Abstract
Inclusion complex formation between β-cyclodextrin and Naproxen was investigated using differential scanning calorimetry (DSC) as a function of the β-cyclodextrin-to-Naproxen molar ratio, ranging from 0:5:1 to 5:1. When these mixtures are heated above the melting temperature of Naproxen, an exothermic peak is observed at a temperature slightly higher than the melting peak of Naproxen. This peak, which has not been previously reported, has been interpreted as an exothermic energy of inclusion complex formation. The magnitude of this complex formation peak was found to be dependent upon the composition of the β-cyclodextrin and Naproxen mixture and increased in magnitude to a maximum value at a β-cyclodextrin:Naproxen molar ratio of 2:1. In addition, Naproxen recrystallization and re-melting peaks seen in the cooling and re-heating scans, respectively, decreased in magnitude with increasing molar ratio and totally disappeared for the mixture with 5:1 of β-cyclodextrin to Naproxen ratio indicative of complete inclusion of Naproxen in the cyclodextrin cavities. Complete inclusion was further reflected by the disappearance of key Naproxen peaks in Fourier transform infrared spectra of samples recovered from DSC experiments. The large excess of β-cyclodextrin needed to fully complex the Naproxen was found to be due to slow kinetics. Increasing the hold time after the initial melting led to inclusion efficiencies up to 95 % even for the 2:1 mixture. These experiments suggest that ratios of β-cyclodextrin:Naproxen 2:1 or greater facilitate the process by increasing the presence of cyclodextrin molecules in the close proximity of the drug molecules and lead to high inclusion efficiencies.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Naproxen (NA) is a propionic acid derivative and is a type of non-steroidal anti-inflammatory drug commonly administered for the treatment of pain, inflammation and fever [1, 2]. Its selected properties are given in Table 1. The poor aqueous solubility of NA due to its hydrophobicity limits the bioavailability of the drug in the aqueous environment of the human body [3]. Inclusion complex formation with cyclodextrins (CDs) is an approach to improve the aqueous solubility via molecular encapsulation of the drug within the cavity of the more soluble CD molecule [4, 5].
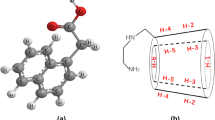
CDs have indeed been explored extensively as additives in the pharmaceutical industry due to their unique ability to host and solubilize hydrophobic guest molecules including drugs [5–8]. CDs are cyclic oligomeric polysaccharides composed of repeat glucose units which have a three dimensional structure similar in shape to a truncated cone or torus which is illustrated in Fig. 1A [9]. The central cavity is relatively hydrophobic, and the outer edge of the molecule is hydrophilic [10, 11]. The three native CDs, α-, β-, and γ-CD, are produced by the enzymatic degradation of starch and differ from one another only in the number of repeat glucose units [7]. βCD, which is illustrated in Fig. 1B, is the most commonly used CD due to the cavity size, availability and low cost [6, 10].
