Abstract
The aim of the present work was to examine ellagic acid hydroxypropyl-β-cyclodextrin complex (EA-HP-β-CD) obtained through the freeze-drying method via FTIR, XRD, SEM, NMR and molecular modeling, as well as to investigate it’s antioxidant and anti-inflammatory activity on carrageenan-induced paw oedema in rats. Phase-solubility study showed that ellagic acid (EA) formed 1:2 stoichiometric inclusion complex with hydroxypropyl-β-cyclodextrin (HP-β-CD). The XRD and SEM analysis were confirmed true inclusion complex formation of EA with HP-β-CD by freeze-drying method when compared with a physical mixture. The FTIR, NMR and molecular modeling studies suggested that the carbonyl groups and hydroxyl groups of EA were involved in the inclusion complexation with HP-β-CD. EA-HP-β-CD exhibited better protection from protein denaturation and lysis of erythrocyte membrane as compared to positive controls. In vivo anti-inflammatory evaluation of EA-HP-β-CD in rats showed significant anti-inflammatory and antioxidant activity on carrageenan-induced rat paw oedema. The present findings suggest that EA-HP-β-CD inclusion complex enhances the anti-inflammatory and antioxidant effect of EA in experimental animal models.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Consumption of phenolic antioxidants available from fruits and vegetables is related with decline in the incidence of several degenerative diseases such as inflammation, cancer, diabetic and coronary heart disease [1]. Ellagitannins (ETs) are bioactive polyphenols that are abundant in some fruits, nuts and seeds such as pomegranates, black raspberries, raspberries, strawberries, walnuts and almonds, and are classified under hydrolysable tannins [2]. Ellagic acid (EA) was first discovered in 1831 by chemist Henri Braconnot, who named it “acide ellagique” [3]. It is a dimeric derivative of gallic acid, which mainly occurs in higher plants, as EA-glycosides, or bound as ETs [4]. Over last two decades, antioxidant nature of EA has been shown to possess wide array of pharmacological activities like prevention and treatment of inflammatory diseases, cancer, diabetic complications, atherosclerosis, hypertension, asthma and as skin whitening agent. Even though EA exhibits therapeutic benefits, it has poor solubility (<10 µg/ml in phosphate buffer pH 7.4) and permeability (0.13 × 10−6). It has been classified as class IV drug under biopharmaceutical classification system [5]. Additionally, orally administered EA displays poor stability at physiological pH (7.4), due to opening of lactone ring and formation of urolithin in colon microbiota, which limits its pharmacological activity. Hence, there is a need to develop drug delivery system which can mitigate the issues associated with EA without compromising its therapeutic benefits.
Cyclodextrins (CDs) are cyclic (α-1,4)-linked oligosaccharides of α-d-glucopyranose designated as α, β and γ-CDs according to the number of glucose units. They form inclusion complexes with variety of molecules. The most important property is the insertion of guest molecule into cavities of CDs leading to complexation, with no involvement of covalent bonding [6]. CDs have emerged as a promising approach to improve the solubility of a number of poorly soluble phytochemicals like hesperetin, hesperidin, resveratrol, quercetin, myricetin, kaempferol and rutin. In addition, the inclusion ability of CDs also potentiates their antioxidant activities [7]. Hydroxypropyl-β-cyclodextrin (HP-β-CD) complex was reported better inclusion ability and water solubility with drugs. Besides, toxicological studies pointed out that HP-β-CD was well tolerated in humans as compare other CDs [8, 9].
Boukharta et al. [10] and Chudasama et al. [11] reported that inclusion complex of EA in cyclodextrin elevates the levels of EA, almost twice in lung and up to 5.8-fold higher in pancreas respectively. In our recent study, we have found that EA-HP-β-CD complex alleviates adjuvant-induced arthritis in rat, which is mediated by attenuation of hyperalgesia, oxidative stress and pro-inflammatory cytokines [12]. However, to the best of our knowledge, characterization and evaluation of antioxidant and anti-inflammatory activities of inclusion complexes of EA with HP-β-CD has not been well explored till date.
The objective of the present study was to synthesize complex of EA with HP-β-CD by freeze-drying method and further to investigate the mechanism of inclusion complexation of EA with HP-β-CD using different analytical techniques including Fourier transform infrared spectroscopy (FTIR), powder X-ray diffractometry (XRD), nuclear magnetic resonance spectroscopy (NMR), scanning electron microscopy (SEM) and molecular modeling. EA-HP-β-CD complex was evaluated for in vitro anti-inflammatory activity by protein denaturation and membrane stabilization assays. Further, evaluation of the antioxidant and anti-inflammatory potential of EA-HP-β-CD inclusion complex on carrageenan-induced rat paw oedema was performed.
Experimental
Materials
Ellagic acid was obtained from Sigma Chemical Co. (St. Louis, MO, USA). HP-β-CD was obtained as a gift sample from Signet, India. Carrageenan (lambda) was purchased from Fluka Chemical (Switzerland) and prepared as a 1 % w/v solution in 0.9 % saline, not more than 24 h before use. Indomethacin (Sigma, USA) was suspended in 2 % (w/v) aqueous carboxy methylcellulose. Hydrocortisone was obtained from HiMedia, Mumbai, India. All other reagents used of analytical grade. Milli Q water (Millpore) was used throughout the studies.
