Abstract
Sulfanilamide belongs to the group of drugs that have a bacteriostatic effect on different pathogenic microorganisms. This activity originates from the competitive antagonism with p-aminobenzoic acid, which is an integral part of folic acid. The safe use of sulfanilamide is limited due to poor solubility in the aqueous medium. Therefore, the aim of this paper is the synthesis of sulfanilamide, as well as preparing and structural characterization of its inclusion complexes with cyclodextrins. The crude sulfanilamide was obtained in the synthesis between acetanilide and chlorosulfonic acid according to the standard procedure. The synthesized sulfanilamide was recrystallized from water in order to obtain the satisfactory purity of the substance. Sufanilamide was complexed with β-cyclodextrin and 2-hydroxypropyl-β-cyclodextrin by the co-precipitation method. A molecular encapsulation of sulfanilamide was confirmed by using FTIR, 1H-NMR, XRD and DSC methods. Phase-solubility techniques were used to assess the formation of the inclusion complex between sulfanilamide and cyclodextrins. The photostability of sulfanilamide and its inclusion complexes was estimated by UVB irradiation in a photochemical reactor by applying the UV–Vis method. Based on the UV–Vis analysis, sulfanilamide:2-hydroxypropyl-β-cyclodextrin complex was presented as more photostable than sulfanilamide:β-cyclodextrin complex and sulfanilamide. The obtained results enable the potential use of these inclusion complexes for the preparation of oral formulations due to the enhanced solubility of sulfanilamide.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Sulfonamides are the synthetic drugs which are broadly used in the treatment of defined infections caused by Gram-positive and Gram-negative bacteria, some fungies and some protozoa. In addition to the antibiotics development, the application of sulfonamides in the treatment of microbial infections is important due to the efficient and broad action, as well as safe use [1, 2]. Sulfanilamide, 4-aminobenzenesulfonamide, is the simplest representative in the group of sulfonamide drugs [3]. The chemical structure of sulfanilamide with marked C atoms is presented in Fig. 1a.
Sulfonamides are competitive inhibitors of p-aminobenzoic acid which is necessary for the synthesis of folic acid, a compound that is important for the growth and reproduction of bacteria. In this way, sulfonamides achieve a bacteriostatic effect [4]. Due to poor water solubility of sulfonamides, their application in therapy as pharmaceutical active substances is limited [2].
Cyclodextrins are cyclic oligosaccharides that are composed from glucopiranosyl units interconnected by α-1,4-glicoside bonds [5, 6]. The chair conformation of glucopyranosyl units gives cyclodextrin molecules the form of a truncated cone. The secondary hydroxyl groups are located on the outside, while the primary hydroxyl groups are located on the inside of the molecule. Unlike the other saccharides, cyclodextrins have the hydrophobic cavity where it is possible to incorporate a non-polar molecule of the adequate size [7, 8].
β-Cyclodextrin (βCD) and its derivates (Fig. 1b) are intensively studied by different experimental techniques and used in the pharmaceutical industry with the aim to increase the solubility, stability and bioavailability of drugs [9]. Ristić et al. [10] acetyled the OH groups of βCD by using acetic anhydride with iodine as the catalyst to synthesize acetyl-βCD. They used the obtained cyclodextrin for preparing the inclusion complex with poly(ethylene oxide) with the 4,000 g/mol molecular mass.
The inclusion complexes with βCD and their derivates are more used in order to improve physical and chemical characteristics (solubility, volatility, odor, unstability) of pharmacological active substances. The improvement of the solubility and stability of atenolol [11], nifedipine [12], amlodipine [13], allicin [14], iron(II) fumarate [15], usnic acid [16], antazoline, naphazoline and xylometazoline [17] due to the complexation with βCD, and nabumetone with γCD [18] has been described in literature. Recent researches show the improved photostability of nifedipine and piroxicam in the inclusion complexes with 2-hydroxypropyl-β-cyclodextrin (HP-βCD) [19, 20], as well as antazoline, xylometazoline and naphazoline with βCD [21]. Al-Rawashdeh et al. [22] investigated the effect of the HP-βCD concentration on the physicochemical properties of the sunscreen agents (oxybenzone, octocrylene, ethylhexyl-methoxy-cinnamate) in the aqueous solution and cream formulations.
