Abstract
The present study is focused on the characterization of the interaction between (−)-epigallocatechingallate (EGCG) and cyclodextrins like β-cyclodextrin (βCD), heptakis(2,6 di-O-methyl)-β-cyclodextrin (DMβCD), and hydroxypropyl-β-cyclodextrin (HPβCD) in aqueous solution. These inclusion complexes previously demonstrated improvements in the antioxidant activity respect to free EGCG. The structural evidence obtained by 2D-ROESY and selective 1D-ROESY experiments was rationalized by autodock studies and indicates that all the complexes have similar inclusion geometries, but the difference resides on the exposition degree of the antioxidant rings of EGCG, such as pyrogallol and galloyl groups. The thermodynamic study allowed estimating that the inclusion process is entalpically driven for the derivatized cyclodextrins complexes and entropically driven for βCD complexes due to the predominance of hydrophobic interactions with EGCG.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
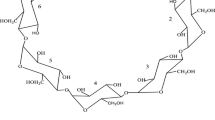
Cyclodextrins (CDs) are cyclic oligosaccharides composed of glucose units connected by α-1,4-glycosidic linkages. With a shape like a truncated cone, they form a hydrophobic cavity, capable of creating inclusion complexes with various molecules by incorporating them into the cavity [1, 2]. These inclusion complexes can lead to alterations of physical, chemical and biological properties of the guest molecule and may eventually have considerable medicinal potential. The ability of a CD to form an inclusion complex with a guest molecule is a function of steric as well as thermodynamic factors. The driving forces for complex formation are attributed to the removal of water molecules from the hydrophobic cavity and the formation of van der Waals, hydrophobic, and hydrogen bond interactions [3]. Molecular encapsulation of drugs and food ingredients with CDs improves the stability of flavours, vitamins, colourants, unsaturated fats, etc., in both a physical and chemical sense, leading to extended product shelf-life [4–6]. HPβCD has been shown to be well tolerated by humans, with diarrhea as the main adverse event, and no adverse effects there have been reported on kidney function. In fact, it is an alternative to βCD, with improved water solubility and it may be more toxicologically benign [7].
Tea is a beverage made from the leaves of Camellia sinensis species of the Theaceae family. This beverage is one of the most ancient and, next to water, the most widely consumed liquid in the world. Green tea is the non-oxidized/non-fermented product and it has been suggested to possess broad physiological activities such as antioxidant, antiallergic, anticarcinogenic, antibacterial, and antiviral effects [8]. Green tea contains several polyphenolic components such as catechin (CA), epicatechin (EC), epicatechin gallate (ECG), epigallocatechin (EGC), epigallocatechin gallate (EGCG) and gallocatechin gallate (GCG). Catechin abundance in tea leaves is EGCG > EGC > ECG > EC, with EGCG accounting for about 60 % of the catechin content [9]. This flavonoid is the most biologically active tea catechin and it has been reported as a preventive agent of prostatic and esophageal cancer, has sown intestinal protective effects, and has been proposed as a mediator of beneficial cardiovascular actions [10–15]. All these properties are related to the ability to preserve the redox balance due to the pro- and antioxidant activities of EGCG.
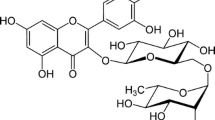
EGCG (Fig. 1) is subject to oxidation, with the formation of reactive oxygen species and dimers. Several factors, including pH, temperature, oxygen level, antioxidant level, metal ions, concentration, and other ingredients in tea, may affect the stability of EGCG [16–19].
Moreover, tea catechin powders are bitter, brown, and easily oxidized, and hence difficult to use as a medicine or as a natural food additive. In fact, when incubated in various solutions and soft drinks, these catechins showed poor stability and decayed by at least 50 % during the first month of storage at room temperature [20]. For these reasons, we have proposed the encapsulation of EGCG, the major bioactive constituent of green tea catechins, in different cyclodextrins to investigate the fundamental properties of the inclusion complexes.
Previously we reported the antioxidant activity of EGCG, CA and GCG forming complexes with βCD, DMβCD and HPβCD studied by ORAC-FL and ORAC-PGR assays [21]. We found increased or maintained antioxidant activity with respect to their respective free flavonoids, and conclude that this antioxidant activity depends mainly on the inclusion modes of tea catechin in the cyclodextrin cavity.
