Abstract
The stabilities of the inclusion compounds of three chemotherapeutic agents, camptothecin (CPT), docetaxel (DOC) and idarubicin (IDA), plus a model compound 1,4-dihydroxyanthraquinone (DHA) with several β-cyclodextrin (β-CD) derivatives were investigated by solubility measurements, isothermal titration microcalorimetry and fluorescence anisotropy measurements. Ionic heptakis-(6-deoxy-6-thioethers) of β-CD were found to exhibit very high binding potentials for these drugs making them to good candidates for advanced drug delivery.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Cyclodextrins (CDs) 1 are 1 → 4 α-linked cyclic oligomers of glucopyranose. Those CDs consisting of 6, 7, and 8 glucose units are produced on an industrial scale and called α-, β- and γ-CD, respectively. CDs are able to include hydrophobic or amphiphilic guest molecules in aqueous solution whereby they recognize both their size and polarity patter [1, 2]. Binding data are available for numerous CD complexes, called inclusion compounds. Inclusion of active substances in CDs gives rise to many applications [3], such as formulation of pharmaceutical drugs [4, 5], and cosmetics [6], as well as chromatographic separations [7, 8]. Applications of CDs and CD derivatives in the pharmaceutical field are especially interesting since they allow solubilization of drugs in water [5, 9]. In this way, CDs can increase the plasma-level of the complexed drugs and thus increase the therapeutic effect. The use of native β-CD is limited by the low solubility in water and the nephrotoxicity especially in parenteral drug delivery [10]. Through chemical modification of the hydroxyl groups it is possible to synthesize CD derivatives with better solubility and lower toxicity, for example hydroxypropyl-β-CD (HPβCD, Encapsin®) [11] and sulfobutyl-β-CD [12] (Captisol®). Furthermore both selectivities and affinities of CDs can be increased by chemical modifications of CDs [13, 14].
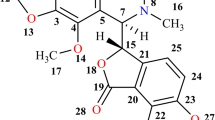
Since many anti-cancer drugs are not sufficiently soluble in water, complexation by CDs appears to be a very promising strategy to improve bioavailability. For instance, camptothecin (CPT) represents an interesting antineoplastic agent against several types of cancer, including colorectal and ovarian cancer, but administration is problematic because of its low solubility and stability [15]. Therefore inclusion of CPT in CDs and CD ethers was already recommended for diminishing these problems [16]. Recently we demonstrated that functional heptakis-(6-deoxy-6-S-thioethers) of β-CD are able to solubilize CPT to a much higher degree [17]. In the following we describe the inclusion and solubilization of other poorly water soluble chemotherapeutics docetaxel (DOC) and idarubicin (IDA), depicted in Fig. 1, by these CD derivatives.
Docetaxel (tradename Taxotére®) is a semi-synthetic analogue of paclitaxel (Taxol). It exerts its cytotoxic efficiency by binding to free tubulin, simultaneously promoting the assembly of tubulin into stable microtubules leading to inhibition of cell division [18, 19]. Because of the extremely low solubility of DOC, its administration requires a formulation with the detergent tween 80 acting as a dispersant. While rather weak solubilization of DOC was observed with native β-CD, already monosubstituted β-CDs and β-CD dimers showed significant solubilization effects [20, 21].
Idarubicin (trade names Zavedos, Idamycin) is a highly toxic anti-neoplastic drug, which is used for the therapy of acute myeloid leukemia. To best of our knowledge, inclusion of IDA in CDs was not previously described. In the following, solubilizations of CPT, DOC and IDA by inclusion in various cationic, neutral and anionic heptakis-(6-S-6-deoxy-thioethers) of β-CD are pointed out in detail. In addition, 1,4-dihydroxy-anthraquinone (DHA) was chosen as a simple model compound for IDA, to investigate whether this moiety is an appropriate binding site for the complexation by β-CD and its derivatives.
Experimental
Materials
The drugs were purchased in pharmaceutical quality from TCI Europe (CPT), AK Scientific (DOC), Sigma–Aldrich (IDA) and Fluka (DHA). The CD derivatives were synthesized as described previously [14, 17]. Hydroxypropyl-β-CD (HPβCD) and randomly methylated β-CD (RAMEB) were supplied from Wacker Chemie AG, Burghausen, Germany.