β-cyclodextrin A 3-dimensional torus structure and B chemical structure [35]
Complex formation is an equilibrium interaction described by the following equation:
where [CD] and [drug] represent uncomplexed CD and drug, respectively, [CD · drug] represents the complex, K is the stability constant for the complex, and a and b are the stoichiometric coefficients. The inclusion complex formation of NA with βCD and its derivatives has been studied extensively by various techniques, including potentiometry [1], conductivity [1], fluorescence [1, 12, 13], nuclear magnetic resonance (NMR) [12, 14], mass spectroscopy [14], differential scanning calorimetry (DSC) [13, 15–21], X-ray diffraction (XRD) [13, 15, 18–20], thermogravimetric analysis (TGA) [15, 16, 19], ultraviolet–visible spectroscopy (UV–Vis) [13, 15, 17, 19–23], Fourier transform infrared spectroscopy (FTIR) [18–21], hot stage microscopy [23], scanning electron microscopy [23], and molecular modeling [13, 22]. Stability constants of the NA-CD inclusion complexes, which can be used to calculate complexation stoichiometries and thermodynamic parameters of inclusion complex formation, including enthalpy and entropy changes, have previously been calculated based on spectroscopic characterizations utilizing a phase solubility method described by Higuchi [13, 15, 23] or a modified Benesi-Hildebrand equation [12, 14, 22]. Complex formation is generally associated with a large negative enthalpy change (ΔH) and either a positive or negative entropy change (ΔS), making complex formation largely an enthalpy-driven exothermic process [7]. In addition NA complexes with βCD, γCD and βCD derivatives formed in solutions have generally been reported as having a 1:1 stoichiometric ratio (where a = b) [1, 12, 14, 15, 22, 24, 25]. The stoichiometric ratios in nearly all guest-CD inclusion complexes that are reported in the literature are either 2:1, 1:1 or 1:2 [26]. Stoichiometries that differ from these have also been reported [27] but are attributed to poor inclusion efficiencies rather than any physical significance. It should be noted that these previously reported studies are all based on the behavior of CD solutions, and, to the authors’ knowledge, stoichiometry of the inclusion complex, stability constants and thermodynamic parameters between NA and βCD, or the requirements of cyclodextrin-drug ratios to be used for efficient complexation have not been previously reported for complex formation from physical mixtures in the absence of solvents.
DSC is an analytical technique commonly employed in the characterization of CD-drug inclusion complexes [28, 29]. In a typical DSC analysis for determination of complex formation, crystalline compounds which usually display distinct melting peaks in their DSC heating scans, appear amorphous if all the drug is included into the CD cavity. Observation of a drug melting peak is an indication of incomplete complex formation [28]. To the authors’ knowledge, no additional peaks, which are not attributed to drug melting or recrystallization transitions, have been previously reported during or after the event of inclusion complex formation between Naproxen and βCD. FTIR is another analytical technique useful in the characterization of inclusion complexes. Spectra of CD-drug physical mixtures results in an overlay of the two pure component spectra, while spectra of CD-drug inclusion compounds can display new peaks which are not present in either pure component spectra [30, 31], or they can appear as the CD spectra alone, with no observation of the IR peaks of the included compound [32, 33].
The primary goal of this research was to examine the inclusion complex formation behavior of βCD and NA by DSC from their physical mixture via melting of the drug molecule. A comprehensive evaluation by DSC of the complex formation between βCD and NA at a wide range of βCD:NA molar ratios (from 0.5:1 to 5:1) is now reported. Further characterization by FTIR was carried out to verify observations from the DSC data. The results show that complexation via melting in physical mixtures is influenced by transport limitations and ratios of βCD:NA greater than 2:1 facilitate the process by increasing the presence of cyclodextrin molecules in the close proximity of the drug molecules and lead to high efficiencies.
Materials and methods
Naproxen (NA) and β-cyclodextrin (βCD) were purchased from Sigma-Aldrich and used as received. DSC experiments were carried out in a Pyris Diamond DSC. 10 mg samples were prepared in the appropriate molar ratio, then mixed gently with a spatula and contained in a crimped aluminum DSC pan with a lid. The heating and cooling scans were carried out at a rate of 20 °C/min with a nitrogen purge of 10 ml/min. All heat values that are reported were normalized to the NA mass in the sample mixtures. FTIR spectra were acquired with a Digilab FTS 3100 spectrometer using a resolution of 4 cm−1 and 32 scans averaged in the wavenumber range of 4,000–400 cm−1. Samples were prepared at 1 wt % compositions into KBr discs for analysis. Four repeat samples were analyzed.