Animals
Healthy adult male Sprague–Dawley rats weighing 180–220 g were obtained from the animal house of Haffkine Bio-Pharmaceutical Corporation Ltd., Mumbai. The animals were fed with a rodent standard diet with free access to water ad libitum and were housed in room maintained at 22 ± 2 °C temperature and humidity 55 ± 5 % with a 12 h light/dark cycle. The Institutes Animal Ethics Committee (IAEC) of the Department of Pharmaceutical Science, Institute of Chemical Technology, Mumbai approved experimental protocol (ICT/IAEC/2013/P38) in accordance with Committee for the Purpose of Control and Supervision of Experiments on Animals (CPCSEA) guidelines.
Phase solubility study
Phase solubility study was carried out using the method reported by Higuchi and Connors [13]. Increasing concentrations of HP-β-CD solutions such as 0, 4, 8, 12, 16, 20 and 24 mM were prepared in distilled water and filled in screw capped bottles. Excess EA was added to these solutions to attain saturation. Each bottle was capped and shaken for 72 h in a constant temperature water bath at 30 ± 2 °C. Following equilibrium, these solutions were filtered using 0.45 μm nylon disk filter, diluted suitably and assayed for the total dissolved EA content by using UV spectrophotometer (Shimadzu UV-160, Japan) at 254 nm. Experiments were performed in triplicate. The phase solubility diagram was constructed by plotting concentrations of dissolved EA against cyclodextrin concentration. The stability constants (K1:1 and K1:2) for the complexation were calculated from the plot using following Eqs. (1) and (2).
where [S0] is the intrinsic solubility of EA, slope of initial straight line obtained from phase solubility diagram and [St] and [Lt] are the concentrations of EA and HP-β-CD in solution, respectively [6].
Preparation of physical mixture
Physical mixtures (PM) were prepared by homogeneous blending of previously sieved and weighed EA and HP-β-CD in a mortar at a molar ratio of 1:2.
Preparation of inclusion complexes
The inclusion complex was prepared by freeze-drying technique. EA and HP-β-CD in 1:2 molar ratio, was obtained by adding 0.302 g EA to 20 ml Milli Q water containing 2.75 g HP-β-CD. The mixture was first agitated in rotary orbital shaker for 24 h at room temperature and then filtered through 0.45 µm filter. The resulting solution was frozen by keeping it in a repository at −60 °C and was lyophilized in a freeze-dryer (Labconco FreeZone 4.5, USA) for 24 h.
Fourier transform infrared spectroscopy (FTIR)
Solid state FTIR studies of EA, PM and EA-HP-β-CD were carried by a FTIR spectrometer (Shimadzu, IRAffinty-1, FTIR 8400S, Japan). All the FTIR spectra were collected over the spectral region 4000–450 cm−1.
Powder X-ray diffraction (XRD)
XRD studies were performed by using D8 Advance X-ray diffractometer (Bruker, Germany), with Cu kα radiation of wavelength 1.54060 Å. EA, HP-β-CD, PM and EA-HP-β-CD were subjected to XRD studies. The sample powders were placed in a glass sample holder and scanned from 4° to 60° (2θ) at a speed and step size of 2°/min and 0.01°, respectively.
1H Nuclear magnetic resonance (1H NMR) spectroscopy
All 1H NMR spectra were measured at 25 °C on Bruker AV 500 MHz (Bruker BioSpin, Switzerland) using DMSO-d6 as a solvent and TMS as an internal standard. The spectra were processed using Bruker’s TOPSPIN software.
Scanning electron microscopy (SEM)
Surface topology of EA, PM and EA-HP-β-CD were individually evaluated with analytical scanning electron microscope (JEOL-JSM-6380LA, Japan). The analysis was carried out at magnification 200× for higher spatial resolution. Prior to imaging, samples were sputter coated with platinum using (JEOL-JFC-1600, Japan) auto fine coater to render them electrically conductive.
In vitro dissolution studies
Dissolution studies were carried out by using USP dissolution apparatus type II (Electrolab, India). The test samples were examined at the paddle rotation speed of 75 rpm in 900 ml phosphate buffer (pH 6.8) as the dissolution medium at 37 ± 0.5 °C. Each formulation contained 100 mg EA. The samples were withdrawn at 5, 10, 15, 30, 45 and 60 min, filtered through syringe-filter (0.45 µm) and analyzed by using spectrophotometer at 254 nm.
Molecular modeling studies
Molecular structure of EA was obtained from Pubchem [14], converted to 3D from build panel of Maestro [15] and further energy minimized using MacroModel [16]. The HP-β-CD structure was drawn by adding 2-hydroxypropyl groups on the 3D structure of beta-cyclodextrin (β-CD), obtained from the protein data bank (accession id: 1JL8) [17] and then subjected to energy minimization by Impact [18] using Truncated Newton Conjugate Gradient (TNCG) method and OPLS_2005 force field.
