Abstract
Calix[4]arene based podands 1a of cone conformation and 1b of 1,3-alternate conformation possessing imine units and bearing anthracene moieties have been synthesized by a 1 + 2 Schiff base condensation in good yields and examined for their cation recognition abilities towards cations such as lithium, sodium, potassium, nickel, cadmium, copper, zinc, lead, silver and mercury ions by UV–vis and fluorescence spectroscopy. The calix[4]arene derivative 1b shows a selective fluorescence enhancement in presence of Cu2+ ions among the various metal ions tested (Li+, Na+, K+, Ni2+, Cd2+, Cu2+, Zn2+, Pb2+, Ag+ and Hg2+ ions). The colour of the solution changes from colourless to light yellow in the presence of Cu2+ ions. The stoichiometry of the complex formed between 1b and Cu2+ was found to be 1:1 as established by Job’s plot.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Recently, there has been a lot of interest in the design and synthesis of functional molecules having optical sensing ability for different types of analytes [1–4]. Thus, a large number of optical sensors have been developed for the alkali and alkaline earth metal ions [5–7]. However, there are, relatively few examples of designed optical sensors for the copper, which is the third most abundant element (after Fe2+ and Zn2+) amongst essential heavy metal ions present in human body. The selective signaling of copper ion is a very important topic for the detection and treatment of this ion in various chemical systems including living systems [8–11]. It plays an important role in fundamental physiological processes in organisms ranging from bacteria to mammals [8, 9]. However, copper can be toxic if the level exceeds cellular needs. It is also capable of displacing other metals which act as co-factors in enzyme-catalysed reactions [10, 11]. Thus, designing sensors for copper has recently drawn worldwide attention. In most of the fluorescent sensors reported for copper so far, the binding of the copper with the ionophore results in nonspecific fluorescence quenching via photo-induced electron transfer [12–20], however, fluoroionophores which undergo fluorescence enhancement as a result of metal-ion binding are preferred over those which show fluorescence quenching. Recently, some examples of fluoroionophores which undergo fluorescence enhancement upon binding with copper have been reported [21–23].
Calix[4]arenes with appropriately appended groups have been good candidates for cation and anion sensing [24–31]. There are some examples of calixarene based fluoroionophores [32] selective for alkali metal ions [33–42], Tl+ [43], Hg2+ [44–46], Pb2+ [32, 47, 48], Co2+ [49], Ni2+ [49], Cd2+ [50], and Zn2+ [51, 52], respectively. Apart from this Liu et al. [53] reported a calix[4]arene based receptor with imino anthracene appendage on the upper rim which undergoes fluorescence enhancement in the presence of Ca2+ ions. Gao et al. [54] reported an upper rim calix[4]arene salicylidene derivative as an effective fluorogenic sensor for Cu2+ ions. From our laboratory, we have also reported a number of calix[4]arene [55–57] and thiacalix[4]arene [58–60] based receptors possessing imine units at the lower rim which selectively interact with silver ions. Recently, Rao et al. reported calixpodands with imine units by condensation of O,O′-bis(2-aminoethyl)-p-tert-butylcalix[4]arene with 2-hydroxy-1-naphthaldehyde [51] and with 9-anthraldehyde [61]. It was found that these receptors bound Zn2+ ions by imino nitrogens and by the hydroxyl groups of the naphthalene moiety, while Cu2+ ions bind through imino nitrogens and through alkoxyphenyl oxygens, however, Fe2+ ion binds through the phenolic oxygens apart from the imino nitrogens and the alkoxyphenyl oxygens. Based on these reports, we planned to change the coordination environment by employing O,O″-bis(2-aminoethoxy)-O′,O′′′-dipropoxy-p-tert-butylcalix[4]arene 2 of cone and 4 of 1,3-alternate conformation as a molecular scaffold for the preparation of calix[4]podands 1a and 1b, respectively. Preliminary studies on the complexation abilities of these calixpodands have shown that the modification actually changes the coordination sites and that calixpodand 1b of 1,3-alternate conformation has selective fluorescent enhancement in the presence of Cu2+ ions, while calixpodand 1a of cone conformation shows indiscriminate fluorescent enhancement in presence of all metal ions tested. While this work was in progress, Li et al. [62] reported a fluorescent sensor for Cu2+ ion based on calix[4]arene bearing imine units on the upper rim.
