Abstract
The aim of the present work was to investigate the interaction between bile salts present in the intestine of man, dog and rat with the negatively charged cyclodextrin (CD), sulfobutylether-β-cyclodextrin (SBEβCD). The interactions between bile salts and CDs are of importance for the release of CD-complexed drugs upon oral administration. This makes a good understanding of this particular interaction important for rational drug formulation. SBEβCD is a modified CD, which has attracted particular interest in formulation science. It is unique in the sense that it carries approximately seven negatively charged side chains, which can potentially interact electrostatically with the guest molecule. Bile salts are negatively charged at physiological pH, and the concomitant repulsion from SBEβCD could potentially reduce their affinity for this CD and hence their ability to expel drugs delivered as SBEβCD complexes. However, this study has demonstrated that the interaction, between a bile salts and SBEβCD is only slightly weaker than the corresponding interactions with natural βCD. Significant differences between the thermodynamics of bile salt complexes with respectively HPβCD and SBEβCD were found, when comparing the same degree of substitution. This underscores the importance of the substituents on the interactions of modified CDs with bile salts.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Cyclodextrins (CDs) are macrocyclic oligosugars commonly composed of 6, 7 or 8 glucosidic molecules, α-, β- and γ-CDs, respectively [1–4]. The outer hydrophilic surface and the inner hydrophobic cavity make the CDs capable of forming inclusion complexes with molecules of appropriate shape and size. Although not exclusively, the formed ligand–CD complexes are often of 1:1 stoichiometry [1]. A variety of chemically modified CDs have been synthesised in order to optimise physicochemical and toxicological properties as compared to the native CDs. The inclusion of guest molecules into CDs and their derivatives facilitates many applications such as solubilisation [5–10], targeting of drugs [11], and chromatographic separations [12, 13].
Commonly, the modified βCDs, methyl-, 2-hydroxypropyl- (HPβCD) and sulfobutylether-β-cyclodextrin (SBEβCD), are used in commercial pharmaceutical products [14, 15]. The driving forces for the formation of CD inclusion compounds are mainly hydrophobic and van der Waals interactions [16, 17], but for the negatively charged SBEβCD Coulombic interactions have also been demonstrated to contribute significantly [18]. Generally, SBEβCD has been demonstrated to form stronger inclusion complexes than HPβCD [19–28], although some exceptions are published [29, 30]. The differences between the two modified CDs are, however, specially pronounced for positively charged molecules [19, 24, 27]. Many drugs are bases and, hence, positively charged at low pH, which makes SBEβCD particularly interesting in drug discovery and development as it can induce a charge–charge interaction increasing the complexation efficiency significantly [10].
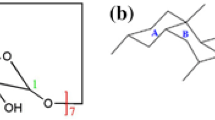
Upon oral administration, xenobiotics complexed with CD must be displaced from the complex to be available for absorption, as CDs are only absorbed from the gastrointestinal tract to a very small extent [2]. In the small intestine this displacement is thought to depend upon bile salts [31–33], which may bind competitively to the CD. For SBEβCD, it may be speculated whether the negative charge may repel the negatively charged conjugated bile salts (see Fig. 1) present in the intestine. If indeed so, this would lead to a slower or lower release of the drug from the CD complex. Therefore, knowledge about the interaction between SBEβCD and bile salts are of special interest for rational drug formulation design using these modified CDs.
Studies investigating the interaction between bile salts and CDs have mainly been exploited by nuclear magnetic resonance (NMR) [34–37], calorimetry [16, 33, 38–41] or affinity capillary electrophoresis (ACE) [42–44]. The highly negatively charged SBEβCD poses a special challenge in ACE experiments due to the need for correction for changes in ionic strength and temperature and the effect it has on electrophoretic as well as complexation behavior as a function of SBEβCD concentration. Østergaard et al. [45] have in a recent study developed a method to correct for the ionic strength effects for SBEβCD, but the results have not been evaluated by a method less affected by differences in ionic strength. In this work, this correction protocol is tested by comparisons of data from respectively ACE and isothermal calorimetry (ITC). The overall purpose of this study is to investigate possible effects of the negatively charged side chains on SBEβCD on the stability and structure of its complexes with bile salts by the use of a combination of calorimetric, eletrophoretic and molecular dynamics (MD) simulation methods.
Experimental procedures
Materials
The sodium salts of the bile acids were purchased from various sources. Taurocholate (TC; CAS no. 83830-80-2) was purchased from Fluka (Buchs, Switzerland). Tauro-β-muricholate (TβMC; CAS no. 25696-60-0) was purchased from Makaira Ltd (London, GB). Taurochenodeoxycholate (TCDC, CAS no. 516-35-8), taurodeoxycholate (TDC; CAS no. 516-50-7), glycocholate (GC; CAS no. 475-31-0), glycodeoxycholate (GDC; CAS no. 16409-34-0), glycochenodeoxycholate (GCDC; CAS no. 640-79-9) and deuterium oxide (99.95 %) were purchased from Sigma-Aldrich (St. Louis, MO, USA). All compounds were used as provided. SBEβCD was purchased from CyDex (Lenexa, USA). Sodium phosphate buffer used in the CE experiments, 0.050 M, pH 7.00, was obtained from Agilent (Palo Alto, USA). Ultra pure water for the ACE experiments was obtained from Agilent whereas water for the ITC experiments was from a Millipore purification system (Billerica, MA, USA). All other chemicals were of analytical grade and used as received.