Sulfanilamide is sensitive to photo-oxidation due to the presence of aniline in its structure [23]. Therefore, the aim of this paper was the synthesis of sulfanilamide and the preparation of the inclusion complexes of sulfanilamide with βCD and HP-βCD in order to improve the solubility and photostability of the drug. The structural characterization of inclusion complexes was performed by using suitable methods.
Experimental
Materials and methods
Reagents
Chlorsulfonic acid 99 %, acetanilide 97 %, HP-βCD 97 % (Sigma Aldrich, Taufkirchen, Germany), βCD 98 % (Merck, Darmstadt, Germany), the ammonia solution 25 %, hydrochloric acid (conc.) and sodium hydroxide (Alkaloid, Skopje, Macedonia). Other solvents and reagents used were of p.a. purity.
Procedure of sulfanilamide synthesis
Chlorosulfonic acid (18.5 cm3) was firstly added into a dried round flask of 100 cm3 and then acetanilide (7.5 g) was gradually added because of the releasing gas. The temperature of the reaction mixture was maintained at 20 °C. After the release of gas, the reaction mixture was heated for 1 h at 65 °C and then poured onto the pieces of ice (75 g). The separated crystals of 4-(acetylamino)benzenesulfonyl chloride were dissolved in 60 cm3 of water. Ammonia (15 cm3) was added and the mixture was left for 24 h. The filtered crystals in the round flask were treated with 2.75 mol/dm3 sodium hydroxide (50 cm3) and heated under reflux. The concentrated hydrochloric acid (1.5 cm3) was carefully added into the mixture, and then neutralized to pH 7. The native sulfanilamide was recrystallized from the water in order to obtain the pure substance. The determined melting point of 163 °C was in accordance with the literature data [24].
The preparation of inclusion complexes by the co-precipitation method
Sulfanilamide (172 mg) and βCD (1,135 mg) or HP-βCD (1,540 mg) were suspended in 150 cm3 of water. The solutions were mixed at room temperature for 24 h, evaporated in a vacuum evaporator at 60 °C to 20 cm3, and then dried in a desiccator above the concentrated sulfuric acid at 25 °C. After drying, the obtained complexes were further analyzed by using suitable methods.
The preparation of the physical mixture
Physical mixtures were prepared by simple mixing of sulfanilamide with βCD or HP-βCD in the molar ratio of 1:1.
Infrared Fourier transformation (FTIR)
FTIR spectra of the samples were recorded in the wavenumber range of 4,000–400 cm−1 on the FTIR spectrophotometer of Bomem Hartmann & Braun MB-series by preparing KBr tablets (1 mg of the sample, 150 mg of KBr).
X-ray crystallography
X-ray diffraction was performed on the Phillips PW1030 automatic powder diffractometer under the following conditions: the samples were exposed to monochrome CuKα radiation and analyzed under the angle 2θ between 5 and 55° with 0.05° increments and recording time τ = 5 s. The voltage and the strength of the electric current were 40 kV and 20 mA, respectively.
1H-NMR spectrometry
1H-NMR spectra of the samples were made on a Bruker AC 250 NMR spectrometer with operating frequencies of 250 MHz, in a glass cuvette (5 mm) at room temperature, by the pulse method with multiple pulse repetitions. D2O was used as the solvent for all analyzed samples.