Previous work has described the formation of EGCG and native βCD in which the aromatic A ring and a part of the heterocyclic ring were included from the wide secondary hydroxyl group side of the βCD cavity in aqueous solution, while the trihydroxyphenyl and 3-O-gallate groups were left outside the cavity [22, 23].
In the present study the structure in solution of the inclusion complexes of EGCG with different CDs was investigated. Association constants, estimated from fluorescence studies at different temperatures, were analyzed in order to get information on the thermodynamic mechanisms involved in the association processes. Nuclear magnetic resonance (NMR) spectroscopy was used to obtain detailed information about the structure of the inclusion complexes in aqueous solution, and it was rationalized with docking studies.
Experimental
Materials
(−)-Epigallocatechin gallate, (EGCG), β-cyclodextrin, (βCD), heptakis (2,6-di-O-methyl)-β-cyclodextrin, (DMβCD), (2-hydroxypropyl)-β-cyclodextrin [D.S. = 7] (HPβCD), and deuterated water (D2O) were purchased from Sigma-Aldrich, Inc., St. Louis, MO. All other reagents were analytical grade and double distilled water was used throughout.
Method
Fluorescence spectroscopy
Fluorescence spectra were recorded on a Perkin-Elmer LS 55 spectrofluorometer equipped with a xenon lamp source and thermostated bath. Fluorescence measurements were made with excitation and emission slits of 10 and 10 nm, respectively.
In the In the case of a 1:1 complex, the following equation is applicable for the Benesi-Hildebrand method [24]:
where F ∞ is the fluorescence intensity when total EGCG has been complexed in CDs, F o is the fluorescence of EGCG in the absence of CDs, and F is the observed fluorescence at each CD concentration tested. If the stoichiometry is 1:1, the representation of 1/(F − F o) versus 1/CD should give a linear plot. The K a values were obtained by simply dividing the intercepts by the slopes.
For the determination of association constants (K a), suitable aliquots of the CD solutions (10 mM) were added to 50 μM of EGCG, keeping the final volume of the system constant at 3 ml. The resulting mixture was equilibrated in a Julabo thermostatic shaking water bath for 24 h at variable temperature (298–313 K) after which equilibrium was reached and the emission fluorescence spectra were recorded in the 320–480 nm range (1 nm step), at a fixed excitation wavelength of 276 nm.
The fluorescence intensity at any wavelength (F) can be related to the CD concentration by a non-linear regression described by the following equation [25]:
Experimental data of F as a function of [CD] can be fitted to Eq. 2, using as initial K a and F ∞ parameters those obtained from the analysis of the experimental data using the Benesi-Hildebrand equation for 1:1 complexes.
NMR spectroscopy
2D Rotating-frame Overhauser Effect SpectroscopY (2D-ROESY) spectra were acquired at 30 °C in the phase-sensitive mode using a Bruker Avance DRX instrument operating at a proton NMR frequency of 300.13 MHz and Bruker standard parameters (pulse program roesygpph19). Each spectrum consisted of a matrix of 16 K (F2) by 8 K (F1) points covering a spectral width of 3,000 Hz using a spin-lock mixing time of 400 ms and relaxation delay of 2 s, recording 32 scans. One-dimensional 1H NMR spectra were recorded at 300 K in unbuffered D2O solutions. The resonance of residual solvent (HDO) at 4.7 ppm was used as internal reference and the proton chemical shifts were referenced to TMS.
Selective 1D-ROESY
Experiments were performed at 30 °C in D2O on a Bruker AVANCE 400 NMR spectrometer (400.133 MHz for 1H) and were obtained by selective 180° pulse excitation and selective refocusing with shaped pulses using the gradient selroegp pulse sequence, with a mixing time of 400 ms for ROESY spin-lock, recycle delay of 1.0 s, and 512 transients. In each case, the shaped pulses (Gaussian) were chosen according to the desired selectivity (20, 40 or 60 ms, depending on more or less isolated spin systems). The spectra were acquired under the same conditions as 1H NMR. The 1H and 1D ROESY NMR spectra were processed by applying a Fourier transform with zero-filling to 64 K data points.