Measurements
Isothermal titration calorimetry (ITC) was performed at 25 °C with an AutoITC isothermal titration calorimeter (MicroCal Inc., Northampton, USA) as described before [14].
The solubility measurements were performed according to standard procedures [16]. Solutions of CD derivatives (0–6 mM, 5 mL) in water (20 mM HCl (pH = 1.7) in case of CPT) were stirred with an excess of the drug at 25 °C for 18 h. The resulting solutions were filtered through a Teflon (0.25 μm) syringe filter. The concentration of dissolved drug was determined by UV using the extinction coefficients ε listed in Table 1. The solubility of the drug was plotted versus the concentration of the CD derivative (see Fig. 3). The binding constant K was calculated from the solubility of the drug [G]0 without CD and the slope B according to Eq. 1.
Fluorescence intensities I of 15 μM solutions of IDA in 0.1 M saline phosphate buffer pH = 7.4 (PBS) were measured with a Jasco FT 6500 fluorescence spectrometer at an excitation wavelength 480 nm and emission wavelength 570 nm for horizontal (h) and vertical (v) positions of both polarizer and analyzer for various concentration 5–500 μM of the CD derivative at 25 °C as described elsewhere [22]. From the resulting intensity values I hh , I vv , I hv , and I vh the anisotropy r was calculated for each CD concentration according to Eq. 2. The anisotropy r was plotted versus the molar ratio of CD derivative and the drug G. This plot was fitted with the standard equation for a 1:1 complex [23], shown in Eq. 3, with the ratio of the total molar concentrations of CD and drug, x = [CD] t /[G] t and the reduced dissociation constant k = 1/(K[G] t ), by non-linear regression using the program OriginPro 7.5G.
Results and discussion
A series of heptafunctional 6-S-substituted CD derivatives 3–9 (see Fig. 2) was synthesized by nucleophilic displacement reactions of heptakis-(6-iodo-6-deoxy)-β-CD [24] with functional thiols according to published procedures, since these compounds already showed very promising binding constants for other guests and very high aqueous solubilities [14, 17]. The hepta-6-amino derivative 2 and the commercially available statistical derivatives hydroxypropyl-β-CD (HPβCD) and randomly methylated β-CD (RAMEB) were also tested for comparison.
Since compounds DOC and DHA show only a very small solubility in water (see Table 1) the solubility isotherm (phase solubility method) [25] is the best method for the measurement of the binding constants of the corresponding inclusion compounds. A dramatic increase of the solubility of DOC in water was found due to the complexation of DOC by ionic β-CD derivatives 4 and 5 as shown in Fig. 3. Apparent binding constants K were calculated from the initial straight line portion of the phase solubility diagram (Eq. 1, Fig. 3) assuming that a 1:1 complex is initially formed. Since the concentration of CD extend only up to 6 mM, formation of 1:2 complexes (guest:CD) was rather unlikely.
Since IDA hydrochloride is soluble in water, binding constants K were determined by isothermal titration calorimetry (ITC). In all cases 1:1 stoichiometries and high binding constants, listed in Table 2, were found. Since the ITC data for the complexation of IDA within amino derivative 4 were not sufficiently accurate, the binding constant K was determined by fluorescence anisotropy measurements. Fluorescence anisotropy is a highly sensitive method to determine diffusion coefficients and hydrodynamic radii of fluorescent guests. It was already used in a few cases for the determination of complex stabilities [22]. Because of its high sensitivity it becomes favorable as soon as the guest is very costly and toxic like IDA. The anisotropy value r is close to zero for unbound fluorophores in solution due to rapid rotational diffusion. Complexation of the fluorophore by CD slows it down leading to an increase of r. We observed, that fluorescence anisotropy of IDA indeed increased with increasing concentration of 4, as shown in Fig. 4. The dependence of r on the concentration of CD was fitted by Eq. 3, appropriate for 1:1 complex formation [23], leading to a binding constant K = 9,739 M−1.