Differential scanning calorimetry (DSC)
DSC curves of the samples were recorded using differential scanning calorimeter DSC Q10 from TA Instrument with the scanning rate of 10 °C/min and the temperature range of 30–230 °C. Thermal properties were studied by heating about 5 mg of the samples in closed aluminum containers in the nitrogen atmosphere. The samples (4–5 mg) were hermetically sealed in an aluminium pan and placed in a DSC cell together with empty aluminium pans as a reference and heated with continous nitrogen purge at the flow of 50 cm3/min.
Phase solubility
Phase-solubility studies were performed by using the method described by Higuchi and Connors [25]. An excess amount of sulfanilamide was added to 5 cm3 of distilled water containing increased CD concentrations. The concentration range was 0–10 mmol/dm3 for βCD and HP-βCD. The samples were mixed at room temperature for 24 h, and then filtered through the membrane filter with the pore size of 0.45 μm (Econofilters, Agilent Technologies, Germany). The amount of dissolved sulfanilamide was determined by using the UV–Vis method based on the constructed calibration curve. The absorbance of the samples was measured at Varian Cary-100 Conc. at room temperature in the quartz cells (1 × 1 × 4.5 cm). Distilled water was used as the blank sample. The presence of CDs did not interfere with the spectrophotometric assay of sulfanilamide. The apparent 1:1 stability constants (K 1:1) of the inclusion complexes were calculated from phase-solubility diagrams according to the following equation (Eq. 1):
where S 0 is the solubility of sulfanilamide at 25 °C in the absence of cyclodextrins and the slope means the corresponding slope of the phase solubility diagrams.
Photostability study
The solutions of the inclusion complexes and sulfanilamide (equivalent to 5 μg/cm3 sulfaniliamide) were prepared in distilled water. The samples were treated by UV irradiation in a period of 0–300 s in the cylindrical photochemical reactor “Rayonnet” with 8 symmetrically placed UVB lamps having an emission maximum at 300 nm. The quartz cells (1 × 1 × 4.5 cm) placed on a circular rotating holder were used. The total measured energy flux (hitting the samples) was about 12.0 W/m2 at a distance of 10 cm from the lamps. The samples were filtered through the cellulose membrane of 0.45 μm (Econofilters, Agilent Technologies, Germany) and analyzed by the UV–Vis method.
Results and discussion
The structural characterization of inclusion complexes (sulfanilamide:βCD and sulfanilamide:HP-βCD) was provided using the different methods: Fourier transform infrared spectroscopy (FTIR), the proton nuclear magnetic resonance spectroscopy (1H-NMR), X-ray diffraction analysis (XRD) and differential scanning calorimetry (DSC).
FTIR analysis of inclusion complexes
FTIR spectra of sulfanilamide, βCD, sulfanilamide:βCD complex and the adequate physical mixture of sulfanilamide and βCD are presented in Fig. 2. The characteristic bands of valence N–H vibrations of primary amino group should be expected in the wavenumber range of 3,500–3,200 cm−1. Three sharp bands of medium intensity at 3,478, 3,376 and 3,268 cm−1 in the spectrum are the results of valence asymmetric νas(NH2) (aniline) and asymmetric vibrations νas(NH2) (sulfonamide), as well as symmetric vibrations νs(NH2) (sulfonamide), respectively [26–28]. The basic band of NH deformation vibration at 1,629 cm−1 occured in the spectrum. The out-of-plane N–H deformation vibration γ(N–H) at primary amines is in the range of 900–650 cm−1. In this case the band at 695 cm−1 is broad and medium intensity, the position of which depends on the presence of hydrogen bonds. The C–N valence vibration ν(C–N) of aromatic amines gives the sharp band at higher wavenumbers, because the C–N bond is stronger due to delocalization of free electronic pair. This band occured at 1,294 cm−1, while the bands of valence vibrations of SO2 ν(SO2) were noticed to be very intesive at 1,314 and 1,148 cm−1. The expected spectral range for these vibrations is 1,230–1,120 cm−1. Also, there are different intensity bands of C=C bonds from benzene at 1,595 and 1,504 cm−1. Due to 1,4-disubstitution in the aromatic ring, the out-of-plane deformation vibration of C–H bond γ(C–H) occured as one band at 837 cm−1, which is in the expected range of 860–800 cm−1. Based on the comparison of the obtained IR spectra for sulfanilamide and different sulfanilamide polymorphic forms [29, 30] in the wavelength range of 4,000–3,000 and 700–400 cm−1, it can be concluded that the synthesized sulfanilamide corresponded to the β-polymorphic form.