For these studies, complexes of EA with HP-β-CD were constructed using Maestro in ratios of 1:1 and 1:2 respectively. The free EA and HP-β-CD, as well as the complexes were individually soaked in SPC water in orthorhombic boundary conditions in system builder panel of Desmond [19]. Molecular dynamics using Impact were further pursued on these systems to relax them to minimum energies and optimum geometries of the EA-HP-β-CD complexes using OPLS_2005 force field. Velocity Verlet algorithm was used for integrating the equations of motion in standard Cartesian-space MD with NVT (constant number of molecules, temperature, and volume) ensemble and the results were viewed in trajectory panel using appropriate frame steps and optimum speeds. The complexation energies (ΔE) of each of the geometrically refined structures of complexes in the minimized systems were calculated by molecular mechanics using MacroModel as a measure of the complexation effect of HP-β-CD with EA according to the Eq. (3)
where the energies of HP-β-CD, EA and their inclusion complex were represented by Ehost, Eguest and Ecomplex in kJ/mol respectively. The magnitude of the energy change would be an indication of the driving force towards complexation. The larger the complexation energy change, the more thermodynamically favorable and stable would be the inclusion complex.
Inhibition of bovine serum albumin denaturation
Test solutions (1 ml) containing different concentrations of drug was mixed with 1 ml of 1 % albumin solution in phosphate buffer and incubated at 27 ± 1 °C for 15 min. The reaction mixture was keep at 60 ± 1 °C in a water bath for 10 min to induced denaturation. The solution was allowed to cool and turbidity was measured at 660 nm using UV spectrophotometer. Indomethacin was used as standard drug. Percentage inhibition of denaturation was calculated from control where no drug was added [20].
Membrane stabilizing activity
Fresh whole human blood was collected into heparinized tube to prevent clotting. The blood centrifuged at 3000 rpm for 5 min, and was washed three times using equal volume of 0.9 % saline. The 10 mM sodium phosphate buffer (pH 7.4) was used to reconstitute 40 % v/v suspension of erythrocyte and used for this test. The heat induced haemolysis and hypotonic solution induced haemolysis assays were performed to access membrane stabilizing activity describe by Shinde et al. [21].
Lambda (λ) Carrageenan-induced paw oedema in rats
The experiment was carried out using the method of Winter et al. [22]. Adult male Sprague–Dawley rats (180–200 g), with free access to water, but fasted overnight (8 h) were used. The animals were randomly divided into 6 groups (n = 6) and oedema was induced by injection of 0.1 ml of 1 % suspension of λ carrageenan into the right hind paw. EA (20 mg/kg), EA-HP-β-CD (10 and 20 mg/kg), Indomethacin (10 mg/kg), or vehicle were administrated orally 1 h before injection of carrageenan. Paw volumes were measured at 0, 1, 2, 3 and 4 h after carrageenan injection, with a plethysmometer (Model No. 7140, Ugo Basile, Italy) and the volume of oedema at each time point was calculated by subtracting the initial paw volume (0 h). The percentage inhibition of paw volume was calculated as follows:
where Vc is the mean volume of rat paw oedema on carrageenan-induced group, and Vt is the mean volume of rat paw oedema in treated group.
Preparation of tissues homogenates
The SOD, CAT, GSH and MPx levels were determined in rat paw oedema tissues after sacrifice. To prepare the tissue homogenates, paw oedema tissues of each rat were treated with 2 ml solution containing 0.05 M Tris buffer, 0.15 M NaCl and 0.1 % (v/v) Triton X-100. The mixtures were homogenised on ice for 15 min. Homogenates were filtered and centrifuged by using a refrigerated centrifuge at 4 °C. The supernatants were used for the determination of the enzymatic activities [23].
Determination of antioxidant enzyme and myeloperoxidase (MPx) activity in rats paw tissue
The superoxide dismutase (SOD) activity was determined as described by Misra and Fridovich [24]. The catalase (CAT) activity was determined as described by Aebi [25] while the method of Sedlak and Lindsay [26] was adopted for the estimation of glutathione (GSH). MPx activity was measured according to the modified method of Bradley et al. [27].
Statistical analysis
All values were expressed as Mean ± Standard Error Mean (S.E.M.). The statistic significance of differences between the groups were analyzed by one-way ANOVA tests followed by Dunnett’s test. A p values (p < 0.05) was considered statistically significant.
