydrin was purchased from Fluka. EHMC and DBM were gifted from Merck Thailand. Ethanol and acetone were reagent grade purchased from Labscan (Bangkok, Thailand). Deionized water was used for all experiments.
Methods
Synthesis of β-cyclodextrin polymer cross-linked by epichlorohydrin (pbCDE)
Synthesis of pbCDE was done following a previous report [25]. Typically, 10 g of beta-cyclodextrin (βCD) was dissolved in 20 mL of 33% NaOH solution with a magnetic stirrer and left overnight at room temperature. Epichlorohydrin (Ep, 7 eq.) was added to the solution and stirred at room temperature for 2.5 h. The polycondensation was stopped by addition of 20 mL of acetone. The acetone was removed by decantation. Water was added and the pH of the aqueous solution was adjusted to neutral by HCl then dialyzed overnight (12 kDa cellulosic membrane). The solution was finally freeze-dried. The 80% yield of white solid product was obtained.
Synthesis of β-cyclodextrin polymer cross-linked by citric acid (pbCDC)
The synthesis of pbCDC was carried out following a literature procedure [21]. Briefly, an aqueous solution of NaH2PO4, βCD and citric acid with concentration of 0.2, 0.09, 0.45 mol/L was prepared. The water was removed using a rotary evaporator and the resulting mixture dried. The dried mixture was heated to 140 °C for 30 min. The polymer was recovered after addition of water to the solution. The gel fraction was filtered off. The soluble fraction was dialyzed against water (12 kDa cellulosic membrane) and finally freeze-dried, The 16% yield of white powder was obtained.
UV–Vis spectra were recorded in a quartz cell (light path 10 mm) on a UV–Vis spectrophotometer (Lamda35, Perkin Elmer instrument, Shelton). All 1H-NMR spectra were obtained in D2O on a Varian Mercury NMR spectrometer, which operated at 400.00 MHz for 1H nuclei (Varian Company, CA). Fourier transform infrared (FT-IR) spectra were studied by using FT-IR spectrometer SYSTEM 2000 (Perkin Elmer, USA). The molecular weight determination was performed using a Malvern Zetasizer Nano ZS instrument running a static light scattering (SLS) method [31]. Briefly, The samples were prepared at different concentrations at 25 °C applying the Rayleigh Eq. (1) below:
where \({R}_{{\Theta }}\), the Rayleigh ratio which is the ratio of scattered light to incident light of the sample; M w is the weight-averaged molecular weight; A 2 is the 2nd virial coefficient; c is concentration; \(P\left(\varTheta \right)\) is the angular dependence of the sample scattering intensity; K is the optical constant as defined as Eq. (2):
where N A is Avogadro’s constant; λ0 is the laser wavelength; n0 is the solvent refractive index; dn/dc is the differential refractive index increment.
M w and A 2 can be obtained by measuring the intensity of the scattered light \((Kc/{R_\Theta })\) of various sample concentrations compared with the scattering produced from a standard sample (toluene was used as a reference). The Debye-plot was plotted wherein Mw is determined from the intercept at zero concentration (c = 0; \(Kc/{R_\Theta }\) = 1/M w ). \({n}_{0}\) and dn/dc were obtained from an Abbe refractometer (Karl Zeiss, Jena) at 25 °C.
Preparation of the complexes
The complexes with pbCDE and pbCDC were prepared by adding an excess of curcumin, DBM and EHMC and dissolving in 1 mL acetone into a solution of pbCDE and pbCDE in deionized water (10 mL). The mixture was stirred at room temperature for 24 h and shielded from light. Each mixture solution was filtered through 0.45 µM cellulose membrane filters and finally freeze dried. The amount of curcumin, DBM and EHMC loaded into the inclusion complexes were determined by quantifying curcumin in the freeze-dried solid and dissolving in ethanol. Subsequently, the obtained solution was analyzed by UV–Vis spectroscopy.