In the FTIR spectrum of sulfanilamide:βCD inclusion complex, there is a broad band in the wavenumber range of 3,500–3,200 cm−1 with the pronounced maximum at 3,400 cm−1 that corresponds to the valence vibration of OH group from βCD. The bands of ν(N–H) vibration are not present in the spectrum of the inclusion complex, but are present in the spectrum of the physical mixture. In the spectrum of sulfanilamide:βCD complex, the bands of δ(NH2) at 1,629 cm−1, ν(SO2) at 1,148 cm−1, γ(C–H) at 837 cm−1 and γ(NH2) at 695 cm−1 are absent, while the bands intensity of ν(C=C) at 1,595 and 1,504 cm−1, ν(SO2) at 1,314 cm−1 is lower compared with the spectrum of sulfanilamide.
The FTIR spectra of sulfanilamide, HP-βCD, sulfanilamide:HP-βCD inclusion complex and the adequate physical mixture of sulfanilamide and HP-βCD are presented in Fig. 3. Unlike the FTIR spectrum of the physical mixture, significant changes in the spectrum of the inclusion complex were noticed by comparing with the spectra of pure substances. The broad band with the maximum at 3,395 cm−1 in the inclusion complex spectrum is the result of the valence vibration of OH groups from HP-βCD. The same band in the spectrum of HP-βCD occurred at 3,410 cm−1. The shift of this band for 15 units to lower values of the wavenumber indicates the involvement of this group in the formation of the hydrogen bond. The characteristic bands of valence and deformation vibrations of N–H bond from sulfanilamide are not present in the spectrum of the complex. The intensity of bands for the valence vibration ν(C=C) and ν(SO2) are significantly reduced in the spectrum of the inclusion complex compared with the spectra of sulfanilamide. The results of the FTIR analysis indicate the achieved inclusion of sulfanilamide in the cavity of HP-βCD.
1H-NMR analysis of inclusion complexes
Further investigations of inclusion complexes were performed by 1H-NMR analysis. The marked C-atoms in sulfanilamide and βCD, i.e. HP-βCD are given in Fig. 1. Chemical shifts and the changes of the chemical shifts of protons in sulfanilamide:βCD complex compared with the pure compounds of sulfanilamide and HP-βCD are given in Table 1.
The protons from sulfanilamide at C2 and C6, as well as C3 and C5, have the chemical shift changes (Δδ) of 0.0232 and 0.0049, respectively. The H-atoms at C2 and C6 were more involved in the interaction with βCD. The highest changes in the chemical shifts of βCD protons were noticed at C5, C3 and C6 atoms wich is in accordance with the involvement of these protons in the formation of hydrogen bonds. This analysis indicates the achievement of noncovalent interaction between sulfanilamide and βCD inside the complex.
Chemical shifts and the chemical shifts changes of protons in sulfanilamide:HP-βCD inclusion complex compared with sulfanilamide and HP-βCD are presented in Table 2.
The sulfanilamide protons in the spectrum of the complex at C2 and C6, C3 and C5 atoms have Δδ of 0.0036 and 0.0037, respectively. These shifts are almost the same so that all protons are involved equally in the interaction with HP-βCD. The highest proton shifts of HP-βCD in the complex have the protons at C3 atom, and slightly lower for the protons at C5 and C6 atoms. The methyl protons of HP-βCD (−0.0022) have the lowest shifts. Based on the obtained results of FTIR and NMR analysis, the part of sulfanilamide (phenyl with NH2 group) was probably included in the cavity of the host.