Results and discussion
Phase-solubility study
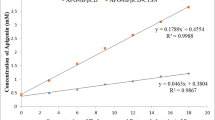
The stoichiometry of EA-HP-β-CD complex was determined by phase solubility study (Fig. 1). The phase solubility plot is a useful technique for evaluation of interactions between CDs and poorly soluble drug molecules, because it provides not only the solubilizing ability of CDs but also the stability constant of complex formation. The plot shows a linear increase in aqueous solubility of EA as a function of HP-β-CD concentration up to 12 mM followed by negative deviation from linearity up to 24 mM. This solubility curve of EA in the presence of HP-β-CD can be classified as AN type according to Higuchi and Connors [13], and may be attributed to the formation of soluble 1:2 EA-HP-β-CD inclusion complex [28]. The calculated stability constants K1:1 and K1:2 of EA with HP-β-CD were 201.61 and 18.91/M respectively, which indicated that cavity size of HP-β-CD is optimal for entrapment of the EA molecules and it provides better solubilization effect [6]. Furthermore, it was observed that increasing the HP-β-CD concentration resulted in a downward trend in the negative values of Gibbs free energy at 30 °C (−2263.91 to −4353.22 cal/mol), which indicates that the process is spontaneous, and HP-β-CD solutions put forward a more favourable environment than water for EA.
FTIR analysis
The formation of inclusion complex of EA with HP-β-CD was studied by FTIR spectroscopy. It is generally observed that FTIR spectrum of inclusion complex showed major peaks of CDs along with fewer guest peaks, but found to be shifted to higher or lower wavenumber with reduced intensity or change in shape [28]. Figure 2 and Table 1S in Supplementary Material shows the FTIR spectra and spectral assignments of EA, HP-β-CD, PM and the EA-HP-β-CD inclusion complex. The FTIR spectrum of EA (Fig. 2a), shows prominent absorption bands at 3468/cm attributed to the OH stretching, while the band observed at 1710/cm corresponds to C=O stretching [29]. The band observed at 1616 and 1506/cm are due aromatic ring vibrations, while the bands at 1320–1000/cm imply ester linkage. The FTIR spectrum of HP-β-CD (Fig. 2b) illustrated intense absorption bands at 3340/cm (for O–H stretching vibrations), 2931/cm (for C–H stretching vibrations) and 1149, 1010/cm (C–O, C–C, C–O–C stretching vibration). The FTIR spectrum of the PM (Fig. 2c) did not differ significantly from a combination of the spectra of EA and HP-β-CD. The spectrum of inclusion complex was very similar to that of HP-β-CD (Fig. 2d). However, the characteristic C=O stretching band of EA at 1710/cm shifted to 1703/cm with reduced intensity and the OH stretching band at 3468/cm was masked by an intense band corresponding to the OH stretching vibrations of HP-β-CD. This is probably due to inclusion complexation of EA into the HP-β-CD cavity. This suggests that the carbonyl and hydroxyl groups of EA were involved in the inclusion complexation with HP-β-CD.
Powder X-ray diffraction (XRD) analysis
Further evidence for the formation of EA-HP-β-CD complex was obtained from XRD. As shown in Fig. 3, HP-β-CD exhibited two broad peaks (2θ = 11.37° and 18.61°) confirming its amorphous nature, whereas EA displayed numerous sharp peaks characteristic of its crystallinity, which are consistent with the previous reports [30]. The XRD pattern of PM showed approximate superimposition of the individual patterns of HP-β-CD and EA (Fig. 3c). The diffractogram of the complex differed from that of the corresponding PM, where the characteristic peaks of EA, particularly at 10.3°, 20.6°, 27.8° and 31.3° (2θ) nearly disappeared, indicating the formation of a true inclusion complex.
1H NMR spectroscopy
1H NMR technique reflects direct confirmation of the inclusion of guest into the hydrophobic HP-β-CD cavity. Inclusions of EA in HP-β-CD cavity are indicated by the changes in chemical and electronic environments of protons, which are affected during complexation, and are reflected through changes in the chemical shift (Δδ) values (Supplementary Material Fig. S1). Such chemical shift gives evidence about which part of the guest molecule is inserted into the CD cavity. The 1H NMR spectrum of HP-β-CD, EA and their inclusion complex are shown in Fig. 4. The 1H chemical shifts of free HP-β-CD [31] and EA [32] corresponded to the previous literature and shown in Table 1. It shows that the inclusion complexation of EA with HP-β-CD has a negligible effect on the δ values of the H-1, H-2, H-3 and H-4 protons of HP-β-CD (0.001–0.004). However, H-5 (narrow side) and H-6 protons exhibit significant chemical shift changes, 0.087 and −0.017 ppm respectively. It was observed that the variation in chemical shift of H-3 was significantly smaller than H-5 after the inclusion complexation. The interior of HP-β-CD cavity contains both the H-3 and H-5 protons present near the wide and narrow edges of the cavity respectively [33]. The 1H NMR spectrum of EA at δ7.47 ppm (s, 2H, ArH) and δ3.36 ppm (s, 4H, OH) are shown in Fig. 4 and Table 1. On formation of inclusion complex of EA with HP-β-CD a change in chemical shift (0.009–0.015) was seen. Thus, this 1H NMR data reveals that EA was included in the HP-β-CD cavity and should penetrate inside the cavity from the narrow side of HP-β-CD (Fig. 5).