Phase solubility study
The phase solubility studies were investigated using Higuchi and Connors method [32]. Briefly, the different concentrations of pbCDE and pbCDC were prepared in DI water in a volumetric flask. The β-CD concentrations of pbCDE and pbCDC were calculated by taking in account the β-CD content of the polymer estimated by 1H-NMR. To each vial 10 mM of guest was added. The solution was shaken for 24 h at room temperature. Samples were filtered through 0.45 µm membrane filters. The absorption of dissolved guest was measured by UV–Vis spectrophotometry. The stability constant (Ks) was calculated following the equation below:
where S0 is the concentration of guest in the absence of the cyclodextrin polymers.
Photostability test
The photostability of each compound was tested in water or ethylene glycol following a previous method [33]. Briefly, 4 mL of fresh sample solutions in a quartz cuvette with the same concentration were irradiated by using a broadband UVB lamp (9 W, Daavlin, OH) for EHMC and a broadband UVA lamp (9 W, Dermalight_80, OH) for DBM and curcumin at room temperature (35–37 °C). The distance between a cuvette and a UV lamp was 5 cm. The UV absorption profiles of the irradiated samples were analyzed on a UV–Vis spectrometer. The photostability of each compound was expressed as percentage relative to absorbance at the maximum absorption wavelength of the compound. All experiments were done in triplicate. Percentage photostability was calculated as follows:
Results and discussion
Synthesis and characterization of pbCDE and pbCDC
The CD contents of the pbCDE polymer were studied by 1H-NMR in D2O (Fig. 3b). The broad signals at 5.1–5.3 ppm were assigned to the seven anomeric protons of βCD and the broad signals between 3.4 and 4.2 ppm correspond to the twelve protons of βCD and five protons of the 2-hydroxypropyl ether moiety. These results suggest that the ratio of Ep/βCD is 5. The value for the molecular weight (Mw) is 12.4 ± 0.4 kDa. The correlation between Ep/βCD ratio and Mw is in agreement with the previous report [34].
Figure 3c shows the 1H-NMR spectrum of pbCDC, the broad signal at 5.1 ppm was assigned to the anomeric protons of βCD (7 protons per unit). The signal for the citric acid methylene protons is displayed as two broad signals at 2.7–3.3 ppm. The citric acid/βCD ratio in the pbCDC products was estimated by integrating the peaks assigned to the four protons of the citric acid methylene group divided by the seven anomeric protons of the pbCDC units. The Mw of pbCDC was found to be 8.44 ± 0.49 kDa. The βCD content in the oligomer was determined by dividing the Mw of the pbCDC by the Mw of the repeating unit calculated according to the citric acid/βCD ratio. The degree of substitution (DS) calculated from this data was 3–4. The substitution degree of pbCDE was higher than that of pbCDC due to the reactivity of citric acid is lower than Ep [35].
The FTIR spectra of pbCDE and pbCDC compared to βCD is shown in Fig. 4. The OH group stretching vibrations at 3300–3500 cm−1 are characteristic peaks of CDs. The OH group stretching peak of pbCDC shifts to 3426 cm−1, slightly higher than that of pbCDE at 3407 cm−1 and βCD monomer at 3378 cm−1. These results indicated the OH groups of βCD in pbCDE and pbCDC reacted with the Ep and citric acid, respectively, then substitution reaction occurred. Moreover, a sharp peak at 1736 cm−1 for pbCDC is assigned to the C=O stretching of the ester moiety in pbCDC, which was not present in the spectra of the starting materials. This result is in agreement with previous work reported by Monti et al. [21]. The H–O–H stretch of the water present in CDs is seen at 1651 cm−1. The C–O–C stretching vibration at 1156 cm−1 was clearly appeared for pbCDE due to ether bonds were formed. Unfortunately, the drug content in the complex is very low, the signals of drug in the inclusion complexes could not detected by FT-IR. Other evidence that usually appears is the signal for the C=C of aromatics at 1644 cm−1.
Inclusion complex formation
All guests, curcumin, DBM and EHMC, were formed as inclusion complexes with pbCDE and pbCDC resulting in a clear colorless solution for DBM and EHMC and yellow aqueous solution. Table 1 show the relative amounts of active compounds in the inclusion complexes measured by UV–Vis spectroscopy and their stability constant (Ks). As a result, the contents of all active compounds in pbCDE are higher amounts than those in pbCDC, probably due to the steric of pbCDC is greater than that of pbCDE leading to lower stabilization of all active compounds in the CD cavity. EHMC can be detected as the highest amount in both pbCDE and pbCDC inclusion complexes.