Results
DSC analyses were carried out for pure βCD, pure NA and for βCD:NA physical mixtures over a range of molar ratios from 0.5:1 to 5:1 (mol βCD:mol NA). Figure 2 shows the results for pure NA and pure βCD, displaying a Tm of 159 °C and a heat of melting of 129 J/g for NA in the first heating scan, and showing the removal of moisture from βCD above 100 °C. The variation between the melting temperatures obtained in DSC experiments from the literature data shown in Table 1 is due to differences in the heating rates employed during DSC experiments. Typically, a heating rate of 10 °C/min is employed in melting point determination by DSC; however, in these experiments a heating rate of 20 °C/min was used to sufficiently sharpen and resolve all observed peaks. A faster rate of heating is known to cause a shift of the melting peak to higher temperatures, due to the kinetics of melting. The physical mixtures were first heated to 140 °C and held for 3 min to remove water from βCD, eliminating the broad dehydration peak. After cooling to 30 °C the samples were heated above the melting temperature of NA (Tm = 159 °C) to 180 °C and held at 180 °C for 1 min, or heated to 165 °C and held for 60 or 120 min. The samples were then cooled to 30 °C, held at 30 °C for 1 min and finally reheated to 180 °C.
DSC scans for 0.5:1 and 5:1 βCD:NA are individually shown in Figs. 3, 4, respectively, to indicate that although the peaks for the 5:1 βCD:NA sample are significantly less pronounced than those for the 0.5:1 βCD:NA sample in the comparative plots shown in Fig. 5, the peaks are indeed present and visible with the enlarged scale used in Fig. 4. These scans were all generated with a 1 min hold time at 180 °C. The heating scan shown in plot A of each figure (in Figs. 3, 4) contains two distinct peaks: (1) the endothermic melting peak of NA at about 159 °C and (2) an exothermic peak at a temperature just higher than the NA melting peak. This peak has not been previously reported, to the authors’ knowledge, and is evidence of the exothermic complex-formation interaction occurring between βCD and NA upon NA melting. In the cooling scan of 0.5:1 βCD:NA shown in Fig. 3B, an exothermic peak is seen, which is centered around 106 °C and is indicative of NA recrystallization. The heat of recrystallization (ΔHc) for the 0.5:1 molar ratio sample was found to be significantly lower than the ΔHm observed in the first heating scan, and ΔHc for the 5:1 molar ratio sample was found to be zero, indicating that no fraction of the melted NA recrystallized in the cooling scan. Both observations are in contrast to the same DSC experiment carried out for pure NA (Fig. 2A), where NA after undergoing its melting in the heating scan recrystallized completely in the cooling scan. Similarly, in the second heating scan in the pure NA case, a re-melting peak is observed which is similar in magnitude (but opposite in sign) to the respective recrystallization peak. In these DSC experiments on mixtures with βCD, when NA enters its molten state in the first heating scan, the drug molecules are able to enter the empty βCD cavities forming the complex. Then, in the cooling scan, only free uncomplexed NA is able to recrystallize, and in the re-heating scan, only the free NA is able to re-melt, causing a reduction in ΔHc and in ΔHm2, respectively.
Examination of the thermal behavior of the intermediate molar ratios between 0.5:1 and 5:1 provides further information on inclusion complex formation of NA with βCD. Figure 5 shows the comparative DSC scans during the 1st heating, cooling and the second heating scans for each mixture. Figure 6 shows the variation of the heat of melting, heat of complexation, heat of recrystallization, and heat of re-melting during the respective scans. The heat of complex formation, ΔHcf, was found to increase in magnitude with increasing βCD molar composition up to a maximum value at a molar ratio of 2:1 (Fig. 6A). At molar ratios higher than 2:1, ΔHcf maintained a value around −20 J/g. This initial increase in magnitude indicates an increase in the energy of complexation since more NA enters the βCD cavities; however, stabilization of the value may indicate a finite heat of complexation which exists for complex formation of the mixture. The magnitudes of the heat of recrystallization, ΔHc and heat of re-melting, ΔHm2, decrease with increasing βCD molar composition, and the values are similar for each mixture. It should be noted that the peaks shown in Fig. 5B, C generally shift to lower temperatures with increasing molar ratio. A possible explanation for the peak shift is the disruption of crystallinity and crystallizability due the increasing presence of the amorphous inclusion compound in the mixture after the first heating scan with increasing molar ratio. This would cause slower crystallization kinetics resulting in lower recrystallization temperatures, as well as a defect-laden semi-crystalline structure resulting in lower re-melting temperatures. Thus, higher molar ratios were found to promote inclusion complexation, leaving less free NA to recrystallize and re-melt. Since the magnitudes of recrystallization and re-melting peaks are similar at the same molar ratio, all of the inclusion complex formation must occur after the first melting of NA, and no further complex formation occurs upon melting in the second heating scan. The recrystallization and re-melting peaks completely disappear only for the 5:1 molar ratio mixture, suggesting that a large excess of βCD is needed for complete amorphization attributed to complex formation of NA with βCD by melt processing at 180 °C with a holding time of 1 min at this temperature. Inclusion efficiencies can then be calculated based on the heat of re-melting and pure NA heat of melting (ΔH NAm = 129 J/g) using the following relationship.