Characterisation of SBEβCD by NMR
The NMR experiments were carried out at 25 °C on a Bruker Avance-600 NMR (Bruker Biospin, Rheinstetten, Germany) spectrometer operating at 14.1 T and equipped with a cryogenically cooled probe: 32 scans were acquired for the 1D 1H experiments, 5,120 scans were acquired for the attached proton test (APT) experiments, while for the two dimensional HSQC and HMBC experiments, 32 and 64 scans, respectively, were acquired for each of the 128 t1 increments. The SBEβCD samples were run at 40 mM in D2O.
Capillary electrophoresis
As BGE a 50.0 mM phosphate buffer (pH 7.00, I = 0.110 M) was prepared by weighing adequate amounts of disodium hydrogen phosphate dodecahydrate and potassium dihydrogen phosphate, and dilution with water. SBEβCD stock solutions with a concentration of 1.00 × 10−2 M were prepared in 50 mM phosphate buffer (pH 7.00, I = 0.110 M) as well as in water. Three series of BGE solutions containing SBEβCD in the concentration range of 0–7.50 × 10−3 M were prepared for the ACE experiments. BGE series 1 was prepared by suitable dilution of the 1.00 × 10−2 M SBEβCD stock solution in 50.0 mM phosphate with the 50 mM phosphate buffer.
The ACE experiments were executed using a Beckman PACE 5510 CE with a diode array detector [45]. Uncoated fused silica bubble cell capillaries (150 μm light path) of 37 cm × 50 μm inner diameter, with a length of 30 cm to the detector (Agilent Technologies, Waldbronn, Germany) were used in the CE experiments. Conditioning of new capillaries was performed by flushing with 1 M NaOH and water for 30 min each. UV detection was performed at 200 nm and the capillary cassette temperature was set to 22 °C. The applied voltage was set to 10 kV in the normal polarity mode. Samples in buffer without SBEβCD, containing 5–8 × 10−5 M bile salt and 3 × 10−4 % (v/v) formamide as a marker of the electroosmotic flow (EOF), were introduced by applying a pressure of 0.5 psi for 5 s. Each sample was run in triplicate. The capillary was flushed with 0.1 M NaOH, 0.05 M phosphate buffer, the back ground electrolyte (BGE), with or without SBEβCD for 1 min each between measurements.
Electrophoretic mobilities, μ, determined at constant voltage needs to be corrected for changes in temperature (Joule heating), viscosity and ionic strength to a selected reference state prior to the estimation of stability constants [46, 47]. The measured effective electrophoretic mobility, μ, was therefore recalculated to the reference temperature T0 at 25 °C [41, 42] assuming the mobilities have the same temperature dependence as found for the conductivity of the BGE (α th = 0.022 K−1):
The temperature corrected electrophoretic mobilities μ 0 were subsequently corrected for viscosity variations, due to the presence of CD in the BGE and changes in ionic strength as previously described by Østergaard et al. [45].
The binding isotherms were constructed by plotting the corrected effective electrophoretic mobility μ’ as a function of the CD concentration ([CD]) instead of μ versus [CD] in order to correct for viscosity, ionic strength, and temperature changes. The binding parameters K 1:1 and μ BS–CD were determined by non-linear regression analysis according:
using SigmaPlot (version 9.0, Systat Software, Point Richmond, CA, USA).
Isothermal titration calorimetry
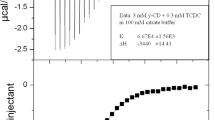
The energetics of the interaction between the bile salts and SBEβCD was evaluated on a VP-ITC from MicroCal (Northhampton, MA, USA). The experiments were carried out at 25 °C in triplicate in 50 mM phosphate buffer, pH 7.0, and all solutions were degassed using a ThermoVac (MicroCal, Northhampton, MA, USA) before the titration experiments. An aliquot of 1 mM bile salt was filled into the reaction cell, while the titration syringe was filled with a 10 mM SBEβCD solution. The binding experiment involved 27 sequential additions of small aliquots (10 μl) of the SBEβCD solution into the reaction cell under continuous stirring (307 rpm). For TβTC, however, only 0.25 mM bile salt was in the reaction cell and 2.5 mM SBEβCD in the syringe, due to the very strong interaction between the two species. The heat of dilution was estimated from the heat change at high CD concentrations, where practically all bile salt had interacted, and subtracted from the raw data prior to non-linear regression analysis. The latter was performed with the ORIGIN software package (version 7.0). The fitted parameters were the binding constant (K 1:1), the change in standard binding enthalpy (ΔH 0) and the binding stoichiometry (n) and calculated by the one site model in ORIGIN. Knowledge of the binding constant and molar reaction enthalpy enabled calculation of the standard Gibbs free energy of binding (ΔG 0) and the change in standard entropy (ΔS 0) according to:
where R is the gas constant, and T is the absolute temperature.