Scanning electron microscopy (SEM) analysis
The scanning electron microscopy (SEM) images of EA, HP-β-CD, EA-HP-β-CD inclusion complex and their PM were represented in Fig. 6. The SEM images are used to study the external morphology of drugs, HP-β-CD and their inclusion complex [34]. EA existed in irregular shaped crystal particles (Fig. 6a), whereas HP-β-CD was observed as amorphous ‘‘shrinked” spherical particles (Fig. 6b). In PM, the characteristic HP-β-CD microspheres are observed with EA crystals or adhered to their surface (Fig. 6c). In contrast, the EA-HP-β-CD inclusion complex (Fig. 6d) showed a change in morphological appearance, with loss of the typical structure of EA and HP-β-CD spherical particles (Fig. 6a, b), thus confirming the formation of the inclusion complex.
In vitro dissolution studies
The dissolution profiles of EA alone, PM and EA-HP-β-CD are reported in Fig. 7. The release rate profiles were drawn as the percentage of drug dissolved vs time. According to these results, the HP-β-CD inclusion complex released up to 55 % of the drug in 15 min, and up to 60 % after 30 min; whereas EA exhibited a release of 10 % after 15 min and up to 13 % after 60 min. The dissolution rate of EA-HP-β-CD shows a marked fivefold increase as compared to that of EA alone. The faster dissolution rate obtained with inclusion complex can be attributed to the decrease in EA crystallinity as confirmed by XRD analysis. Improvement in EA wettability and formation of readily soluble complexes in the dissolution medium are plausible reasons cited in literature [35].
Molecular modeling studies
Computer aided molecular modeling has found widespread use in the study of three dimensional structures of the inclusion complexes with cyclodextrin especially by molecular dynamics simulations [36, 37]. EA-HP-β-CD systems were studied in ratios of 1:1 and 1:2 using molecular dynamics. The structures of EA, HP-β-CD and the different complexes generated were relaxed and their energies were calculated using molecular mechanics by MacroModel. The energies of EA and HP-β-CD were found to be −10325.861 and −15.750.867 kJ/mol respectively. The complexation energies were then calculated as per Eq. 3 and are shown in Table 2.
It was observed that EA-HP-β-CD in ratio of 1:2 yields a more stable complex than in 1:1 inclusion complex. This in turn is favored by large number of hydrophobic interactions on either faces of EA, with the HP-β-CD molecules in the 1:2 complex. Hydrogen bonding between carbonyl groups and hydroxyl groups of EA with that of the hydroxyl groups of HP-β-CD, along with hydrophobic interactions enhance stability of the 1:2 complex (−24723.52), almost more than twice as that in 1:1 complex (−10905.29; in Supplementary Material Fig. S2). Thus, in silico studies indicate that the 1:2 inclusion complex of EA with HP-β-CD is more stable than the 1:1 complex and the carbonyl and hydroxyl groups of EA are involved in hydrogen bonding with HP-β-CD as shown in Fig. 8. These results are in agreement with in vitro FTIR (Fig. 2) and 1H NMR (Fig. 4) studies.
Effect of EA and EA-HP-β-CD on in vitro anti-inflammatory activity
The preliminary anti-inflammatory activity was evaluated in vitro by protein denaturation and membrane stabilization assays. Denaturation of tissue proteins and membrane stabilization is one of cause of inflammation and rheumatoid arthritis. In inflammatory disease, lysozomal enzymes like bactericidal enzymes and proteases are releases from neutrophils that caused tissue inflammation and damage. The erythrocyte membrane was similar to lysozomal membrane components and the stabilization of membrane was taken as a measure of anti-inflammatory activity of test drugs [38]. Compared to the indomethacin and hydrocortisone, test group showed acceptable anti-inflammatory activity and represented in Table 3. EA-HP-β-CD (20 µg/ml) exhibited maximum 75.85 % inhibition of albumin denaturation, 43.03 % heat induced and 61.62 % hypotonic induced haemolysis.
Effect of EA and EA-HP-β-CD on carrageenan-induced paw oedema in rats
The carrageenan group shows maximum phlogistic response from 1st to 4th h, while the indomethacin treatment showed significant inhibition (p < 0.001) of paw oedema for the same duration with percentage of inhibition from 48.82 to 76.68 as illustrated in Table 4. Treatment with EA-HP-β-CD at dose of 10 and 20 mg/kg showed dose-dependent reduction of carrageenan-induced paw oedema, whereas plain EA had little effect at 20 mg/kg. Peak inhibitory effects 51.31 and 59.48 % were recorded with EA-HP-β-CD at doses of 10 and 20 mg/kg at 4 h post-carrageenan administration respectively, comparable to that of indomethacin (10 mg/kg, 76.68 %). EA was previously shown to regulate lipopolysaccharide-induced inhibitory κB (IκB), tumor necrosis factor (TNF-α), interleukin-1β (IL-1β), and iNOS in murine macrophage RAW 264.7 cells [39]. Moreover, Corbett et al. [40] reported that EA was effective against carrageenan-induced rat paw oedema. Our results agree with previously reported results that EA has anti-inflammatory activity. This study demonstrates that due to the complexation with HP-β-CD, significant potentiation of the anti-inflammatory activity occurs.