The phase-solubility diagrams for DBM, EHMC and curcumin with pbCDE and pbCDC showed in Fig. 5. The stability constant (Ks) of curcumin, DBM and EHMC inclusion complexes with βCD were reported in Table 1. All three guests were practically insoluble in water (< 3 µg/mL) at room temperature. The Ks was calculated from the linear fit of the curve according to Eq. (3). The observed Ks indicated the AL type solubility diagram suggest that the stoichiometry of DBM, EHMC and curcumin inclusion complexes with pbCDE and pbCDC are 1:1 and increase in the stability compared with the values of β-CD. Moreover, the Ks values of the inclusion complexes of DBM, EHMC and curcumin with pbCDE are greater than those of pbCDC indicating that the capability of complexes in improving the solubility and stability of all three compounds with pbCDE > pbCDC.
1H-NMR of all inclusion complexes in D2O are shown below. Figure 6 shows the 1H-NMR spectra of EHMC, DBM and curcumin inclusion complexes in pbCDE in D2O. It clearly shows that the aromatic proton signals of EHMC appearing in the 6–8 ppm region indicate that the EHMC is located inside CD cavity. In the case of DBM and EHMC (Fig. 6b, c) the broad signal of aromatic protons could be detected between 7 and 8.5 ppm but not clearly. These results suggested that the inclusion complexes of DBM–pbCDE and EHMC–pbCDE could be formed but the content of DBM and EHMC were very low. Unfortunately, the amount of all compounds in pbCDC were very low, the signals from the aromatic moieties of all guests could not be detected by 1H-NMR.
Figure 7 shows the UV–Vis absorption spectra of all active compound inclusion complexes with pbCDE and pbCDC in aqueous solutions. The maximum absorption wavelength of the EHMC, DBM and curcumin inclusion complexes in water showed a strong absorption throughout the UV–Vis spectra and similar shape to their free compounds. There was no absorption of the free active compounds in water because of their poor water solubility. The maximum absorption wavelength of all compound inclusion complexes with pbCDE showed a slight red shift compared to those with pbCDC and the free compounds. These results indicated that pbCDE and pbCDC improve the aqueous solubility of all active compounds and form inclusion complexes, where all active compounds penetrate into the βCD cavity by hydrophobic interactions.
Photostability
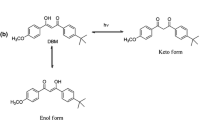
Upon exposure to UV lamps, Fig. 8 shows the percentage photostability of all inclusion complexes compared with the free compounds in water and ethylene glycol irradiated for 60 min. As the results, all inclusion complexes with pbCDE in ethylene glycol were more photostable compared to the free compounds. The photostability of curcumin increased when forming the inclusion complexes with pbCDE both in water and ethylene glycol. On the other hand, curcumin inclusion complexes with pbCDC showed a decrease in the photostability, probably due to an effect of the pH of the aqueous solution. It could be observed that the pH of pbCDC in aqueous solution was 3.2 whereas a pH of 5.5 was observed for pbCDE in aqueous solution. The degradation kinetics of curcumin under various pH conditions was reported by Lin et al. [37]. Curcumin degraded faster in a citrate–phosphate buffer pH 3 (k 5.842 × 10−5 min−1) than in a phosphate buffer pH 6 (k 3.541 × 10−5 min−1) at 37 °C. The DBM inclusion complexes with pbCDE and pbCDC in EG showed a slight increase in their photostability. In water, the photostability of the DBM inclusion complexes with pbCDE is similar to free DBM, whereas DBM–pbCDC showed a dramatic drop to 55%. These results indicated that the enol-form of DMB could be stabilized in ethylene glycol, whereas in water DBM is located inside the CD cavity, which is the more hydrophobic, preferred keto-form [38, 39]. The H-bond between the carbonyl group and the solvent cannot be stabilized, resulting in a proportionate increase in the keto-form [40]. The photostability of the EHMC inclusion complexes with pbCDE dramatically increased in water to 84 and 93% in ethylene glycol. This increased photostability was probably a result of less exposure of the EHMC molecules to UV light due to their sides being suitable to be inside the CD cavity where the EHMC could also be protected from the oxidizing environment e.g. reactive oxygen species. Whereas the photostability of the EHMC–pbCDC was similar to free EHMC both in water and EG, suggesting that the more acidic citric acid could not stabilize the photostability of EHMC [41].