For two of the complexes, SBEβCD:GDC and SBEβCD:GCDC, titrations were carried out at six temperatures in the interval 5–55 °C. This enabled calculation of the heat capacity, ΔC p , which has previously been interpreted with respect to the extent of non-polar dehydration that takes place upon complexation [48, 49]. Precise values of ΔC p were obtained by simultaneously fitting the enthalpograms at all temperatures, assuming that ΔC p is constant over the experimental temperature range and using the van’t Hoff equation to link the temperature dependence of the binding constants to the enthalpy changes, as previously described [50].
Molecular dynamics simulations
Molecular dynamics simulations were carried out to study the behaviour of the SBE-chains and the complexation induced changes in water accessible surface area (ASA). 10 ns simulations of the non-complexed bile salts, GDC and GCDC were carried out in 34 × 34 × 34 Å3 water boxes, while a 25 ns simulation of SBEβCD and 30 ns simulations of its complexes with GDC and GCDC were carried out in water boxes measuring 40 × 40 × 40 Å3. The SBEβCD sample used in the experiments had on the average 6.4 SBE-chains on each CD molecule. Therefore, CDs with six substituents were used in the MD simulations. The systems were neutralised by adding six sodium ions (counter ions for the six negative changes on the to the SBE chains) to the simulation box. Simulations were done with NAMD version 2.7 [51] using the CHARMM27 force field [52]. Simulation details and parameters for the SBE chains have previously been described by Schönbeck et al. [53].
Calculations of polar and non-polar surface area of the free and complexed species were conducted in VMD [54] by averaging ASA of individual frames in the simulation intervals 2–10 ns for the free bile salts, 5–25 ns for the free SBEβCD and 5–30 ns for the two complexes.
Results and discussion
Though SBEβCD are frequently used for oral administration due to the CDs high solubilisation capacity [10], very little is known about the CDs thermodynamic behaviour, which will be investigated in the present work.
Degree and pattern of substitution of SBEβCD
The NMR analysis of the used SBEβCD suggested average DS of 0.91 and a position of the distribution of the on approximately 50, 40 and 10 % attached to O3, O2 and O6, respectively. A more detailed description can be found in the supplementary materials.
Binding stoichiometry
An electropherogram from the experiments can be seen in the supplementary information. Correlation coefficients, R 2, were above 0.99, see Table 1, after fitting to the 1:1 model described in Eq. 2, indicating that the 1:1 complexation model adequately described the binding data obtained by CE. However, as reported previously [55], a high correlation coefficient is not sufficient to demonstrate that the 1:1 model is the best to describe the data. Linear x-reciprocal plots [55–57] and physically unrealistic binding parameters when a 1:2 model was applied to the ACE data (negative K 1:2 and positive 1:2 complex mobilities) supported that 1:1 binding is dominating at the investigated concentration range.
The stoichiometric ratios (n value) observed from the fitting curve in the ITC experiments was within the range of 0.9–1.1 at the investigated concentration. This clearly indicate that the majority of the inclusion complexes had a 1:1 stoichiometry of bile salt and SBEβCD, which is in accordance with the stoichiometry from the ACE experiments and previously reported stoichiometries for the interaction between bile salts and βCD [39, 40, 42, 58–60] and other modified βCDs [35, 37, 38, 40, 42, 53, 61]. Figure 2 shows a typical enthalpogram for a complex with a high stability constant from which the stoichiometry was estimated.
Stability constants
The stability constants determined by ACE and ITC are presented in Table 1 and 2, respectively. The data showed a high degree of agreement of the results determined by ACE and ITC, demonstrating that the correction methods for the influence of differences in ion strength and viscosity in the ACE experiments previously defined by Østergaard et al. [45] ensured accurate measurements. This means that the ACE method efficiently and with very little material consumption could be used for screening cyclodextrins in a preformulation setup to define the most suited cyclodextrin for the compound instead of conducting the more classical phase-solubility profiles with a much higher compound spenditure.
Stability constants for two bile salts–SBEβCD complexes have previously been reported in the literature [45], while the majority of the relevant bile salts for human, dog and rat remain unexplored. The stability constants for all investigated bile salts were lower than the those reported for binding to natural βCDs [33, 34, 39, 58, 59]. This trend is in accordance with what has been found for HPβCDs, where the substitution also had a negative effect on the stability of bile salt complexes [61]. Specifically, HPβCD with a DS of 0.54 [62] showed comparable stability to the SBEβCD complexes for two different bile salts. However, when comparing the current stability constants for SBEβCD complexes to the results reported by Schönbeck et al. [61] for HPβCD with a similar degree of substitution (DS = 1.0), it is clear that SBEβCD produces more stable inclusions. The repulsive effects between the negatively charged bile salt and the negatively charged sulfobutylether group therefore seem to affect the interaction to a very limited extent if at all. Assuming that the bile salts are oriented similarly as in complexes with βCD, methyl- and HPβCD [41, 61–63], the charged glycine or taurine chain on the bile salts protrudes from the narrow rim of the CD where only 10 % of the sulfobutyl chains are attached, as discussed in the supplementary information. Additionally, the negative charge on the SBEβCD is placed on a highly flexible butyl chain, which in total seems to be oriented in a way that ensures sufficient separation of the charges to support a good interaction between the two species, as also previously reported by Zia et al. [28, 64]. This explanation is confirmed by the MD simulations and is illustrated in Fig. 3.