Molecular modeling
In silico build-up of the βCD and its derivative forms were carried out using the Builder module of the Insight II program by adding fourteen methyl groups to βCD in positions 2 and 6 (DMβCD). The models were subjected to optimization using a protocol of 300 steps of conjugate gradients to avoid steric hindrance and clashes that can appear in the building process. The EGCG was built using Gaussview and it was then optimized using PM3 as implemented in the Gaussian 98 package of programs [26]. HPβCD was not carried out because a precise structural description of this CD is very difficult, due to the wide range of substitution patterns at the O-2 and O-6 positions that always occur randomly and are distributed among the different glucose units. The nature of the substitution pattern can directly affect the affinity and selectivity of molecular complexation behaviour of HPβCD [27] and the possibility of getting wrong representations of the complexes by Autodock are higher than for the other CDs.
Autodock 3.0.5 [28] with Lamarckian Genetic Algorithm (LGA) was used to generate the starting complexes. The parameters used for the global search were an initial population of 50 individuals, with a maximum number of energy evaluations of 1.5 × 106 and a maximum number of generations of 5.0 × 104 as an end criterion. An elitism value of one was used, and mutation and crossing-over probabilities of 0.02 and 0.08, respectively, were used. From the best solutions obtained according to these parameters, some of them defined by the user as the best probabilities, in our case 0.06, were further refined by the pseudo-Solis and Wets (pSW) local search method. Autodock defines the conformational space implementing grids all over the space of the possible solutions. With the aim of testing the ability of Autodock to converge into solutions that are inside the βCD, an 80 by 80 Å grid with 0.3 Å spacing between each point was setup in such a way that it covered both the external surface and the internal cavity of the βCD. The following procedure was used in the βCD docking simulations: 250 runs were made for each βCD. At the end of each run the solutions were separated into clusters according to their lowest RMSD and the best score value based on a free empiric energy function. Cluster solutions whose average score was not over 1 kcal mol−1 with respect to the best energy obtained in the corresponding run were selected. Then, the solution that represents most of the complexes obtained in the run was compared with the NMR experimental data, ensuring that this solution is able to represent them accurately. The selected final complexes were optimized using the semiempirical PM3 method as a refining procedure with Gaussian 98.
Results and discussion
Based on our previous work [21] we continue with the study of EGCG and cyclodextrins. Taking advantage of the fact that the inclusion is 1:1, we decided to get information on the association mechanism determining the thermodynamic parameters, and determining the inclusion geometry to understand why EGCG enhances its antioxidant activity once it forms the inclusion complex.
Addition of various amounts of CDs in aqueous solution of EGCG leads to a weak modification of the absorption which confirms the formation of inclusion complexes between EGCG and the different cyclodextrins. The observed changes in the absorption spectra are too small to allow for the determination of the binding constant. However, the effect of CDs on the fluorescence spectra of EGCG is more pronounced than the corresponding effect on the absorption spectra.
To get the association constant K a we used non-linear least-squares regression analysis [12], an alternative and more appropriate approach than the graphical methods. The initial parameter estimates needed for this method were obtained from the linear plots.
The association constants, K a, of the three complexes at different temperatures (298, 303, 308 and 313 K) were determined and the results are summarized in Table 1, which shows that the association constant for the EGCG–HPβCD and EGCG–DMβCD complexes decreases with increasing temperature, as expected for an exothermic process, which might be interpreted as a lower degree of interaction at higher temperatures, possibly due to the fact that hydrogen bonds are usually weakened by heating. However, for EGCG–βCD, the association constant increases as temperature rises, as expected for an endothermic process. This results disagrees with that obtained by Ishizu et al. [29], and the difference may be due to the temperature at which they work. Previous work showed the oxidation of EGCG in aqueous solution at high temperatures by HPLC methodologies [30], and we observed the decrease of the fluorescence band of EGCG at temperatures over 40 °C. For this reason we consider that this temperature must be the highest experimental value to use for these tests. This evidence can explain the decrease of the association constant at high temperatures, interpreted as an exothermic process, because this effect may not have been observed by NMR measurements. The problem is not the decrease of the interactions, but it is rather due to the previous oxidation of the flavonoid before the formation of the inclusion complex.