Effect of EA and EA-HP-β-CD on antioxidant enzymes and Myeloperoxidase (MPx) activity on carrageenan-induced paw oedema (4th h) in rats
Carrageenan (phlogistic agent) is a seaweed polysaccharide and it is believed that injection of carrageenan induces biphasic response [22], the initial phase (1 h) involves the release of histamine and serotonin, and the second phase (over 1 h) is due to the release of prostaglandin-like substances. Based on this, the second phase is associated with increase in oxidative stress in rat paw oedema as well as leads to the release of other neutrophil derived mediators like myeloperoxidase [41]. The levels of antioxidant enzymes (SOD, CAT and GSH) were estimated to study if there was any amelioration of oxidative damage caused due to inflammation in the rat paw tissue.
Reactive oxygen species (ROS) are normal by-products of cellular metabolism. Imbalance between ROS and antioxidant occurs in a number of diseases. Among ROS, the superoxide anion (O2 −) plays a pivotal role in inflammation including endothelial cell damage, increased microvascular permeability, activation of leukotrienes B4, recruitment of neutophils at sites of inflammation, lipid peroxidation and formation of peroxynitrite (ONOO−). The enzyme superoxide dismutase (SOD) neutralizes O2 − by transforming it into hydrogen peroxide (H2O2), thereby preventing the formation of highly aggressive compounds such as ONOO− and hydroxyl radical (HO−) [42, 43]. In present study we found that EA-HP-β-CD (20 mg/kg) significantly (p < 0.05) increased SOD activity, which was reduced in carrageenan injected rat paw tissues (Table 5).
Catalase is a cytoplasmic 240 kD homotetrameric protein and is an important intracellular antioxidant enzyme detoxifying the H2O2 to water and non-reactive oxygen species thus preventing generation of hydroxyl radical and protecting the cells from oxidative damage [44, 45]. As shown in Table 5, there was a significant increase in CAT activity after EA-HP-β-CD treatment in vivo.
Glutathione in its reduced form (GSH) demonstrates pleiotropic effect on cell function, it act as an electron donor for antioxidative enzymes like glutathione peroxidase and prevents the formation of conjugates with some harmful endogenous and xenobiotic compounds via catalysis of glutathione S-transferase [41]. Treatments with EA-HP-β-CD restore the GSH level in rat paw which decreased the inflammation induced by carrageenan.
Myeloperoxidase (MPx) is an enzyme stored in azurophilic granules of neutrophils and macrophages, acts as powerful pro-oxidant in the setting of inflammatory process. In this study injection of carrageenan significantly (p < 0.001) increased the MPx level, whereas as treatment with 20 mg/kg EA (p < 0.05), 10 mg/kg EACD (p < 0.01), 20 mg/kg EAHPCD (p < 0.001) and 10 mg/kg indomethacin significantly (p < 0.001) reduced the MPx level. Restored levels of antioxidant enzymes (SOD, CAT and GSH) and MPx has been attributed to EA [46, 47], however, we find that this activity increases due to complexation with HP-β-CD.
Conclusion
In this study, we formed ellagic acid inclusion complex with HP-β-CD by freeze-drying method. The results of 1H NMR, FTIR, XRD and SEM conclusively proved the formation of EA-HP-β-CD inclusion complex. 1H NMR and molecular modeling confirmed that the carbonyl groups and hydroxyl groups of EA were involved in complexation into HP-β-CD cavities from its narrow edge. The result of molecular modeling suggested that 1:2 complex is more stable than 1:1 complex. Moreover, this study demonstrates that HP-β-CD inclusion is an effective strategy to enhance the anti-inflammatory and antioxidant activity of EA. Investigations on whether the approach developed in the present work could improve the oral bioavailability and be useful in treatment of chronic inflammation, are underway.