In accordance with previous observations for neutral modified βCDs, the affinity of the tauro-conjugated bile salts for SBEβCD was slightly lower than its glycol-conjugated counterparts [53, 61], a difference suggested to emerge as the conjugation of the bile salts is situated in or at the proximity of the extended CD cavity potentially leading to interactions. The stability constants for TCDC, GCDC and TβMC are significantly higher than for TC, GC, TDC and GDC, which is consistent with earlier reports concerning mβCD and HPβCD [42, 53]. The hydroxyl group at C12 is highly important for the structural positioning of the bile salt within the modified CDs due to steric hindrances, hence explaining the distinct negative impact on the stability constant of this group. Adding a charge to the modification of the CDs has no significant implication on this as it happens within the CD cavity.
Enthalpy and entropy changes
Compensatory relationships for the changes in enthalpy and entropy have been reported for a series of guest molecules and CDs [3, 65, 66], including bile salts [41, 53, 61]. This compensation may be illustrated in plots of TΔS 0 versus ΔH 0, as shown in Fig. 4, where linear relationships are indicative of a common underlying mode of interaction. This type of analysis for βCD (41) and modified βCDs [53, 61] suggested that the weakly bound bile salts (the cholates and deoxycholates) fall into one group with a mode of interaction qualitatively different from the strongly bound bile salts. For SBEβCD a similar pattern was observed and apparently the charges on SBEβCD do not affect the mode of interaction. Figure 4 suggests a relative large intercept (TΔS 0), which is in accordance with reported values for simple modified CDs possessing a flexible hydrophilic sidearm [3]. It has been suggested that the slope (α) and the intercept (TΔS 0) of the entropy–enthalpy compensation plot can be related to the degree of conformational change and the extent of desolvation induced upon complexation, respectively [3, 65–67]. Assuming that this correlation exits, the data in Fig. 4 suggests extensive desolvation effect in the interaction between SBEβCD and the bile salts.
The complexes of the BSs with SBEβCD (X) lie on the three lines that appear in the entropy-enthalpy compensation plot for the interaction of BSs with natural and methylated (open symbols [50, 55]) and hydroxypropylated (closed symbols [66]) βCDs. Squares denote complexes with TC and GC, circles with TDC and GDC and triangles with TCDC and GCDC
The interaction with SBEβCD is favoured by both enthalpic and entropic contributions for all seven bile salts. ΔH 0 is the dominant contribution to ΔG 0, with values in the −13 to −27 kJ/mol range. In general, it was found that the net affinity was essentially proportional to the magnitude of the (negative) enthalpy change. Comparison of the data in Table 2 with analogous results for HPβCD with a degree of substitution of 0.6 [53, 61] shows some, but not major, differences in ΔH 0 and ΔS 0 nor their relative contribution to the total ΔG 0 when values for a specific bile salt are considered. If, however, the comparison is made towards the highly substituted HPβCD having a DS of 1.02 [61], differences are observed for the relative contribution of ΔH 0 and ΔS 0 for TC, GC, TDC and GDC whereas the similarity is kept for TCDC and GCDC. These differences probably reflect modifications of the mode of interaction at high densities of substituents on the CD. The thermodynamic effects of methyl and hydroxypropyl substituents have been interpreted in terms of buried surface area [63]. The substituents form an extended hydrophobic cavity, as shown in Fig. 4, which upon complexation causes a larger number of ordered water molecules to be released from non-polar surfaces of both bile salt and substituents. This gives a large and positive contribution to both ΔH 0 and ΔS 0, leading to the entropy–enthalpy compensation observed in Fig. 4, in which the complexes with highly substituted CDs are found in the upper right corner. The density and hydrophobicity of substituents on SBEβCD are expected to result in a significant dehydration of non-polar surface upon complexation. This is supported by the MD simulations from which it is determined that around 500 Å2 of non-polar surface is buried (see Table 3). This is around 150 Å2 more than for the analogous complexes with natural βCD [53]. ΔC p was determined for GDC and GCDC by the use of a global fit, and an example of such global fit is shown in Fig. 5. The large and negative ΔC p s, derived from this analysis (−600 to −700 J mol−1 K−1, Table 2) are about twice as large (numerically) as those for complexes of natural βCD, which again points towards dehydration of a significant hydrophobic surface area. A rough estimate of the hydrophobic surface area dehydrated by the substituents may be calculated from the experimental values of ΔC p , assuming that each Å2 of dehydrated hydrophobic surface area contributes with approximately −2 J K−1 mol−1 to ΔC p [53], i.e. 150 Å2, which is in agreement with the values obtained from the MD simulations. Further, the magnitude of the ΔC p ’s for SBEβCD are similar in magnitude to the ΔC p s observed for complexation of bile salts with highly substituted HPβCDs, i.e. with a degree of substitution of approximately 1.0 [53]. This indicates that the overall behaviour of the sulfobutyl chains is similar to the behaviour of hydroxypropyl chains. The hydrophobic surface seems predominantly to be buried within the complex as the substituents interacts with the parts of the bile salt that protrudes from the wider rim of the CD. However, although the calculated ΔASA values and the experimentally determined ΔC p ’s are similar to what is observed for HPβCD with the same number of substituents (DS ≈ 1), the rest of the thermodynamic functions, K, ΔH 0 and ΔS 0, resembles those of HPβCDs with considerably fewer substituents (DS ≈ 0.6). These differences may be attributed to the different character of the two types of substituents. The more favourable interaction for the sulfobutyl than the hydroxypropyl substitution could reflect a more optimal structural placement of the hydrophilic/hydrophobic groups for the interaction, i.e. at the end or the middle of the substitution chain, respectively.