The thermodynamic parameters (∆G, ∆H and ∆S) for the formation of the inclusion complex were determined from the temperature-dependence of the association constants, using the classical van’t Hoff equation and plotting ln K a versus 1/T. The thermodynamic parameters enthalpy changes (∆H) and entropy changes (∆S) of the binding reaction are important to confirm the driving force for the interactions of EGCG with cyclodextrins. It has been generally accepted that the main driving forces for complex formation are hydrogen binding between the hydroxyl groups of the CD and the guest, van der Waals force interactions between host and guest molecules, hydrophobic interactions, and the release of “high energy water” molecules from the cavities of CD to bulk water. Hydrophobic interaction essentially involves favorable positive entropy together with a slightly positive enthalpy change. Generally, solute inclusion in the cyclodextrin cavity is associated with large negative values of ∆H, and ∆S values are either negative or slightly positive, indicating inclusion complexation of the guest without extensive desolvation, because the inclusion reactions are primarily enthalpy-driven processes [3]. As shown in Fig. 2, the EGCG–DMβCD and EGCG–HPβCD complexes show this behavior. By inspection of Table 1, ∆H and ∆S for EGCG–HPβCD are negative in the experimental temperature range, and for EGCG–DMβCD ∆H is negative and ∆S is positive, which indicates that for both complexes the inclusion process is an exothermic and enthalpically controlled process. A different behavior is shown by the EGCG–βCD complex, where complexation gave positive enthalpy changes and a positive entropy term (Fig. 2), indicating that this inclusion is mainly entropically driven. βCD has the lowest aqueous solubility of all the cyclodextrins due to the formation of hydrogen bonds between the C2–OH and C3–OH groups of the adjacent glucopyranose units, forming a complete secondary belt, turning it into a rigid structure [31]. This fact may explain that the process is governed mainly by hydrophobic interactions by removing the polar hydration shell of EGCG and restoring the structure of water around the exposed part of the guest. In fact, this exposed part (B and B′ rings) is located near the hydrophobic secondary rim as discussed below.
This same effect was observed by us in the complexation of morin [32], galangin [33], kaempferol [34] and luteolin [35] with βCD. Although the behavior of these flavonoids complexed with DMβCD is enthalpically favored, regardless of the positive or negative entropy. However, for these same flavonoids with HPβCD the behavior is not similar, and in some cases complex formation is enthalpy driven (luteolin, kaempferol) and in others it is entropy driven (galangin, morin). No reason for these differences was found.
NMR studies allowed us to distinguish between inclusion and other possible external interaction processes. In fact, NMR is the most powerful tool used to determine the inclusion of a guest molecule into the hydrophobic CD cavity in solution. It is well known that the chemical shifts of the hydrogen atoms located inside the CD cavity (H-3 and H-5) become shielded and usually show significant upfield shifts in the presence of a guest molecule, whereas the hydrogen atoms on the outer surface (H-1, H-2 and H-4) are not affected or experience only a marginal shift upon complexation.
1H NMR spectra of EGGC dissolved in deuterated water, Fig. 3, show well resolved signals in two distinct spectral regions: two double-doublets corresponding to methylene H-4α and H-4β at 3.02 and 2.88 ppm, respectively, a singlet-like at 5.07 ppm which corresponds to methine proton H-2, and a broad signal which corresponds to methylene H-3 at 5.55 ppm. In the high frequency region, the two spin systems corresponding to H-6 and H-8 protons of the A-ring at 6.12 and 6.09 ppm, respectively, and the two singlets corresponding to the aromatic proton of the B and B′ rings, respectively. This assignment is in agreement with that of previous work [29].
The formation of the inclusion complexes can be shown from the chemical shift changes of EGCG or CDs in 1H-NMR spectra. Figure 3 illustrates that most of the aromatic protons of EGCG are influenced due to the presence of CDs. Table 2 lists the detailed changes of the aromatic chemical shifts of EGCG before and after forming inclusion complexes with CDs. Downfield shifts are observed for all aromatic protons of the B and B′ rings with the three CDs. However, upfield shifts are observed for almost all the A-ring protons once the complex is formed. The broadening of all the proton signals of EGCG in the HPβCD–EGCG complex is indicative of slow molecular motion relative to the NMR timescale, suggesting that this molecule fits tightly in the cyclodextrin cavity.