References
Ma, Q., Kinneer, K., Ye, J., Chen, B.J.: Inhibition of nuclear factor kappaB by phenolic antioxidants: interplay between antioxidant signaling and inflammatory cytokine expression. Mol. Pharmacol. 64, 211–219 (2003)
Landete, J.M.: Ellagitannins, ellagic acid and their derived metabolites: a review about source, metabolism, functions and health. Food Res. Int. 44, 1150–1160 (2011)
Grasser, G.: Synthetic Tannins. Enna F. G. A. (trans.) (3rd ed.) p. 20, London (1922)
Seeram, N.P., Lee, R., Heber, D.: Bio availability of ellagic acid in human plasma after consumption of ellagitannins from pomegranate (Punica granatum L.) juice. Clin. Chim. Acta 348, 63–68 (2004)
Sonaje, K., Italia, J.L., Sharma, G., Bhardwaj, V., Tikoo, K., Ravi Kumar, M.N.V.: Development of biodegradable nanoparticles for oral delivery of ellagic acid and evaluation of their antioxidant efficacy against cyclosporine A-induced nephrotoxicity in rats. Pharm. Res. 24, 899–908 (2007)
Brewster, M.E., Loftsson, T.: Cyclodextrins as pharmaceutical solubilizers. Adv. Drug Deliv. Rev. 59, 645–666 (2007)
Fang, Z., Bhandari, B.: Encapsulation of polyphenols: a review. Trends Food Sci. Technol. 21, 510–523 (2010)
Kfoury, M., Auezova, L., Fourmentin, S., Greige-Gerges, H.: Investigation of monoterpenes complexation with hydroxypropyl-β-cyclodextrin. J. Incl. Phenom. Macrocycl. Chem. 80, 51–60 (2014)
Gould, S., Scott, R.C.: 2-Hydroxypropyl-β-cyclodextrin (HP-β-CD): a toxicology review. Food Chem. Toxicol. 43, 1451–1459 (2005)
Boukharta, M., Jalbert, G., Castonguay, A.: Biodistribution of ellagic acid and dose-related inhibition of lung tumorigenesis in A/J mice. Nutr. Cancer 18, 181–189 (1992)
Chudasama, Y.N., Lugea, A., Lu, Q.Y., Pandol, S.J.: Beta-cyclodextrin increases bioavailability of ellagic acid in rats. Gastroenterology 140, S-860 (2011)
Bulani, V., Kothavade, P., Nagmoti, D., Juvekar, A.: Ellagic acid hydroxypropyl-ß-cyclodextrin inclusion complex alleviates adjuvant-induced arthritis: attenuation of oxidative stress and inflammatory mediators. Cytokines 70, 32 (2014)
Higuchi, T., Connors, K.A.: Phase solubility techniques. In: Reiley, C.N. (ed.) Advance in Analytical Chemistry and Instrumentation, pp. 117–212. Wiley, New York (1965)
Maestro version 9.4, Schrödinger, LLC, New York, NY (2013)
MacroModel version 10.0, Schrödinger, LLC, New York, NY (2013)
Impact version 5.9, Schrödinger, LLC, New York, NY (2013)
Desmond version 3.4, D. E. Shaw Research, New York, NY (2013)
Manohara Reddy, S.A., Mudgal, J., Bansal, P., Vasanthraju, S.G., Srinivasan, K.K., Rao, C.M., Gopalan Kutty, N.: Antioxidant, anti-inflammatory and anti-hyperglycaemic activities of heterocyclic homoprostanoid derivatives. Bioorg. Med. Chem. 19, 384–392 (2011)
Shinde, U.A., Phadke, A.S., Nair, A.M., Mungantiwar, A.A., Dikshit, V.J., Saraf, M.N.: Membrane stabilizing activity—a possible mechanism of action for the anti-inflammatory activity of Cedrus deodara wood oil. Fitoterapia 70, 251–257 (1999)
Winter, C.A., Risley, E.A., Nuss, G.W.: Anti-Inflammatory and antipyretic activities of indomethacin, 1-(P-Chlorobenzoyl)-5-Methoxy-2-Methylindole-3-Acetic Acid. J. Pharmacol. Exp. Ther. 141, 369–376 (1963)
Anderson, A.J.: Lysosomal enzyme activity in rats with adjuvant-induced arthritis. Ann. Rheum. Dis. 29, 307–313 (1970)
Misra, H.P., Fridovich, I.: The role of superoxide anion in the autoxidation of epinephrine and a simple assay for superoxide dismutase. J. Biol. Chem. 247, 3170–3175 (1972)
Aebi, H.: Catalase in vitro. In: Packer, L. (ed.) Methods in Enzymology, pp. 121–126. Academic Press, New York (1984)
Sedlak, J., Lindsay, R.H.: Estimation of total, protein bound and non protein sulfhydryl groups in tissue with Ellman’s reagent. Anal. Biochem. 25, 192–205 (1968)
Bradley, P.P., Priebat, D.A., Christensen, R.D., Rothstein, G.: Measurement of cutaneous inflammation: estimation of neutrophil content with an enzyme marker. J. Invest. Dermatol. 78, 206–209 (1982)
Dandawate, P.R., Vyas, A., Ahmad, A., Banerjee, S., Deshpande, J., Swamy, K.V., Jamadar, A., Dumhe-Klaire, A.C., Padhye, S., Sarkar, F.H.: Inclusion complex of novel curcumin analogue CDF and β-Cyclodextrin (1:2) and its enhanced in vivo anticancer activity against pancreatic cancer. Pharm. Res. 29, 1775–1786 (2012)
Goriparti, S., Harish, M.N.K., Sampath, S.: Ellagic acid: a novel organic electrode material for high capacity lithium ion batteries. Chem. Commun. 49, 7234–7236 (2013)