Titrations of GDC with SBEβCD conducted at 10 degree intervals from 15 °C (upper) to 55 °C (lower). All five titrations were fitted simultaneously using K and ΔH 0 at 25 °C and ΔC p as global fitting parameters and c as local fitting parameters. A titration was conducted at 5 °C but was not included in the fit since the complexation enthalpies at this temperature are very small and overshadowed by other heat effects
ΔC p for GCDC was more negative than for GDC, which indicates a larger contribution of dehydration to the process. As demonstrated earlier by Holm et al. [41, 62] using NMR, GDC and GCDC are positioned differently within the CD complex, where GCDC have the entire D- and part of the C-ring of the steroid skeleton within CD, whereas for GDC only the D-ring in the CD due to the steric hindrance of the hydroxyl group on C12 in the bile salt. This difference in physical orientation seems to be reflected in the difference in ΔC p as also suggested by the ASA calculations. The deeper inclusion and concomitant larger dehydration of GCDC is supported by the entropy–enthalpy compensation plot in which the intercept obtained for the group of GCDC complexes is larger than what is obtained for the GDC complexes.
In conclusion, this study has presented 1:1 stability constants for the binding of seven biologically relevant glyco- and tauro-conjugated bile salts to SBEβCD. A good agreement between the stability constants obtained by ACE and ITC is observed suggesting that the two techniques are equally effective for obtaining stability constants. The size of the obtained stability constants were in the same range as those previously reported for the interaction of the same bile salts with less substituted HPβCDs, but significantly higher than those reported for HPβCD with a similar DS. Also ΔH 0 and ΔS 0 were similar to those of less substituted HPβCDs. In contrast to this, the observed ΔC p s were in the same range as those reported for HPβCD with a similar degree of substitution, indicating that the sulfobutyl substituents behave similarly to HP-substituents and results in significant dehydration of hydrophobic surface upon complexation as also indicated by the ASA data extracted from the MD simulations. These results demonstrate the importance of the substituents on the thermodynamic parameters for the interaction with a host compound.
References
Szejtli, J.: Cyclodextrin Technology. Kluwer Academic Publishers, Dordrecht (1988)
Szejtli, J.: Chemistry, physical and biological properties of cyclodextrins. In: Szejtli, J., Osa, T. (eds.) Cyclodextrins, pp. 189–204. Elsevier Science Ltd, Oxford (1996)
Rekharsky, M.V., Inoue, Y.: Complexation thermodynamics of cyclodextrins. Chem. Rev. 98, 1875–1917 (1998)
Dodziuk, H.: Cyclodextrins and Their Complexes: Chemistry, Analytical Methods, Applications. Wiley, Weinheim (2006)
Uekama, K.: Design and evaluation of cyclodextrin-based drug formulation. Chem. Pharm. Bull. 52, 900–915 (2004)
Uekama, K., Hirayama, F., Irie, T.: Cyclodextrin drug carrier systems. Chem. Rev. 98, 2045–2076 (1998)
Maas, J., Kamm, W.H.G.: An integrated early formulation strategy—from hit evaluation to preclinical candidate profiling. Eur. J. Pharm. Sci. 66, 1–10 (2007)
Neervannan, S.: Preclinical formulation for discovery and toxicology: physicochemical challenges. Expert Opin. Drug Metab. Toxicol. 2, 715–731 (2006)
Strickley, R.G.: Solubilizing excipients in oral and injectable formulations. Pharm. Res. 21, 201–230 (2004)
Loftsson, T., Brewster, M.E.: Cyclodextrins as functional excipients: methods to enhance complexation efficiency. J. Pharm. Sci. 101, 3019–3032 (2012)
Davis, M.E., Brewster, M.E.: Cyclodextrin-based pharmaceutics: past, present and future. Nat. Rev. Drug Disc. 3, 1023–1035 (2004)
Desiderio, C., Fanali, S.: Use of negative charged sulfobutyl ether-β-cyclodextrin for enantiomeric separation by capillary electrophoresis. J. Chromatogr. A 716, 183–196 (1995)