Conclusions
Water soluble beta-cyclodextrin polymers were synthesized by polycondensation using Ep and citric acid as crosslinking agents. The substitution degree including the yield of pbCDC was lower than that of pbCDE due to the reactivity of citric acid is lower than Ep. FTIR spectra confirmed that hydroxyl groups of βCD had crosslinked with Ep for pbCDE and with the carboxyl group of citric acid for pbCDC. The inclusion complexes of curcumin, DBM and EHMC with pbCDE and pbCDC were also successfully prepared. The water solubility of these three compounds increased dramatically. These two linkers affect the loading capacity and photostability of these three guest compounds. EHMC was detected as the highest amount in both pbCDE and pbCDC. The amounts of curcumin, DBM and EHMC loading in pbCDE were higher than those in pbCDC. The photostability of curcumin, DBM and EHMC increased when they form the inclusion complexes with pbCDE. On the other hand, a decrease in photostability of curcumin and DBM were observed significantly by complex formation with pbCDC. The substituents on the CD with the different linkers seem to have some effect on the photodegradation. The mechanism of interactions between all compounds and CDs is under further investigation. This is useful for development of a water-soluble and stable organic molecules complexes.
References
De Miranda, J.C., Martins, T.E.A., Veiga, F., Ferraz, H.G.: Cyclodextrins and ternary complexes: technology to improve solubility of poorly soluble drugs. Braz. J. Pharm. Sci. 47, 665–681 (2011)
Gref, R., Amiel, C., Molinard, K., Daoud-Mahammed, S., Sébille, B., Gillet, B., et al.: New self-assembled nanogels based on host–guest interactions: characterization and drug loading. J. Control Release 111, 316–324 (2006)
Kurkov, S.V., Loftsson, T.: Cyclodextrins. Int. J. Pharm. 453, 167–180 (2013)
Liu, Y., Chen, G.-S., Chen, Y., Ding, F., Chen, J.: Cyclodextrins as carriers for cinchona alkaloids: a pH-responsive selective binding system. Org. Biomol. Chem. 3, 2519–2523 (2005)
Fenyvesi, É, Otta, K., Kolbe, I., Novák, C., Szejtli, J.: Cyclodextrin complexes of UV filters. J. Incl. Phenom. Macrocycl. Chem. 48, 117–123 (2004)
Scalia, S., Casolari, A., Iaconinoto, A., Simeoni, S.: Comparative studies of the influence of cyclodextrins on the stability of the sunscreen agent, 2-ethylhexyl-p-methoxycinnamate. J. Pharm. Biomed. Anal. 30, 1181–1189 (2002)
Pattanaargson, S., Munhapol, T., Hirunsupachot, P., Luangthongaram, P.: Photoisomerization of octyl methoxycinnamate. J. Photochem. Photobiol. A 161, 269–274 (2004)
Scalia, S., Simeoni, S., Barbieri, A., Sostero, S.: Influence of hydroxypropyl-β-cyclodextrin on photo-induced free radical production by the sunscreen agent, butyl-methoxydibenzoylmethane. J. Pharm. Pharmacol. 54, 1553–1558 (2002)
Chen, J., He, Z., Wang, F., Zhang, Z., Liu, X., Zhai, D., et al.: Curcumin and its promise as an anticancer drug: an analysis of its anticancer and antifungal effects in cancer and associated complications from invasive fungal infections. Eur. J. Pharmacol. 772, 33–42 (2016)
Stani, Z.: Curcumin, a compound from natural sources, a true scientific challenge—a review. Plant Foods Hum. Nutr. 72, 1–12 (2017)
Tønnesen, H.H., Másson, M., Loftsson, T.: Studies of curcumin and curcuminoids. XXVII. Cyclodextrin complexation: solubility, chemical and photochemical stability. Int. J. Pharm. 244, 127–135 (2002)