The results that are plotted in Fig. 7 show that the inclusion efficiency rapidly increases with increasing molar ratio of βCD to NA, basically approaching efficiencies of 90 % and higher for CD:NA ratios of higher than 2.
The FTIR spectra shown in Fig. 8 compare the spectra of pure βCD and NA with the spectra of the samples recovered after DSC experiments in the wavenumber range of 2,000–800 cm−1. Key NA peaks observed in this region include peaks at 1729, 1685, indicating the –C=O stretch, and the peak at 1,228 cm−1, indicative of the –O– stretch. The key βCD peaks include the asymmetric R–O–R stretch observed at 1,158 cm−1 and the C–OH stretch observed at 1,029 cm−1. As the βCD:NA molar ratio is increased, the NA peaks become diminished. At molar ratios of 2:1 and higher, and in particular at the 5:1 molar ratio, NA peaks are no longer visible and the spectrum appears as pure βCD. The disappearance of the NA peaks in the FTIR spectra is a further indication of inclusion complex formation, and these data support the DSC results showing full inclusion being achieved only in the very high 5:1 molar ratio sample with the 1 min hold time at 180 °C.
FTIR spectra of pure components compared to spectra of samples recovered from DSC experiments held at 180 °C for 1 min. Arrows show the key peaks at 1,729, 1,685, indicating the –C=O stretch and 1,228 cm−1, indicative of the –O– stretch in NA; and the asymmetric R–O–R stretch observed at 1,158 cm−1 and the C–OH stretch observed at 1,029 cm−1 in βCD
Some degree of inclusion was achieved with all of the tested physical mixtures; however, only the 5:1 molar ratio mixture yielded a 100 % inclusion by melt processing at 180 °C for 1 min. The large excess of βCD needed to include all of the NA was thought to be required due to transport or kinetic limitations in the static environment of the DSC pans or the short, 1 min, hold time after the initial melting. Thus, a series of additional experiments were performed by heating samples to 180 °C for 1 min in glass jars under a nitrogen purge with manual mixing to observe the effect of mixing on the inclusion efficiencies of 1:1, 2:1 and 3:1 βCD:NA mixtures. In addition, two more sets of DSC experiments were conducted in which the samples were heated and held above the melting temperature of NA, to 165 °C for 60 and 120 min to further observe whether kinetics were limiting the extent of inclusion complex formation. A lower temperature was chosen since these long exposure times to 180 °C were found to lead to some changes in the thermal behavior of NA, including doublet melting and recrystallization peaks. These changes were suspected to be due to changes in the crystalline form of NA from 60 to 120 min exposures to high temperatures rather than thermal degradation, since changes in FTIR spectra were not observed. No changes were observed in NA samples exposed to 180 °C for only 1 min, however.
Figure 9 shows the cooling and reheating scans for 1:1, 2:1 and 3:1 βCD:NA mixtures exposed to 165 °C for 60 min and compares the data with the samples exposed to 180 °C for 1 min. The recrystallization and remelting peaks are clearly less intense for samples exposed to 165 °C for 60 min when compared to the same molar ratio mixture exposed to 180 °C for 1 min.