Mikus, P., Kanlansky, D., Fanali, S.: Separation of multicomponent mixtures of 2,4-dinitrophenyl labelled amino acids and their enantiomers by capillary zone electrophoresis. Electrophoresis 22, 470–477 (2001)
Brewster, M.E., Loftsson, T.: Cyclodextrins as pharmaceutical solubilizers. Adv. Drug Deliv. Rev. 59, 645–666 (2007)
Loftsson, T., Brewster, M.E., Masson, M.: Role of cyclodextrins in improving oral drug delivery. Am. J. Drug Deliv. 2, 175–261 (2004)
Liu, L., Guo, Q.-X.: The driving force in the inclusion complexation of cyclodextrins. J. Incl. Phenom. Macrocycl. Chem. 42, 1–14 (2002)
Connors, K.A.: The stability of cyclodextrin complexes in solution. Chem. Rev. 97, 1325–1357 (2002)
Schneider, H.J., Yatsimirsky, A.K.: Selectivity in supramolecular host-guest complexes. Chem. Soc. Rev. 37, 263–277 (2008)
Nagase, Y., Hirata, M., Wada, K., Arima, H., Hirayama, F., Irie, T., Kikuchi, M., Uekama, K.: Improvement of some pharmaceutical properties of DY-9760e by sulfobutyl ether β-cyclodextrin. Int. J. Pharm. 229, 163–172 (2001)
Savolainen, J., Järvinen, K., Matilainen, L., Järvinen, T.: Improved dissolution and bioavailability of phenytoin by sulfobutylether-β-cyclodextrin ((SBE)7m-β-CD)) and hydroxypropyl-β-cycloextrin (HP-β-CD) complexation. Int. J. Pharm. 165, 69–78 (1998)
Järvinen, T., Järvinen, K., Schwarting, N., Stella, V.J.: β-Cyclodextrin derivatives, SBE4-β-CD and HP-β-CD, increase the oral bioavailability of cinnarizine in beagle dogs. J. Pharm. Sci. 84, 295–299 (1995)
Larsen, K.L., Aachmann, F.L., Wimmer, R., Stella, V.J., Kjølner, U.M.: Phase solubility and structure of the inclusion complexes of prednisolone and 6α-methyl prednisolone with various cyclodextrins. J. Pharm. Sci. 94, 507–515 (2005)
Rajendrakumar, K., Madhusudan, S., Pralhad, T.: Cyclodextrin complexes of valdecoxib: properties and anti-inflammatory activity in rats. Eur. J. Pharm. Biopharm. 60, 39–46 (2005)
Zia, V., Rajewski, R.A., Stella, V.J.: Effect of cyclodextrin charge on complexation of neutral and charged substrates: comparison of (SBE)7M-β-CD to HP-β-CD. J. Pharm. Sci. 18, 667–673 (2001)
Johnson, M.D., Hoesterey, B.L., Anderson, B.D.: Solubilization of a tripeptide HIV protease inhibitor using a combination of ionization and complexation with chemically modified cyclodextrins. J. Pharm. Sci. 83, 1142–1146 (1994)
Másson, M., Loftsson, T., Jónsdóttir, S., Fridriksdóttir, H., Petersen, D.S.: Stabilisation of ionic drugs through complexation with non-ionic and ionic cyclodextrins. Int. J. Pharm. 164, 45–55 (1998)
Okimoto, K., Rajewski, R.A., Uekama, K., Jona, J.A., Stella, V.J.: The interaction of charged and uncharged drugs with neutral (HP-β-CD) and anionically charged (SBE7-β-CD) β-cyclodextrins. Pharm. Res. 13, 256–264 (1996)
Zia, V., Rajewski, R.A., Bornancini, E.R., Luna, E.A., Stella, V.J.: Effect of alkyl chain length and degree of substitution on the complexation of sulfoalkyl ether β-cyclodextrins with steroids. J. Pharm. Sci. 86, 220–224 (1997)
Arima, H., Yunomae, K., Miyake, K., Irie, T., Hirayama, F., Uekama, K.: Comparative studies of the enhancing effects of cyclodextrins on the solubility and oral bioavailability of tacrolimus in rats. J. Pharm. Sci. 90, 690–701 (2001)
Brewster, M.E., Vandecruys, R., Peeters, J., Neeskens, P., Verreck, G., Loftsson, T.: Comparative interaction of 2-hydroxypropyl-β-cyclodextrin and sulfobutylether-β-cyclodextrin with itraconazole: phase-solubility behavior and stabilization of supersaturated drug solutions. Eur. J. Pharm. Sci. 34, 94–103 (2008)
Ono, N., Hirayama, F., Arima, H., Uekama, K., Rytting, J.H.: Model analysis for oral absorption of a drug/cyclodextrin complex involving competitive inclusion complexes. J. Incl. Phenom. Macrocycl. Chem. 44, 93–96 (2003)
Miyajima, K., Yokoi, M., Komatsu, H., Nakagaki, M.: Interaction of β-cyclodextrin with bile salts in aqueous solutions. Chem. Pharm. Bull. 34, 1395–1398 (1986)
Tan, X., Lindenbaum, S.: Studies on complexation between β-cyclodextrin and bile salts. Int. J. Pharm. 74, 127–135 (1991)