The data in Table 2 clearly demonstrate the superior binding constants of the heptakis-6-thio-ethers 3–9 compared to the ones of native β-CD, and the heptakis-6-amino derivative 2 and the ethers HPβCD and RAMEB. The binding constants were found to be one or two orders of magnitude higher than the binding constants of the reference compounds. This increase was attributed to the higher hydrophobicity of sulfur atoms compared to oxygen and nitrogen atoms. These 7 sulfur atoms elongate the hydrophobic cavity of β-CD. The binding constant K becomes higher the more methylene groups are attached to the CD cavity for the same reason. On the other hand, branching of the attached alkyl chain leads to a reduction of the binding constant, as shown for host 9. These bulky substituents might hinder the guest entering the cavity from the primary side.
The four guests of this investigation have similar hydrophobic binding sites in common. In all cases benzene moieties are prone for complexation by β-CD rings, since they are only mono-substituted (DOC) or ortho-disubstituted (CPT, DHA, IDA). CPT shows the lowest binding constants in Table 2 since the highly hydrophilic quinoline nitrogen, protonated under the experimental conditions (pH = 1.7) appears to disturb inclusion compound formation. The exceptionally high binding constants for the model compound DHA are attributed to the absence of any charged group in the molecule. Also the binding constant of DOC in the hepta-cystaminyl derivative 4 is significantly high coming close to the binding constant of a β-CD dimer (K = 150,000 M−1) [21].
Since IDA is a cationic guest it behaves differently from its model compound DHA. On one hand, the anionic hosts 5, 6, 7 and 9 perform better than for DHA due to Coulomb attraction between host and guest. On the other hand the cationic host 4 performs worse because of Coulomb repulsion. Less pronounced differences between binding of DHA and IDA were found for the neutral hosts 3 and 8. Similar recognition effects caused by Coulomb interactions between hosts and guests, so-called salt bridges, were already described in the past [14, 26].
Conclusion
Ionic heptakis-(6-deoxy-6-thioethers) of β-CD are well suited for both the complexation and solubilization of anti-cancer drugs such as camptothecin, docetaxel and idarubicin. The high binding abilities of these β-CD derivatives were attributed to the hydrophobicity of the seven sulfur atoms which elongate the hydrophobic cavity of β-CD. So-called salt bridges between oppositely charged host and guest further stabilize these inclusion compounds significantly.
Abbreviations
- CD:
-
Cyclodextrin
- CPT:
-
Camptothecin
- DHA:
-
1, 4-Dihydroxyanthraquinone
- DOC:
-
Docetaxel
- HPβCD:
-
Hydroxypropyl-β-cylodextrin
- IDA:
-
Idarubicin
- ITC:
-
Isothermal titration calorimetry
References
Wenz, G.: Cyclodextrins as building blocks for supramolecular structures and functional units. Angew. Chem., Int. Ed. 33, 803–822 (1994)
Müller, A., Wenz, G.: Thickness recognition of bola-amphiphiles by α-cyclodextrin. Chem. Eur. J. 13, 2218–2223 (2007)
Szejtli, J.: Introduction and general overview of cyclodextrin chemistry. Chem. Rev. 98, 1743–1753 (1998)
Wenz, G.: An overview of host–guest chemistry and its application to nonsteroidal anti-inflammatory drugs. Clin. Drug Investig. 19(Suppl 2), 21–25 (2000)
Davis, M.E., Brewster, M.E.: Cyclodextrin-based pharmaceutics: past, present and future. Nat. Rev. Drug Discov. 3, 1023–1035 (2004)
Holland, L., Rizzi, G., Malton, P.: Cosmetic compositions containing cyclic oligosaccharides for long-lasting fragrances. Patent WO 2000067717. The Procter and Gamble Company. PCT Int. Appl., 2000
Desiderio, C., Fanali, S.: Use of negatively charged sulfobutyl ether-β-cyclodextrin for enantiomeric separation by capillary electrophoresis. J. Chromatogr. A. 716, 183–196 (1995)