Table 2 compares the results of the three different DSC experiments and the mixing experiments. The 120 min experiment was not carried out using the 3:1 mixture, since 100 % inclusion was achieved in the 60 min experiment. The results show no significant difference in inclusion efficiencies achieved with mixing versus samples prepared in DSC experiments held at 180 °C for 1 min, indicating that inclusion is not necessarily limited due to absence of mixing in the static DSC experiments. However, a distinct influence of hold time above melting temperature is observed. Inclusions of 57, 90 and 100 % were achieved with 1:1, 2:1 and 3:1 molar ratio βCD:NA samples exposed to 165 °C for 60 min in the DSC. Furthermore, a hold time of 120 min yielded 57 and 95 % inclusion efficiencies for 1:1 and 2:1 molar ratio samples. Inclusions achieved in 60 min and 120 min experiments for 1:1 and 2:1 molar ratio mixtures are within the same margin of error. Thus, exposures longer than 60 min do not seem to promote complex formation beyond the improved efficiency observed between 1 min and 60 min exposures. Figure 10, which compares the heat of remelting after different hold times above the melting temperature, shows the approach to essentially zero above 2:1 molar ratio of CD to NA. In addition, comparison of the FTIR spectra shown in Fig. 11 for pure components and samples recovered from DSC experiments shows the further reduction in Naproxen key peak intensities in samples held at 165 °C for 60 min.
At first sight, a 2:1 stoichiometry seems to be suggested by the data since, as Fig. 10 shows, 90 % inclusion was achieved with those physical mixtures. However, if an ideal stoichiometry of 2:1 existed for βCD:NA complexes, inclusions efficiencies greater than 50 % would not be possible in 1:1 mixtures. For the 1:1 mixture, a 60 min hold time resulted in a higher inclusion efficiency of 57 %, which did not improve further with hold time being increased to 120 min. It is possible that under efficient mixing conditions, inclusion efficiencies higher than 57 % can be achieved which would be consistent with a 1:1 stoichiometry. Stoichiometric ratios of 1:1 are generally reported for CD:NA inclusion complexes based on solution complexation where transport limitations are less pronounced compared to solid state complexation [1, 12, 14, 15, 22, 24, 25].
The present observations indicate that the inclusion complex formation of βCD:NA mixtures by melting NA in DSC experiments is limited by transport kinetics, and sufficient time is needed for the molten NA to diffuse and enter the βCD cavities to achieve high inclusion efficiencies. This is achieved more readily when an excess of βCD is used, which naturally increases the presence of CD molecules in the close proximity of the drug molecules. The 2:1 βCD:NA molar ratio mixture appears to be a practical ratio needed to achieve a relatively high inclusion efficiency without going to high excess of βCD in forming the inclusion complex from melt without using solvents.
Conclusions
NA was found to form an inclusion complex with βCD upon melting in DSC heating scans, which was indicated by a reduction in NA melting peak intensity in repeat DSC heating scans, as well as by the reductions in NA FTIR spectral absorbance observed in samples recovered from DSC experiments. In addition, a unique exothermic peak was identified in the first DSC heating scans of βCD-NA physical mixtures, which was found to be indicative of inclusion complex formation. In cooling and re-heating scans, the amount of free, uncomplexed NA was found to decrease with an increase in βCD:NA molar ratio, based on heats of recrystallization and re-melting, respectively. Full inclusion of NA was achieved by melting the drug in the presence of excess βCD in 3:1 and higher molar ratios of βCD:NA in physical mixtures in the DSC. However, essentially full inclusion of NA could be achieved with 2:1 molar ratio mixtures by holding at 165 °C for 1 h in DSC experiments.