Cabrer, P.R., Alvarez-Parrilla, E., Al-Soufi, W., Meijide, F., Núñez, E.R., Tato, J.V.: Complexation of bile salts by natural cyclodextrins. Supramol. Chem. 15, 33–43 (2003)
Mucci, A., Vandelli, M.A., Salvioli, G., Malmusi, L., Forni, F., Schenetti, L.: Complexation of bile salts with 2-hydroxypropyl-β-cyclodextrin: a 13C-NMR study. Supramol. Chem. 7, 125–127 (1996)
Cabrer, P.R., Alvarez-Parrilla, E., Meijide, F., Seijas, J.A., Núñez, E.R., Tato, J.V.: Complexation of sodium cholate and sodium deoxycholate by β-cyclodextrin and derivatives. Langmuir 15, 5489–5495 (1999)
Mucci, A., Schenetti, L., Salvioli, G., Ventura, P., Vandelli, M.A., Forni, F.: The interaction of biliar acids with 2-hydroxypropyl-β-cyclodextrin in solution and in the solid state. J. Incl. Phenom. Macrocycl. Chem. 26, 233–241 (1996)
Ollila, F., Pentikäinen, O.T., Forss, S., Johnson, M.S., Slotte, J.P.: Characterization of bile salt/cyclodextrin interactions using isothermal titration calorimetry. Langmuir 17, 7107–7111 (2001)
Liu, Y., Yang, Y.-W., Cao, R., Song, S.-H., Zhang, H.-Y., Wang, L.-H.: Thermodynamic origin of molecular selective binding of bile salts by animated β-cyclodextrins. J. Phys. Chem. B 107, 14130–14139 (2003)
Cooper, A., Nutley, M.A., Camilleri, P.: Microcalorimetry of chiral surfactant—cyclodextrin interactions. Anal. Chem. 70, 5024–5028 (1998)
Holm, R., Shi, W., Hartvig, R.A., Askjær, S., Madsen, J.C., Westh, P.: Thermodynamics and structure of inclusion compounds of tauro- and glyco-conjugated bile salts and β-cyclodextrins. Phys. Chem. Chem. Phys. 11, 5070–5078 (2009)
Holm, R., Nicolajsen, H.V., Hartvig, R.A., Westh, P., Østergaard, J.: Complexation of tauro- and glyco-conjugated bile salts with three neutral β-cyclodextrins studied by affinity capillary electrophoresis. Electrophoresis 28, 3745–3752 (2007)
Holm, R., Hartvig, R.A., Nicolajsen, H.V., Westh, P., Østergaard, J.: Characterization of the complexation of tauro- and glyco-conjugated bile salts with γ-cyclodextrin and 2-hydroxypropyl-γ-cyclodextrin using affinity capillary electrophoresis. J. Incl. Phenom. Macrocycl. Chem. 61, 161–169 (2008)
Østergaard, J., Jensen, H., Holm, R.: Use of correction factors in mobility shift affinity capillary electrophoresis for weak analyte—ligand interactions. J. Sep. Sci. 32, 1712–1721 (2009)
Østergaard, J., Jensen, H., Holm, R.: Affinity capillary electrophoresis method for investigation of bile salt complexation with negatively charged sulfobutyl ether-β-cyclodextrin. J. Sep. Sci. 35, 2764–2772 (2012)
Vespalec, R., Bocek, P.: Calculation of stability constants for the chiral selector–enantiomer interactions from electrophoretic mobilities. J. Chromatogr. A 875, 431–445 (2000)
Uselova-Vcelakova, K., Zuskova, I., Gas, B.: Stability constants of amino acids, peptides, proteins, and other biomolecules determined by CE and related methods: recapitulation of published data. Electrophoresis 28, 2134–2152 (2007)
Ross, P.D., Rekharsky, M.V.: Thermodynamics of hydrogen bond and hydrophobic interactions in cyclodextrin complexes. Biophys. J. 71, 2144–2154 (1996)
Olvera, A., Perez-Casas, S., Costas, M.: Heat capacity contributions to the formation of inclusion complexes. J. Phys. Chem. B 111, 11497–11505 (2007)
Schönbeck, C., Holm, R., Westh, P.: Higher order inclusion complexes and secondary interactions studied by global analysis of calorimetric titrations. Anal. Chem. 84, 2305–2312 (2012)
Phillips, J.C., Braun, R., Wang, W., Gumbart, J., Tajkhorshid, E., Villa, E., Chipot, C., Skeel, R.D., Kale, L., Schulten, K.: Scalable molecular dynamics with NAMD. J. Comput. Chem. 26, 1781–1802 (2005)
MacKerell, A.D., Bashford, D., Bellott, M., Dunbrack, R.L., Evanseck, J.D., Field, M.J., Fischer, S., Gao, J., Guo, H., Ha, S., Joseph-McCarthy, D., Kuchnir, L., Kuczera, K., Lau, F.T.K., Mattos, C., Michnick, S., Ngo, T., Nguyen, D.T., Prodhom, B., Reiher, W.E., Roux, B., Schlenkrich, M., Smith, J.C., Stote, R., Straub, J., Watanabe, M., Wiorkiewicz-Kuczera, J., Yin, D., Karplus, M.: All-atom empirical potential for molecular modeling and dynamics studies of proteins. J. Phys. Chem. B 102, 3586–3616 (1998)