Jahed, V., Zarrabi, A., Bordbar, A., Hafezi, M.S.: NMR (1H, ROESY) spectroscopic and molecular modelling investigations of supramolecular complex of β-cyclodextrin and curcumin. Food Chem. 165, 241–246 (2014)
Yadav, V.R., Suresh, S., Devi, K., Yadav, S.: Effect of cyclodextrin complexation of curcumin on its solubility and antiangiogenic and anti-inflammatory activity in rat colitis model. AAPS PharmSciTech. 10, 752–762 (2009)
Yallapu, M.M., Jaggi, M., Chauhan, S.C.: β-Cyclodextrin-curcumin self-assembly enhances curcumin delivery in prostate cancer cells. Colloids Surf. B Biointerfaces 79, 113–125 (2010)
Patro, N.M., Sultana, A., Terao, K., Nakata, D., Jo, A., Urano, A., et al.: Comparison and correlation of in vitro, in vivo and in silico evaluations of alpha, beta and gamma cyclodextrin complexes of curcumin. J. Incl. Phenom. Macrocycl. Chem. 78, 471–483 (2014)
Loftsson, T., Jarho, P., Másson, M., Järvinen, T.: Cyclodextrins in drug delivery. Expert Opin. Drug. Deliv. 2, 335–351 (2005)
Wei, H., Yu, C.: Cyclodextrin funtionalized polymer as drug carriers for cancer therapy. Biomater. Sci. 3, 1050–1060 (2015)
Li, J., Xiao, H., Li, J., Zhong, Y.: Drug carrier systems based on water-soluble cationic β-cyclodextrin polymers. Int. J. Pharm. 278, 329–342 (2004)
Martel, B., Ruffin, D., Weltrowski, M., Lekchiri, Y., Morcellet, M.: Water-soluble polymers and gels from the polycondensation between cyclodextrins and poly(carboxylic acid)s: a study of the preparation parameters. J. Appl. Polym. Sci. 97, 433–442 (2005)
Martel, B., Weltrowski, M., Ruffin, D., Morcellet, M.: Polycarboxylic acids as crosslinking agents for grafting cyclodextrins onto cotton and wool fabrics: study of the process parameters. J. Appl. Polym. Sci. 83, 1449–1456 (2002)
Anand, R., Malanga, M., Manet, I., Manoli, F., Tuza, K., Aykaç, A., et al.: Citric acid–γ-cyclodextrin crosslinked oligomers as carriers for doxorubicin delivery. Photochem. Photobiol. Sci. 12, 1841–1854 (2013)
Garcia-fernandez, M.J., Tabary, N., Chai, F., Cazaux, F., Blanchemain, N., Flament, M., et al.: New multifunctional pharmaceutical excipient in tablet formulation based on citric acid-cyclodextrin polymer. Int. J. Pharm. 511, 913–920 (2016)
Renard, E., Deratani, A., Volet, G., Sebille, B.: Preparation and characterization of water soluble high molecular weight β-cyclodextrin-epichlorohydrin polymers. Eur. Polym. J. 33, 49–57 (1997)
Wang, C.X.: Fragrance-release property of b-cyclodextrin inclusion compounds and their application in aromatherapy. J. Ind. Text. 34, 157–166 (2005)
Zhang, W., Gong, X., Cai, Y., Zhang, C., Yu, X., Fan, J., et al.: Investigation of water-soluble inclusion complex of hypericin with β-cyclodextrin polymer. Carbohydr. Polym. 95, 366–370 (2013)
Zhu, X., Ping, W.: Optimization of β-cyclodextrin cross-linked polymer for monitoring of quercetin. Spectrochim. Acta Mol. Biomol. Spectrosc. 132, 38–43 (2014)
Anand, R., Manoli, F., Manet, I., Daoud-Mahammed, S., Agostoni, V., Gref, R., et al.: β-cyclodextrin polymer nanoparticles as carriers for doxorubicin and artemisinin: a spectroscopic and photophysical study. Photochem. Photobiol. Sci. 11, 1285–1292 (2012)
Ciobanu, A., Mallard, I., Landy, D., Brabie, G., Nistor, D., Fourmentin, S.: Inclusion interactions of cyclodextrins and crosslinked cyclodextrin polymers with linalool and camphor in Lavandula angustifolia essential oil. Carbohydr. Polym. 87, 1963–1970 (2012)