Additional information about the inclusion mode of EGCG in the cyclodextrin cavity can be derived from the evidence of spatial closeness between CD and EGCG protons. This evidence can be obtained from the presence of the NOE effect between protons from two species, which indicate intermolecular contacts within 5 Å. To gain more conformational information about the inclusion complexes, we used 2D-rotating-frame Overhausser effect spectroscopy (2D-ROESY), and selective ROESY techniques. Figure 4 shows a partial contour plot of the 2D-ROESY spectra of the EGCG–βCD inclusion complex. The spectrum shows intermolecular cross-peaks between H-3 βCD protons and B- and B′-ring protons from EGCG. We can also see intramolecular cross-peaks between H-2 and H-3 of EGCG with the B-ring protons. Due to the rapid dynamics of the complexation process, the ROE effects were used only qualitatively and no conclusions on intermolecular distances were extracted. However this information is not conclusive because we did not see the A- and C-ring signals in this spectrum.
For this reason we decided to use selective 1D ROESY NMR experiments. As seen in Fig. 5b, upon irradiation of the H-2′′/H-6′′ protons of EGCG–βCD, a ROE response was observed on the B-ring protons and on the H-3 protons of the β-cyclodextrin located inside the cavity. Upon selective excitation of the H-2 methyne proton of EGCG (Fig. 5c), a ROE response was observed on the H-3 and H-5 protons of βCD. Finally, Fig. 5d shows the ROE response seen on the H-5 and H-6 protons of βCD by selective irradiation of the H-6 and H-8 protons of EGCG. These results indicate the occurrence of intermolecular interactions between the CD and EGCG, with the latter oriented protruding from the B- and B′-rings through the secondary face of the cyclodextrin, and the A and C-rings are inside the cavity. This geometry agrees with that obtained by Ishizu et al. [29].
Figure 6 depicts the 2D ROESY spectrum of the EGCG–DMβCD complex. This complex shows correlations of H-2′′/H-6′′and H-2′/H-6′ of EGCG with H-3 of DMβCD, and H-6/H-8 of EGCG with H-5 of DMβCD, indicating that the pyrogallol and galloyl groups are on the secondary face of cyclodextrin and the A-ring is inserted in the cavity. This geometry was supported by the selective ROESY experiments (data not shown).
For the unambiguous assignment of the H-3, H-5, and H-6 protons of the 2-hydroxypropyl-β-cyclodextrin region, an HSQC spectrum of the EGCG–HPβCD system was ran under the same conditions as those used for the ROESY spectrum (data not shown). The ROESY spectrum of the EGCG–HPβCD complex (Fig. 7) shows interactions of the H-2″/H-6″ protons of EGCG with the H-3, H-5 protons of HPβCD, and interactions between H-2′/H-6′ of EGCG with the H-3, H-5 and apparently H-6 protons of HPβCD. These results are difficult to analyse due to the broadening and decrease in intensity of the proton signals from EGCG, which can be confused with artifacts or can replace other signals. Moreover, the H-3, H-5 and H-6 HPβCD protons appear in the same regions. We observed the same effect with the H-6 proton of EGCG.
For these reasons we have to be carefully in interpreting these results. Primarily we have one broad proton signal which corresponds to the B ring protons, and a narrower signal from the B′ ring protons, both oriented to the same region of the molecule. It is very unlikely that the H-2′ and H-6′ protons of the B ring have interactions with the opposite H-3 and H-6 HPβCD protons, but the B′ ring shows dipolar interaction only with H-3 and H-5 HPβCD protons and a very weak signal at 3.82 ppm that can be confused with an interaction with the H-6 HPβCD protons. This weak interaction seen for the B′ ring can be considered an artifact and will be disregarded by us. For this reason we estimate that the B and B′ rings are oriented to the secondary rim of the cyclodextrin.
On the other hand, we see interaction of the H-6 and H-8 protons of the A-ring with the H-3, H-5 and H6 protons of HPβCD. This probably means that only one geometric orientation cannot explain the resulting cross peak.