Schönbeck, C., Holm, R., Westh, P., Peters, G.: Do 2-hydroxypropyl substituents extend the hydrophobic cavity of β-cyclodextrin? J. Incl. Phenom. Macrocycl. Chem. (submitted) (2013)
Humphrey, W., Dalke, A., Schulten, K.: VMD: visual molecular dynamics. J. Mol. Graph. 14, 33–38 (1996)
Bowser, M.T., Chen, D.D.Y.: Higher order equilibria and their effect on analyte migration behavior in capillary electrophoresis. Anal. Chem. 70, 3261–3270 (1998)
Lynen, F., Borremans, F., Sandra, P.: Practical evaluation of the influence of excessive sample concentration on the estimation of dissociation constants with affinity capillary electrophoresis. Electrophoresis 22, 1974–1978 (2001)
Rundlett, K.L., Armstrong, D.W.: Examination of the origin, variation, and proper use of expressions for the estimation of association constants by capillary electrophoresis. J. Chromatogr. A 721, 173–186 (1996)
Abadie, C., Hug, M., Kübli, C., Gains, N.: Effect of cyclodextrins and undigested starch on the loss of chenodeoxycholate in the feces. Biochem. J. 229, 725–730 (1994)
Tan, Z.J., Zhu, X.X., Brown, G.R.: Formation of inclusion complexes of cyclodextrins with bile salt anions as determined by NMR titration studies. Langmuir 10, 1034–1039 (1994)
Liu, Y., Li, L., Chen, Y., Yu, L., Fan, Z., Ding, F.: Molecular recognition thermodynamics of bile salts by β-cyclodextrin dimers: factors governing the cooperative binding of cyclodextrin dimers. J. Phys. Chem. B 109, 4129–4134 (2005)
Schönbeck, C., Westh, P., Madsen, J.C., Larsen, K.L., Städe, L.W., Holm, R.: Hydroxypropyl substituted β-cyclodextrins: influence of degree of substitution on the thermodynamics of complexation with tauro- and glyco-conjugated bile salts. Langmuir 26, 17949–17957 (2010)
Holm, R., Madsen, J.C., Shi, W., Larsen, K.L., Städe, L.W., Westh, P.: Thermodynamics of complexation of tauro- and glyco-conjugated bile salts with two modified β-cyclodextrins. J. Incl. Phenom. Macrocycl. Chem. 69, 201–211 (2011)
Schönbeck, C., Westh, P., Madsen, J.C., Larsen, K.L., Stade, L.W., Holm, R.: Methylated beta-cyclodextrins: influence of degree and pattern of substitution on the thermodynamics of complexation with tauro- and glyco-conjugated bile salts. Langmuir 27, 5832–5841 (2011)
Zia, V., Rajewski, R.A., Stella, V.J.: Thermodynamics of binding of neutral molecules to sulfobutyl ether β-cyclodextrins (SBE-β-CDs): the effect of total degree of substitution. Pharm. Res. 17, 936–941 (2000)
Inoue, Y., Hakushi, T., Liu, Y., Tong, L.-H., Shen, B.-J., Jin, D.-S.: Thermodynamics of molecular recognition by cyclodextrins. 1. Calorimetric titration of inclusion complexation of naphthalenesulfonates with α-, β-, and γ-cyclodextrins: enthalpy-entropy compensation. J. Am. Chem. Soc. 115, 475–481 (1993)
Inoue, Y., Lin, Y., Tong, L.-H., Shen, B.-J., Jin, D.-S.: Thermodynamics of molecular recognition by cyclodextrins. 2. Calorimetric titration of inclusion complexation with modified β-cyclodextrins. Enthalpy–entropy compensation in host-guest complexation: from ionophone to cyclodextrin and cyclophane. J. Am. Chem. Soc. 115, 10637–10644 (1993)
Tanford, C.: The Hydrophobic Effect: Formation of Micelles and Biological Membranes. Wiley, New York (1980)
Author information
Authors and Affiliations
Corresponding author
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Holm, R., Østergaard, J., Schönbeck, C. et al. Determination of stability constants of tauro- and glyco-conjugated bile salts with the negatively charged sulfobutylether-β-cyclodextrin: comparison of affinity capillary electrophoresis and isothermal titration calorimetry and thermodynamic analysis of the interaction. J Incl Phenom Macrocycl Chem 78, 185–194 (2014). https://doi.org/10.1007/s10847-013-0287-0
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10847-013-0287-0