XRD analysis of inclusion complexes
The diffractograms of sulfanilamide, βCD, sulfanilamide:βCD inclusion complex and the adequate physical mixture of sulfanilamide and βCD are presented in Fig. 4. The diffractogram of the inclusion complex differs from the sulfanilamide diffractogram. The appearance of new peaks (1c–3c), as well as the shifts of some peaks derived from βCD (1b,4b,8b) were noticed in the diffractogram of the inclusion complex. Also, the peaks of sulfanilamide (1s–10s) were not noticed in this diffractogram. On the other hand, the high crystallinity of the synthesized sulfanilamide was confirmed based on very significant peaks in the diffractogram. XRD analysis also indicates the β-polymorphic form of sulfanilamide [31]. Unlike the diffractogram of the inclusion complex, the peaks of sulfanilamide and β-cyclodextrin are present in the diffractogram of the physical mixture.
The diffractograms of sulfanilamide, HP-βCD, sulfanilamide:HP-βCD inclusion complex and the adequate physical mixture of sulfanilamide and HP-βCD are given in Fig. 5.
The diffractogram of the complex is almost identical with the diffractogram of HP-βCD, which is the confirmation of the sulfanilamide incorporation in the cyclodextrin cavity. The diffractogram of the complex does not have the characteristic peaks of sulfanilamide. The broad and not structured peaks in the diffractogram of HP-βCD, which appeared in the 2θ range of 11.516–18.882°, indicate a low the crystal structure. The diffractogram of the physical mixture has the peaks of both compounds that are not noticed in the diffractogram of the complex. Thus, this diffractogram indicates the simple mixture of sulfanilamide and HP-βCD.
DSC analysis of inclusion complexes
DSC curves of both complexes have thermal changes that differ from the host and guest molecules (Fig. 6). Sulfanilamide has a significant endothermic peak with a maximum at 128.70 °C and a smaller intensity peak at about 163 °C. βCD in DSC thermogram has a sharp endothermic peak at 147.05 °C, while HP-βCD has a broad endothermic peak at 132.65 °C. The peaks of thermal changes that are characteristic for sulfanilamide are not present in DSC thermograms of both inclusion complexes. The peaks at 113.17 °C for the sulfanilamide:βCD complex and at 97.29 °C for the sulfanilamide:HP-βCD complex do not correspond either to thermal changes in the guest molecule or to thermal changes in the host molecule. These results indicate the formation of new structures with the altered temperature changes. In the diffractogram, it can be noticed that the enthalpy change of melting sulfanilamide is reduced after the inclusion in the cyclodextrin cavity. Namely, the enthalpy of sulfanilamide is 391.0 J/g, while after the inclusion this value is 186.9 J/g. The enthalpy of sulfanilamide:HP-βCD complex is 249.3 J/g. These changes also indicate the formation of supramolecular structures in which the properties of the included sulfanilamide changed.
The values of melting enthalpy changes, ΔH m, at the investigated samples were used for the determination of the crystallinity degree of sulfanilamide (χ m,SULF) using the Eq. 2:
where ΔH m is the value of the melting enthalpy of sulfanilamide in the inclusion complexes with sulfanilamide:βCD or HP-βCD, while \(\varDelta H_{\text{m}}^{^\circ }\) is the value of the uncomplexed sulfanilamide, which is in a completely crystalline state.
The percentage of sulfanilamide in the complex with βCD and HP-βCD is 47.80 and 63.76 %, respectively. The differences in the crystallinity degree of the samples are in accordance with their appearance (Fig. 7). Otherwise, the sulfanilamide:βCD complex is a white powder with a low visible crystals (Fig. 7b), while the sulfanilamide:βCD complex has flake and transparent crystals (Fig. 7c).
Figure 8 illustrates the most likely way of the sulfanilamide inclusion in the cyclodextrin cavities.