Selective ROESY experiments were performed to obtain the geometric orientation, but the broadening of the EGCG proton signals decreases the response in the spectrum. For this reason we decided to irradiate the HPβCD proton regions. In this way we can estimate the closeness of the HPβCD and EGCG protons. As can be seen from Fig. 8b, upon selective excitation of the H-3/H-6 HPβCD proton region, a ROE response was observed on the H-2″/H-6″, H-2′/H-6′ and H-6 EGCG protons. When we selectively irradiated the H-3/H-6/H-5 HPβCD proton region (Fig. 8c), a ROE response was observed on all the EGCG protons, but a high intensity of the response was obtained for H6 and H8 EGCG protons. The difference between these spectra (Fig. 8b, c) shows the closeness of the H-6 and H-8 protons of EGCG with the H-5 HPβCD protons. Finally, Fig. 8d shows the spectrum obtained by selective excitation of the H-6/H-5 HPβCD proton region, with a weak response except for the H-2 EGCG protons that indicates the proximity of the C ring with the H-5 proton of the cyclodextrin, because when we excited the H-3/H-6 region we saw a very poor response from the H-2 EGCG protons. For these reasons we can estimate that the possible geometric orientation is with the B and B′ rings exposed to the secondary rim of HPβCD, with the A and C rings inside the cavity because of their closeness to the H-5 HPβCD protons.
We can conclude that the inclusion modes of EGCG in βCD, DMβCD and HPβCD are very similar with each other and that the difference resides in the degree of exposure of the B and B′ groups, were the EGCG–HPβCD complex presents the lowest degree of exposure and suggest that the motion of EGCG is restricted, indicating that the molecule fits tightly in the cyclodextrin cavity.
In order to rationalize these experimental results, we carried out molecular modeling of the complexes. Autodock 3.05 results revealed a preferred final orientation for βCD and DMβCD complexes, in spite of their different arbitrarily imposed initial configurations. The minimum energy complexes obtained for βCD and DMβCD are shown in Fig. 9a, b. The complexes were further refined using a semiempirical method such as PM3. In the case of EGCG–βCD, Fig. 9a, the conformation obtained has the A- and C-rings almost completely inserted in the cyclodextrin cavity, while the B′ and B rings are oriented toward the secondary rim, leaving the OH groups of the pyrogallol ring more exposed to the solvent than the galloyl. The theoretical results for the EGCG–DMβCD complex, Fig. 9b, indicate that the inclusion occurs in a similar way to that of the EGCG–βCD complex. The conformation obtained has the B and B′ rings oriented toward the secondary rim and the A and C rings are almost completely inserted inside the cavity, with the OH-7 of EGCG exposed to the outer surface through the primary rim. The major difference observed between both complexes is that the hydroxyl groups of pyrogaloll are more buried than in the EGCG–βCD complex.
All information about the inclusion geometry of these complexes is related to its antioxidant activity [21], because in all the complexes the EGCG molecule is protected, but maintains the antioxidant groups exposed or in the edges of the cyclodextrin cavities. The degree of exposure of these groups does not show an important difference in the antioxidant activity, because in all cases the galloyl group is exposed and determines its activity. In fact, we found by ESR measurements that the EGCG allows the formation of two o-semiquinonic radical species derived from catechol and galloyl groups when forming a complex with βCD and only one semiquinonic radical species derived from the galloyl group when forming a complex with HPβCD (Folch-Cano et al., manuscript in preparation). This complex was the most important in terms of the antioxidant activity, and this fact can account for the stabilization of the radical species derived from EGCG, due to restricted motion of that molecule when the complex is formed, in agreement with the broadening of the proton signals and the ROE effect observed by NMR techniques.