Kim, S., Liu, Y., Gaber, M.W., Bumgardner, J.D., Haggard, W.O., Yang, Y.: Development of chitosan-ellagic acid films as a local drug delivery system to induce apoptotic death of human melanoma cells. J. Biomed. Mater. Res. B Appl. Biomater. 90, 145–155 (2009)
Zhang, Y., Ren, K., He, Z., Li, H., Chen, T., Lei, Y., Xiad, S., Hea, G., Xiea, Y., Zhenga, Y., Song, X.: Development of inclusion complex of brinzolamide with hydroxypropyl-β-cyclodextrin. Carbohydr. Polym. 98, 638–643 (2013)
Li, X.C., Elsohly, H.N., Hufford, C.D., Clark, A.M.: NMR assignments of ellagic acid derivatives. Magn. Reson. Chem. 37, 856–859 (1999)
Qiu, N., Cheng, X., Wang, G., Wang, W., Wen, J., Zhang, Y., Song, H., Ma, L., Wei, Y., Peng, A., Chen, L.: Inclusion complex of barbigerone with hydroxypropyl-β-cyclodextrin: preparation and in vitro evaluation. Carbohydr. Polym. 101, 623–630 (2014)
de Araujo, D.R., Tsuneda, S.S., Cereda, C.M.S., Carvalho, F.D.G.F., Prete, P.S.C., Fernandes, S.A., Fabiano, Y., Franco, M.K.K.D., Mazzaro, I., Fraceto, L.F., de Braga, F.A., de Paula, E.: Development and pharmacological evaluation of ropivacaine-2-hydroxypropyl-beta-cyclodextrin inclusion complex. Eur. J. Pharm. Sci. 33, 60–71 (2008)
Li, N., Zhang, Y.H., Wu, Y.N., Xiong, X.L., Zhang, Y.H.: Inclusion complex of trimethoprim with beta-cyclodextrin. J. Pharm. Biomed. Anal. 39, 824–829 (2005)
Reddy, M.N., Rehana, T., Ramakrishna, S., Chowdary, K.P.R., Diwan, P.V.: Beta-cyclodextrin complexes of celecoxib: molecular-modeling, characterization, and dissolution studies. AAPS J. 6, 68–76 (2004)
Jadhav, G.S., Vavia, P.R.: Physicochemical, in silico and in vivo evaluation of a danazol-β-cyclodextrin complex. Int. J. Pharm. 352, 5–16 (2008)
Kumari, K.D.K.P., Weerakoon, T.C.S., Handunnetti, S.M., Samarasinghe, K., Suresh, T.S.: Anti-inflammatory activity of dried flower extracts of Aegle marmelos in Wistar rats. J. Ethnopharmacol. 151, 1202–1208 (2014)
Lee, J., Kim, S., Namgung, H., Jo, Y.H., Bao, C., Choi, H.K., Auh, J.H., Lee, H.J.: Ellagic acid identified through metabolomic analysis is an active metabolite in Strawberry (‘Seolhyang’) regulating lipopolysaccharide-induced inflammation. J. Agric. Food Chem. 62, 3954–3962 (2013)
Corbett, S., Daniel, J., Drayton, R., Field, M., Steinhardt, R., Garrett, N.: Evaluation of the anti-inflammatory effects of ellagic acid. J. PeriAnesthesia Nurs. 25, 214–220 (2010)
Halici, Z., Dengiz, G.O., Odabasoglu, F., Suleyman, H., Cadirci, E., Halici, M.: Amiodarone has anti-inflammatory and anti-oxidative properties: an experimental study in rats with carrageenan-induced paw oedema. Eur. J. Pharmacol. 566, 215–221 (2007)
Cuzzocrea, S., Salvemini, D.: The role of superoxide in acute and chronic inflammation. In Therapeutic Application of Superoxide Dismutase (SOD), Landes Bioscience, Georgetown, Texas, USA (Chapter 3) (2005)
Afonso, V., Champy, R., Mitrovic, D., Collin, P., Lomri, A.: Reactive oxygen species and superoxide dismutases: role in joint diseases. Joint Bone Spine 74, 324–329 (2007)
Mittal, M., Siddiqui, M.R., Tran, K., Reddy, S.P., Malik, A.B.: Reactive oxygen species in inflammation and tissue injury. Antioxid. Redox Signal. 20, 1126–1167 (2014)
Chaturvedi, P.: Inhibitory response of Raphanus sativus on lipid peroxidation in albino rats. Evid. Based Complement. Altern. Med. 5, 55–59 (2008)
Mo, J., Panichayupakaranant, P., Kaewnopparat, N., Songkro, S., Reanmongkol, W.: Topical anti-inflammatory potential of standardized pomegranate rind extract and ellagic acid in contact dermatitis. Phytother. Res. 28, 629–632 (2014)
Han, D.H., Lee, M.J., Kim, J.H.: Antioxidant and apoptosis-inducing activities of ellagic acid. Anticancer Res. 26, 3601–3606 (2006)
Acknowledgements
Authors are thankful to the University Grant Commission, India (UGC) for the research fellowship awarded under special assistance programme (SAP) and Sophisticated Analytical Instrument Facility, Indian Institute of Technology, Madras, India, for spectral analysis.
Conflict of interest
The authors declare that they have no conflicts of interest.
Author information
Authors and Affiliations
Corresponding author
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Bulani, V.D., Kothavade, P.S., Nagmoti, D.M. et al. Characterisation and anti-inflammatory evaluation of the inclusion complex of ellagic acid with hydroxypropyl-β-cyclodextrin. J Incl Phenom Macrocycl Chem 82, 361–372 (2015). https://doi.org/10.1007/s10847-015-0498-7
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10847-015-0498-7