Phase-solubility studies of sulfanilamide and inclusion complexes
The constructed calibration curve of sulfanilamide A258 = 0.11901C + 0.09964 (r = 0.9996) was linear in the concentration range of 1–10 μg/cm3. During the investigations of the phase-solubility and photostability, the concentration of sulfanilamide in the samples was determined based on the calibration curve. Phase-solubility analyses were performed in order to get the insight into the host–guest interactions in the water solution and determine and compare the solubilizing and the complexing power of the different examined CDs towards sulfanilamide. The obtained parameters for the investigation of phase-solubility are given in Table 3. The phase-solubility diagrams belong to “AL” type (Fig. 9). It indicates that the molar ratio between the host and guest molecules in the inclusion complexes is 1:1 [25].
For both CDs, sulfanilamide solubility was linearly increased with the increase of the CD concentration. Based on the values of the determined stability constants for both prepared complexes, it can be concluded that sulfanilamide:HP-βCD complex is more stable compared with sulfanilamide: βCD complex. The complexing ability of HP-βCD towards sulfanilamide is also better than of βCD.
Due to the increased solubility of sulfanilamide, supramolecular structures of sulfanilamide with cyclodextrins can be used for the preparation of new oral formulations with the modified release of the active substance.
Photostability studies of sulfanilamide and inclusion complexes
Photosensitivity of sulfanilamide and inclusion complexes was investigated by UVB irradiation in a photochemical reactor. The photodegradation of sulfanilamide alone, and in inclusion complexes, was monitored by the decrease in absorbance at 258 nm in a function of time. The absorption spectra of the samples after various irradiation times are presented in Fig. 10.
Based on the decrease in absorbance at 258 nm, it can be noticed that sulfanilamide alone, and in the complexes is a photosensitive substance. The appearance of new absorption maximums in UV–Vis spectra was noticed in the wavelength ranges of 300–350 and 550–600 nm. The intensity of these maximums is increased with increasing the irradiation time and indicate the formation of degradation products. In accordance with literature data, the potential photodegradation products of sulfanilamide are azo and nitro derivatives (Fig. 11) in water after the effect of UVB irradiation [23].
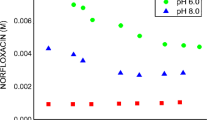
The change of the sulfanilamide concentration in the function of the irradiation time for both inclusion complexes is presented in Fig. 12. After 300 s of irradiation, the remaining content of sulfanilamide was 41.88 %, while in the case of the inclusion complexes with βCD and HP-βCD it was 46.44 and 57.67 %, respectively. The obtained results indicate that the sulfanilamide:HP-βCD complex is the least sensitive to the effect of UVB irradiation.
Conclusion
In this paper, sulfanilamide was successfully synthesized from acetanilide by the standard procedure. In order to improve the solubility of synthesized sulfanilamide, the inclusion complexes with βCD and HP-βCD were prepared by the co-precipitation method. The obtained complexes and initial precursors were structurally characterized by using FTIR, 1H-NMR and XRD methods. Thermal effects that originate from the physical transformation of sulfanilamide in pure and complexed states were analyzed by the DSC method. The interactions of the guest with host molecules were confirmed based on the changes in enthalpy and crystallinity of sulfanilamide. By using phase-solubility techniques, the obtained stability constants for the inclusion complexes at 25 °C indicate that the solubility of sulfanilamide is highest in the presence of HP-βCD. Based on the photostability studies, it can be concluded that sulfanilamide is most protected from the effect of UVB irradiation in the inclusion complex of sulfanilamide:HP-βCD.