References
Uekama, K., Hirayama, F., Irie, T.: Cyclodextrin drug carrier systems. Chem. Rev. 98, 2045–2076 (1998)
Rekharsky, M.V., Inoue, Y.: Complexation thermodynamics of cyclodextrins. Chem. Rev. 98, 1875–1918 (1998)
Del Valle, M.E.M.: Cyclodextrins and their uses: a review. Process Biochem. 39, 1033–1046 (2004)
Brewster, M.E., Loftsson, T.: Cyclodextrins as pharmaceutical solubilizers. Adv. Drug Deliv. Rev. 59, 645–666 (2007)
Szente, L., Szejtli, J.: Cyclodextrins as food ingredients. J. Trends Food Sci. Technol. 15, 137–142 (2004)
Szente, L., Szejtli, J.: Elimination of bitter, disgusting tastes of drugs and foods by cyclodextrins. Eur. J. Pharm. Biopharm. 61, 115–125 (2005)
Gould, S., Scott, R.C.: 2-Hydroxypropyl-β-cyclodextrin (HP-β-CD): a toxicology review. Food Chem. Toxicol. 43, 1451–1459 (2005)
Feng, W.Y.: Metabolism of green tea catechins: an overview. Curr. Drug Metab. 7, 755–809 (2006)
Yamauchi, R., Sasaki, K., Yoshida, K.: Identification of epigallocatechin-3-gallate in green tea polyphenols as a potent inducer of p53-dependent apoptosis in the human lung cancer cell line A549. Toxicol. In Vitro 23, 834–839 (2009)
Henning, S.M., Wang, P., Heber, D.: Chemopreventive effects of tea in prostate cancer: green tea versus black tea. Mol. Nutr. Food Res. 55, 905–920 (2011)
Duhon, D., Bigelow, R., Coleman, D., Steffan, J., Yu, C., Langston, W., Kevil, C., Cardelli, J.: The polyphenol epigallocatechin-3-gallate affects lipid rafts to block activation of the c-Met receptor in prostate cancer cells. Mol. Carcinog. 49, 739–749 (2010)
Connors, S., Chornokur, G., Kumar, N.: New insights into the mechanisms of green tea catechins in the chemoprevention of prostate cancer. Nutr. Cancer 64, 4–22 (2012)
Ye, F., Zhang, G., Guan, B., Xu, X.: Suppression of esophageal cancer cell growth using curcumin, (−)-epigallocatechin-3-gallate and lovastatin. World J. Gastroenterol. 18, 126–135 (2012)
Li, G., Lin, W., Araya, J., Chen, T., Timmermann, B., Guo, G.: A tea catechin, epigallocatechin-3-gallate, is a unique modulator of the farnesoid X receptor. Toxicol. Appl. Pharmacol. 258, 268–274 (2012)
Thakur, V.S., Gupta, K., Gupta, S.: The chemopreventive and chemotherapeutic potentials of tea polyphenols. Curr. Pharm. Biotechnol. 13, 191–199 (2012)
Lin, Q., Akesson, B., Bergenstahl, B.: Effect of colloidal structures on the stability of five flavonoids with different hydrophilicity. Food Hydrocolloids 22, 700–705 (2008)
Yang, C., Lambert, J., Ju, J., Lu, G., Sang, S.: Tea and cancer prevention: molecular mechanisms and human relevance. Toxicol. Appl. Pharmacol. 224, 265–273 (2007)
Mochizuki, M., Yamazaki, S., Kano, K., Ikeda, T.: Kinetic analysis and mechanistic aspects of autoxidation of catechins. Biochim. Biophys. Acta 1569, 35–44 (2002)
Sang, S., Yang, I., Buckley, B., Ho, C., Yang, C.: Autoxidative quinone formation in vitro and metabolite formation in vivo from tea polyphenol (−)-epigallocatechin-3-gallate: studied by real-time mass spectrometry combined with tandem mass ion mapping. Free Radical Biol. Med., 43, 362–371 (2007)
Su, Y.L., Leung, L.K., Huang, Y., Chen, Z.: Stability of tea theaflavins and catechins. Food Chem. 83, 189–195 (2003)
Folch-Cano, C., Jullian, C., Speisky, H., Olea-Azar, C.: Antioxidant activity of inclusion complexes of tea catechins with [beta]-cyclodextrins by ORAC assays. Food Res. Int. 43, 2039–2044 (2010)
Ishizu, T., Kajitani, S., Tsutsumi, H., Sato, T., Yamamoto, H., Hirata, C.: Configurational studies of complexes of tea catechins with caffeine and various cyclodextrins. Planta Med. 77, 1099–1109 (2011)
Ishizu, T., Kajitani, S., Tsutsumi, H., Yamamoto, H., Harano, K.: Diastereomeric difference of inclusion modes between (−)-epicatechin gallate, (−)-epigallocatechin gallate and (+)-gallocatechin gallate, with beta-cyclodextrin in aqueous solvent. Magn. Reson. Chem. 46, 448–456 (2008)