References
Junquera, E., Aicart, E.: A fluorimetric, potentiometric and conductimetric study of the aqueous solutions of Naproxen and its association with hydroxypropyl-beta-cyclodextrin. Int. J. Pharm. 176(2), 169–178 (1999)
Lombardino, J.G.: Nonsteroidal antiinflammatory drugs. Chemistry and pharmacology of drugs, vol. 5. Wiley, New York (1985)
Kawabata, A.: Prostaglandin E(2) and pain-an update. Biol. Pharm. Bull. 34(8), 1170–1173 (2011)
Carrier, R.L., Miller, L.A., Ahmed, I.: The utility of cyclodextrins for enhancing oral bioavailability. J Control Release 123(2), 78–99 (2007)
Challa, R., Ahuja, A., Ali, J., Khar, R.K.: Cyclodextrins in drug delivery: an updated review. Aaps Pharmscitech 6(2), E329–E357 (2005)
Martin Del Valle, E.M.: Cyclodextrins and their uses: a review. Process. Biochem. 39(9), 1033–1046 (2004)
Loftsson, T., Brewster, M.E.: Pharmaceutical applications of cyclodextrins. 1. Drug solubilization and stabilization. J. Pharm. Sci. USA 85(10), 1017–1025 (1996)
Loftsson, T., Duchene, D.: Cyclodextrins and their pharmaceutical applications. Int. J. Pharm. 329(1–2), 1–11 (2007)
Bender, M.L., Komiyama, M.: Cyclodextrin Chemistry. Reactivity and Structure, vol. 6. Springer, Berlin (1978)
Loftsson, T., Brewster, M.E.: Pharmaceutical applications of cyclodextrins: basic science and product development. J. Pharm. Pharmacol. 62(11), 1607–1621 (2010)
Cyclodextrins in Pharmaceutics, Cosmetics, and Biomedicine: Current and Future Industrial Applications. Wiley, Hoboken (2011)
Sadlej-Sosnowska, N., Kozerski, L., Bednarek, E., Sitkowski, J.: Fluorometric and NMR studies of the naproxen-cyclodextrin inclusion complexes in aqueous solutions. J. Incl. Phenom. Macro. 37(1–4), 383–394 (2000)
Bettinetti, G., Sorrenti, M., Negri, A., Setti, M., Mura, P., Melani, F.: Interaction of Naproxen with alpha-cyclodextrin and its noncyclic analog maltohexaose. Pharmaceut. Res. 16(5), 689–694 (1999)
Wang, J., Warner, I.M.: Studies of the naproxen–beta-cyclodextrin inclusion complex. Microchem. J. 48(2), 229–239 (1993)
Bettinetti, G., Mura, P., Faucci, M.T., Sorrenti, M., Setti, M.: Interaction of Naproxen with noncrystalline acetyl beta- and acetyl gamma-cyclodextrins in the solid and liquid state. Eur. J. Pharm. Sci. 15(1), 21–29 (2002)
Bettinetti, G.P., Sorrenti, M., Rossi, S., Ferrari, F., Mura, P., Faucci, M.T.: Assessment of solid-state interactions of Naproxen with amorphous cyclodextrin derivatives by DSC. J. Pharmaceut. Biomed. 30(4), 1173–1179 (2002)
Junco, S., Casimiro, T., Ribeiro, N., Da Ponte, M.N., Marques, H.M.C.: Optimisation of supercritical carbon dioxide systems for complexation of naproxen: beta-cyclodextrin. J. Incl. Phenom. Macro. 44(1–4), 69–73 (2002)
Moribe, K., Fujito, T., Tozuka, Y., Yamamoto, K.: Solubility-dependent complexation of active pharmaceutical ingredients with trimethyl-beta-cyclodextrin under supercritical fluid condition. J. Incl. Phenom. Macro. 57(1–4), 289–295 (2007)
Mura, P., Bettinetti, G.P., Cirri, M., Maestrelli, F., Sorrenti, M., Catenacci, L.: Solid-state characterization and dissolution properties of naproxen-arginine-hydroxypropyl-beta-cyclodextrin ternary system. Eur. J. Pharm. Biopharm. 59(1), 99–106 (2005)
Machin, R., Isasi, J.R., Velaz, I.: Beta-cyclodextrin hydrogels as potential drug delivery systems. Carbohyd. Polym. 87(3), 2024–2030 (2012)