References
Perlovich, G.L., Strakhova, N.N., Kazachenko, V.P., Volkova, T.V., Tkachev, V.V., Schaper, K.J., Raevsky, O.A.: Sulfonamides as a subject to study molecular interactions in crystals and solutions: sublimation, solubility, solvation, distribution and crystal structure. Int. J. Pharm. 349, 300–313 (2008)
Garnero, C., Aiassa, V., Longhi, M.: Sulfamethoxazole:hydroxypropyl-β-cyclodextrin complex: preparation and characterization. J. Pharm. Biomed. 63, 74–79 (2012)
Remko, M., von der Lieth, C.W.: Theoretical study of gas-phase acidity, pK a, lipophilicity, and solubility of some biologically active sulfonamides. Bioorg. Med. Chem. 12, 5395–5403 (2004)
Varagić, V.M., Milošević, M.P.: Farmakologija. Elit Medica, Belgrade (2009)
Nikolic, V.D., Savic, I.M., Nikolic, L.B., Savic, I.M.: Biosynthesis of paclitaxel and its pharmaceutical application for cancer treatment. In: Morales, D. (ed.) Paclitaxel: Biosynthesis, Mechanisms of Action and Clinical Uses, pp. 101–122. Nova Science Publishers, New York (2013)
Szejtli, J.: Introduction and general overview of cyclodextrin chemistry. Chem. Rev. 98, 1743–1754 (1998)
Vyas, A., Saraf, S., Saraf, S.: Cyclodextrin based novel drug delivery systems. J. Incl. Phenom. Macrocycl. Chem. 62, 23–42 (2008)
Mihajlović, L.: Ciklodekstrini—struktura, prirodni izvori, primena. Hemijski pregled 47, 82–86 (2006)
Li, N., Zhang, Y.H., Wu, Y.N., Xiong, X.L., Zhang, Y.H.: Inclusion complex of trimethoprim with β-cyclodextrin. J. Pharm. Biomed. 39, 824–829 (2005)
Ristić, I.S., Nikolić, L., Nikolić, V., Ilić, D., Budmski-Simendić, J.: Synthesis of polyrotaxanes from acetyl-β-cyclodextrin. Russ. J. Phys. Chem. A 85, 20–25 (2011)
Nikolić, V., Nikolić, L., Stanković, M., Kapor, A., Popsavin, M., Cvetković, D.: A molecular inclusion complex of atenolol with 2-hydroxypropyl-β-cyclodextrin; the production and characterization thereof. J. Serb. Chem. Soc. 72, 737–746 (2007)
Nikolić, V., Ilić, D., Nikolić, L., Stanković, M., Cakić, M., Stanojević, L., Kapor, A., Popsavin, M.: The protection of Nifedipin from photodegradation due to complex formation with β-cyclodextrin. Cent. Eur. J. Chem. 8, 744 (2010)
Kapor, A., Nikolić, V., Nikolić, L., Stanković, M., Cakić, M., Stanojević, L., Ilić, D.: Inclusion complexes of amlodipine besylate and cyclodextrins. Cent. Eur. J. Chem. 8, 834–841 (2010)
Nikolić, V., Stanković, M., Kapor, A., Nikolić, L., Cvetković, D., Stamenković, J.: Allylthiosulfinate: β-cyclodextrin inclusion complex: preparation, characterization and microbiological activity. Pharmazie 59, 845–848 (2004)
Kapor, A.J., Nikolić, L.B., Nikolić, V.D., Stanković, M.Z., Cakić, M.D., Ilić, D.P., Mladenović-Ranisavljević, I.I., Ristić, I.S.: The synthesis and characterization of iron (II) fumarate and its inclusion complexes with cyclodextrins. Adv. Technol. 1, 7–15 (2012)
Nikolić, V., Stanković, M., Nikolić, L., Nikolić, G., Ilić-Stojanović, S., Popsavin, M., Zlatković, S., Kundaković, T.: Inclusion complexes with cyclodextrin and usnic acid. J. Incl. Phenom. Macrocycl. Chem. 76, 1–10 (2012)