Benesi, H.A., Hildebrand, J.H.: A spectrophotometric investigation of the interaction of iodine with aromatic hydrocarbons. J. Am. Chem. Soc., 71, 2703–2707 (1949)
Connors, K.A.: Binding Constants: The Measurement of Molecular Complex Stability, 1st edn. Wiley Interscience, New York (1987)
Frisch, M.J., Trucks, G.W., Schlegel, H.B., Scuseria, G.E., Robb, M.A., Cheeseman, J.R., Zakrzewski, V.G., Montgomery, J.A., Stratmann, R.E., Burant, J.C., Dapprich, S., Millam, J.M., Daniels, A.D., Kudin, K.N., Strain, M.C., Farkas, O., Tomasi, J., Barone, V., Cossi, M., Cammi, R., Mennucci, B., Pomelli, C., Adamo, C., Cliford, S., Ochterski, J., Petersson, G.A., Ayala, P.Y., Cui, Q., Morokuma, K., Malick, D.K., Rabuck, A.D., Raghavachari, K., Foresman, J.B., Cioslowski, J., Ortiz, J.V., Stefanov, B.B., Liu, G., Liashenko, A., Piskorz, P., Komaromi, I., Gomperts, R., Martin, R.L., Fox, D.J., Keith, T., Al-Laham, M.A., Peng, C.Y., Nanayakkara, A., Gonzalez, C., Challacombe, M., Gill, P.M.W., Johnson, B.G., Chen, W., Wong, M.W., Andres, J.L., Head-Gordon, M., Replogle, E.S., Pople, J.A.: Gaussian 98 (revision a.7). Gaussian, Inc., Pittsburgh (1998)
Yong, C., Washington, C., Smith, W.: Structural behaviour of 2-hydroxypropyl-beta-cyclodextrin in water: molecular dynamics simulation studies. Pharm. Res. 25, 1092–1099 (2008)
Morris, G.M., Goodsell, D.S., Halliday, R.S., Huey, R., Hart, W.E., Belew, R.K., Olson, A.J.: Automated docking using a Lamarckian genetic algorithm and an empirical binding free energy function. J. Comput. Chem. 19, 1639–1662 (1998)
Ishizu, T., Hirata, C., Yamamoto, H., Harano, K.: Structure and intramolecular flexibility of beta-cyclodextrin complex with (−)-epigallocatechin gallate in aqueous solvent. Magn. Res. Chem. 44, 776–783 (2006)
Proniuk, S., Liederer, B.M., Blanchard, J.: Preformulation study of epigallocatechin gallate, a promising antioxidant for topical skin cancer prevention. J. Pharm. Sci. 91, 111–116 (2002)
Szejtly, J.: Cyclodextrin Technology, 1st edn, vol. 1, pp. 1–78. Kluwer Academic, Dordrecht (1988)
Jullian, C., Orosteguis, T., Pérez-Cruz, F., Sánchez, P., Mendizabal, F., Olea-Azar, C.: Complexation of morin with three kinds of cyclodextrin. A thermodynamic and reactivity study. Spectrochim. Acta A, 71, 269–275 (2008)
Jullian, C., Alfaro, M., Zapata-Torres, G., Olea-Azar, C.: Inclusion complexes of cyclodextrins with galangin: a thermodynamic and reactivity study. J. Sol. Chem. 39, 1168–1177 (2010)
Jullian, C., Brossard, V., Gonzalez, I., Alfaro, M., Olea-Azar, C.: Cyclodextrins–Kaempferol inclusion complexes: spectroscopic and reactivity studies. J. Sol. Chem. 40, 727–739 (2011)
Jullian, C., Cifuentes, C., Alfaro, M., Miranda, S., Barriga, G., Olea-Azar, C.: Spectroscopic characterization of the inclusion complexes of luteolin with native and derivatized [beta]-cyclodextrin. Bioorg. Med. Chem. 18, 5025–5031 (2010)
Acknowledgments
This work was supported by CONICYT Project 24091025 and FONDECYT Project 11080038. Christian Folch-Cano gratefully acknowledges the contribution of CONICYT-CHILE for a Ph.D. fellowship. J. Guerrero gratefully acknowledges the contribution of MECESUP-0007 for an NMR spectrometer.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Folch-Cano, C., Guerrero, J., Speisky, H. et al. NMR and molecular fluorescence spectroscopic study of the structure and thermodynamic parameters of EGCG/β-cyclodextrin inclusion complexes with potential antioxidant activity. J Incl Phenom Macrocycl Chem 78, 287–298 (2014). https://doi.org/10.1007/s10847-013-0297-y
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10847-013-0297-y