Junco, S., Casimiro, T., Ribeiro, N., Da Ponte, M.N., Marques, H.C.: A comparative study of naproxen—beta cyclodextrin complexes prepared by conventional methods and using supercritical carbon dioxide. J. Incl. Phenom. Macro. 44(1–4), 117–121 (2002)
Banik, A., Gogoi, P., Saikia, M.D.: Interaction of Naproxen with beta-cyclodextrin and its derivatives/polymer: experimental and molecular modeling studies. J. Incl. Phenom. Macro. 72(3–4), 449–458 (2012)
Mura, P., Maestrelli, F., Cirri, M.: Ternary systems of Naproxen with hydroxypropyl-beta-cyclodextrin and aminoacids. Int. J. Pharm. 260(2), 293–302 (2003)
Ganzagonzalez, A., Vilajato, J.L., Anguianoigea, S., Oteroespinar, F.J., Blancomendez, J.: A proton nuclear-magnetic-resonance study of the inclusion complex of Naproxen with beta-cyclodextrin. Int. J. Pharm. 106(3), 179–185 (1994)
Arancibia, J.A., Escandar, G.M.: Determination of Naproxen in pharmaceutical preparations by room-temperature phosphorescence. A comparative study of several organized media. Analyst 126(6), 917–922 (2001)
Kurkov, S.V., Ukhatskaya, E.V., Loftsson, T.: Drug/cyclodextrin: beyond inclusion complexation. J. Incl. Phenom. Macro. 69(3–4), 297–301 (2011)
Gines, J.M., Arias, M.J., Perez-Martinez, J.I., Moyano, J.R., Morillo, E., Sanchez-Soto, P.J.: Determination of the stoichiometry of 2,3-dichlorophenoxyacetic acid beta-cyclodextrin complexes in solution and in solid state. Thermochim. Acta 321(1–2), 53–58 (1998)
Singh, R., Bharti, N., Madan, J., Hiremath, S.: Characterization of cyclodextrin inclusion complexes—a review. J. Pharm. Sci. Technol. 2(3), 171–183 (2010)
Mura, P., Maestrelli, F., Cirri, M., Furlanetto, S., Pinzauti, S.: Differential scanning calorimetry as an analytical tool in the study of drug-cyclodextrin interactions. J. Therm. Anal. Calorim. 73(2), 635–646 (2003)
Al-Marzouqi, A., Jobe, B., Corti, G., Cirri, M., Mura, P.: Physicochemical characterization of drug-cyclodextrin complexes prepared by supercritical carbon dioxide and by conventional techniques. J. Incl. Phenom. Macro. 57(1–4), 223–231 (2007)
Al-Marzouqi, A.H., Elwy, H.M., Shehadi, I., Adem, A.: Physicochemical properties of antifungal drug-cyclodextrin complexes prepared by supercritical carbon dioxide and by conventional techniques. J. Pharmaceut. Biomed. 49(2), 227–233 (2009)
Banchero, M., Manna, L.: The use of lysine to enhance the supercritical complexation of ketoprofen and cyclodextrins. J. Supercrit. Fluids 67, 76–83 (2012)
Koester, L.S., Xavier, C.R., Mayorga, P., Bassani, V.L.: Influence of beta-cyclodextrin complexation on carbamazepine release from hydroxypropyl methylcellulose matrix tablets. Eur. J. Pharm. Biopharm. 55(1), 85–91 (2003)
Spyriouni, T., Krokidis, X., Economou, I.G.: Thermodynamics of pharmaceuticals: prediction of solubility in pure and mixed solvents with PC-SAFT. Fluid Phase Equilibr. 302(1–2), 331–337 (2011)
Dodziuk, H.: Rigidity versus flexibility. A review of experimental and theoretical studies pertaining to the cyclodextrin nonrigidity. J. Mol. Struct. 614(1–3), 33–45 (2002)
Acknowledgments
This research was funded by the National Science Foundation (CBET #0929978).
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Grandelli, H.E., Stickle, B., Whittington, A. et al. Inclusion complex formation of β-cyclodextrin and Naproxen: a study on exothermic complex formation by differential scanning calorimetry. J Incl Phenom Macrocycl Chem 77, 269–277 (2013). https://doi.org/10.1007/s10847-012-0241-6
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10847-012-0241-6