Dawoud, A.A., Al-Rawashdeh, N.: Spectrofluorometric, thermal, and molecular mechanics studies of the inclusion complexation of selected imidazoline-derived drugs with β-cyclodextrin in aqueous media. J. Incl. Phenom. Macrocycl. Chem. 60, 293–301 (2008)
Al-Rawashdeh, N.A.: Interactions of nabumetone with γ-cyclodextrin studied by fluorescence measurements. J. Incl. Phenom. Macrocycl. Chem. 51, 27–32 (2005)
Nikolić, V., Ilić-Stojanović, S., Nikolić, L., Cakić, M., Zdravković, A., Kapor, A., Popsavin, M.: Photostability of piroxicam in the inclusion complex with 2-hydroxypropyl-β-cyclodextrin. Chem. Ind. (2014). doi:10.2298/hemind130306034n
Nikolić, V., Stanković, M., Nikolić, L., Nikolić, G., Ilić-Stojanović, S., Popsavin, M., Zlatković, S., Kundaković, T.: Inclusion complexes with cyclodextrin and usnic acid. J. Incl. Phenom. Macrocycl. Chem. 76, 173–182 (2013)
Bani-Yaseen, A.D., Al-Rawashdeh, N.F., Al-Momani, I.: Influence of inclusion complexation with β-cyclodextrin on the photostability of selected imidazoline-derived drugs. J. Incl. Phenom. Macrocycl. Chem. 63, 109–115 (2009)
Al-Rawashdeh, N.A., Al-Sadeh, K.S., Al-Bitar, M.B.: Physicochemical study on microencapsulation of hydroxypropyl-β-cyclodextrin in dermal preparations. Drug Dev. Ind. Pharm. 36, 688–697 (2010)
Albini, A., Fasani, E.: Rationalizing the photochemistry of drugs. In: Tønnesen, H.H. (ed.) Photostability of Drugs and Drug Formulations, Chapter 4. CRC Press, Boca Raton (2004)
Sukul, P., Spiteller, M.: Sulfonamides in the environment as veterinary drugs. Rev. Environ. Contam. Toxicol. 187, 67–101 (2006)
Higuchi, T., Connors, K.: Phase solubility techniques. Adv. Anal. Chem. Instrum. 7, 117–212 (1965)
Popova, A.D., Georgieva, M., Petrov, O., Petrova, K., Velcheva, E.: IR spectral and structural studies of 4-aminobenzenesulfonamide (sulfanilamide)-d0, -d4, and -15N, as well as their azanions: combined DFT B3LYP/experimental approach. Int. J. Quantum Chem. 107, 1752–1764 (2007)
Topacli, C., Topacli, A.: Ab initio calculations and vibrational structure of sulfanilamide. J. Mol. Struct. 644, 145–150 (2003)
Varghese, H.T., Panicker, C.Y., Philip, D.: Vibrational spectroscopic studies and ab initio calculations of sulfanilamide. Spectrochim. Acta A 65, 155–158 (2006)
Lin, H.O., Guillory, J.K.: Polymorphism in sulfanilamide-d4. J. Pharm. Sci. 59, 972–975 (1970)
Borba, A., Gómez-Zavaglia, A., Fausto, R.: Conformational landscape, photochemistry, and infrared spectra of sulfanilamide. J. Phys. Chem. A 117, 704–717 (2013)
Toscani, S., Thorén, S., Agafonov, V., Céolin, R., Dugué, J.: Thermodynamic study of sulfanilamide polymorphism: (I) monotropy of the α-variety. Pharm. Res. 12, 1453–1456 (1995)
Acknowledgments
This work is a part of the research done within the project TR-34012. The authors would like to thank the Ministry of Education, Science and Technological Development of the Republic of Serbia.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Tačić, A., Savić, I., Nikolić, V. et al. Inclusion complexes of sulfanilamide with β-cyclodextrin and 2-hydroxypropyl-β-cyclodextrin. J Incl Phenom Macrocycl Chem 80, 113–124 (2014). https://doi.org/10.1007/s10847-014-0410-x
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10847-014-0410-x