Zarrabi, A., Shokrgozar, M.A., Vossoughi, M., Farokhi, M.: In vitro biocompatibility evaluations of hyperbranched polyglycerol hydrid nanostructure as a candidate for nanomedicine applications. J. Mater. Sci. Mater. Med. 25, 499–506 (2014)
Zarrabi, A., Adeli, M., Vossoughi, M., Shokrgozar, M.A.: Design and synthesis of novel polyglycerol hybrid nanomaterials for potential applications in drug delivery system. Macromol. Biosci. 11, 383–390 (2011)
Puskás, I., Szemjonov, A., Fenyvesi, É, Malanga, M., Szente, L.: Aspects of determining the molecular weight of cyclodextrin polymers and oligomers by static light scattering. Carbohydr. Polym. 94, 124–128 (2013)
Higuchi, T., Connors, K.A.: Phase-solubility techniques. Adv. Anal. Chem. Instrum. 4, 117–212 (1965)
Karpkird, T., Wanichweacharungruang, S.: Synthesis and photostability of cyclodextrin modified with methoxycinnamic acids. J. Photochem. Photobiol. A Chem. 212, 56–61 (2010)
Wintgens, V., Amiel, C.: Water-soluble γ-cyclodextrin polymers with high molecular weight and their complex forming properties. Eur. Polym. J. 46, 1915–1922 (2010)
Zhao, D., Zhao, L., Zhu, C., Tian, Z., Shen, X.: Synthesis and properties of water-insoluble b-cyclodextrin polymer crosslinked by citric acid with PEG-400 as modifier. Carbohydr. Polym. 78, 125–130 (2009)
Uekama, K., Hirayama, F., Esaki, K., Inoue, M.: Inclusion complexes of cyclodextrins with cinnamic acid derivatives: dissolution and thermal behavior. Chem. Pharm. Bull. 27, 76–79 (1979)
Wang, Y.J., Pan, M.H., Cheng, A.L., Lin, L.I., Ho, Y.S., Hsieh, C.Y., et al.: Stability of curcumin in buffer solutions and characterization of its degradation products. J. Pharm. Biomed. Anal. 15, 1867–1876 (1997)
Mturi, G.J., Martincigh, B.S.: Photostability of the sunscreening agent 4-tert-butyl-4′-methoxydibenzoylmethane (avobenzone) in solvents of different polarity and proticity. J. Photochem. Photobiol. A Chem 200, 410–420 (2008)
Kockler, J., Motti, C., Robertson, S., Oelgemöller, M., Glass, B.D.: HPLC method for the simultaneous determination of the UV-filters butyl methoxy dibenzoylmethane and octocrylene in the presence of their photodegradants. Chromatographia 76, 721–727 (2013)
Yamaji, M., Kida, M.: Photothermal tautomerization of a UV sunscreen (4-tert-butyl-4′-methoxydibenzoylmethane) in acetonitrile studied by steady-state and laser flash photolysis. J. Phys. Chem. A 117, 1946–1951 (2013)
Kockler, J., Oelgemöller, M., Robertson, S., Glass, B.D.: Photostability of sunscreens. J. Photochem. Photobiol. C. Photochem. Rev. 13, 91–110 (2012)
Acknowledgements
We would like to thank Dr. István Puskás, CycloLab Research and Development Laboratory Ltd., Hungary for molecular weight determination. The financial supported by Kasetsart University Research and Development Institute (KURDI-78.58) and the Faculty of Science, Kasetsart University (URMF).
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Karpkird, T., Khunsakorn, R., Noptheeranuphap, C. et al. Inclusion complexes and photostability of UV filters and curcumin with beta-cyclodextrin polymers: effect on cross-linkers. J Incl Phenom Macrocycl Chem 91, 37–45 (2018). https://doi.org/10.1007/s10847-018-0796-y
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10847-018-0796-y