Mikus, P., Kaniansky, D., Fanali, S.: Separation of multicomponent mixtures of 2,4-dinitrophenyl labelled amino acids and their enantiomers by capillary zone electrophoresis. Electrophoresis 22, 470–477 (2001)
Connors, K.A.: The stability of cyclodextrin complexes in solution. Chem. Rev. 97, 1325–1357 (1997)
Frijlink, H.W., Eissens, A.C., Hefting, N.R., Poelstra, K., Lerk, C.F., Meijer, D.K.F.: The effect of parenterally administered cyclodextrins on cholesterol level in the rat. Pharm. Res. 8, 9–16 (1991)
Irie, T., Uekama, K.: Pharmaceutical applications of cyclodextrins. 3. Toxicological issues and safety evaluation. J. Pharm. Sci. 86, 147–162 (1997)
Stella, V.J., Rajewski, R.A.: Cyclodextrins: their future in drug formulation and delivery. Pharm. Res. 14, 556–567 (1997)
Kitae, T., Nakayama, T., Kano, K.: Chiral recognition of α-amino acids by charged cyclodextrins through cooperative effects of coulomb interaction and inclusion. J. Chem. Soc., Perkin Trans. 2, 207–212 (1998)
Wenz, G., Strassnig, C., Thiele, C., Engelke, A., Morgenstern, B., Hegetschweiler, K.: Recognition of ionic guests by ionic b-cyclodextrin derivatives. Chem. Eur. J. 14, 7202–7211 (2008)
Lundberg, B.B.: Biologically active camptothecin derivatives for incorporation into liposome bilayers and lipid emulsions. Anti-Cancer Drug Des. 13, 453 (1998)
Kang, J., Kumar, V., Yang, D., Chowdhury, P.R., Hohl, R.J.: Cyclodextrin complexation: influence on the solubility, stability, and cytotoxicity of camptothecin, an antineoplastic agent. Eur. J. Pharm. Sci. 15, 163–170 (2002)
Steffen, A., Thiele, C., Tietze, S., Strassnig, C., Kämper, A., Lengauer, T., Wenz, G., Apostolakis, J.: Improved cyclodextrin based receptors for camptothecin by inverse virtual screening. Chem. Eur. J. 13, 6801–6809 (2007)
Lyseng-Williamson, K.A., Fenton, C.: Docetaxel—a review of its use in metastatic breast cancer. Drugs 65, 2513–2531 (2005)
Figitt, D.P., Wiseman, L.R.: Docetaxel—an update of its use in advanced breast cancer. Drugs 59, 621–651 (2000)
Defaye, J., Ortiz-Mellet, C., Fernandez, J.M.G., Maciejewski, S.: Thioureido-β-cyclodextrins as molecular carriers for the anticancer drug taxotere. In: Coleman, A.W. (ed.) Proceedings of the Ninth International Symposium on Molecular Recognition and Inclusion, pp. 313–316. Kluwer Acad. (1998)
Benito, J.M., Gómez-García, M., Mellet, C.O., Baussanne, I., Defaye, J., García Fernández, J.M.: Optimizing saccharide-directed molecular delivery to biological receptors: design, synthesis, and biological evaluation of glycodendrimer-cyclodextrin conjugates. J. Am. Chem. Soc. 126, 10355–10363 (2004)
Di Marino, A., Rubio, L., Mendicuti, F.: Fluorescence and molecular mechanics of 1-methyl naphthalenecarboxylate/cyclodextrin complexes in aqueous medium. J. Incl. Phenom. Macrocycl. Chem. 58, 103–114 (2007)
Hirose, K.: A practical guide for the determination of binding constants. J. Incl. Phenom. Macrocycl. Chem. 39, 193–209 (2001)
Chmurski, K., Defaye, J.: An improved synthesis of per(6-deoxyhalo)cyclodextrin using N-halosuccinimides-triphenylphosphine in DMF. Supramol. Chem. 12, 221–224 (2000)
Loftsson, T., Hreinsdottir, D., Masson, M.: Evaluation of cyclodextrin solubilization of drugs. Int. J. Pharm. 302, 18–28 (2005)
Schneider, H.-J.: Binding mechanisms in supramolecular complexes. Angew. Chem., Int. Ed. 48, 3924–3977 (2009)
Acknowledgements
The authors thank R. Heisel and A. Engelke for technical assistance and the Federal Ministry of Education and Research of Germany (Project Number 13N9133) for financial support.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Thiele, C., Auerbach, D., Jung, G. et al. Inclusion of chemotherapeutic agents in substituted β-cyclodextrin derivatives. J Incl Phenom Macrocycl Chem 69, 303–307 (2011). https://doi.org/10.1007/s10847-010-9741-4
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10847-010-9741-4