Result and discussions
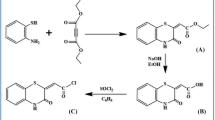
Calix[4]arene based imino receptors 1a (Scheme 1) and 1b (Scheme 2) were synthesized from known precursors 2 [63] and 4 [64], respectively. Condensation of calix-1,3-diamine 2 [63] of cone conformation, with 2.2 mol equiv of 9-anthraldehyde, 3, in refluxing ethanol gave calixpodand 1a in 52% yield (Scheme 1).
Similarly, condensation of calix-1,3-diamine 4 [64] of 1,3-alternate conformation with 2.0 mol equiv of 9-anthraldehyde, 3, in refluxing CH2Cl2/MeOH (1:2) gave calixpodand 1b in 82% yield (Scheme 2). Both the products 1a–b separated out as pure solids, and gave satisfactory elemental analysis after single crystallization.
The structures of compounds 1a–b were confirmed from their spectroscopic and analytical data. The IR spectra of compounds 1a and 1b showed characteristic C=N stretching bands at 1,632 and 1,624 cm−1, respectively. The FAB mass spectra of these compounds showed parent ion peaks corresponding to 1:2 condensation products. The 1H NMR spectra of compounds 1a–b showed two singlets (18H each) corresponding to tert-butyl protons, triplets (4H each) corresponding to OCH2 protons, two singlets (4H each) corresponding to aromatic protons and one singlet (2H) for the imino protons. The bridging methylene protons of compound 1a appear as a AB quartet separated by Δδ > 0.9 ppm. The 1H NMR data suggests a C 2v-symmetric structure that is cone conformation for compound 1a. In the 13C NMR spectrum of compound 1b, all the bridging methylene carbons are equivalent, giving a singlet at 39.2 ppm suggesting a C 2v-symmetric structure that is 1,3-alternate conformation for compound 1b [65].
The cation binding properties of compounds 1a and 1b were investigated by UV–Vis and fluorescence spectroscopy. The titration experiments were carried out in CH2Cl2: CH3CN (1:1 v/v) by adding aliquots of different metal ions. The UV–Vis absorption spectrum of the compound 1b (1 × 10−5 M) exhibits typical anthracenyl absorption bands at λmax 350, 369 and 387 nm, respectively (Fig. 1).
Upon addition of increasing amounts of Cu2+ ions (0–100 equiv) to a solution of 1b, the absorption peak due to the anthracenyl moiety decreases while a new peak gradually moving to longer wavelength finally reaching a maximum value at 451 nm is appeared with an isosbestic point at 405 nm indicating a well defined 1b–Cu 2+ complex (Fig. 1). The colour of the solution of 1b changes from colourless to pale yellow in presence of Cu2+ ions (inset of Fig. 1).
In the fluorescence spectra, compounds 1a and 1b exhibited a very weak emission from locally excited lowest (π → π*) state in CH2Cl2/CH3CN (1:1) as compared to the simple anthracene. This weak emission from these compounds is due to photoinduced electron transfer (PET) from the lone pair of imine nitrogen to the photo-excited anthracene which leads to fluorescence quenching. Upon addition of Cu2+ ions to solution of receptor 1b, a significant fluorescence enhancement was observed (Fig. 2). Under the same conditions as above no significant fluorescence changes (Fig. 2) were observed for other tested metal ions (Li+, Na+, K+, Ni2+, Cd2+, Zn2+, Pb2+, Hg2+, Ag+). These observations indicate that the compound 1b has selectivity for Cu2+ ions.
However, upon addition of different metal ions (Li+, Na+, K+, Ni2+, Cd2+, Cu2+, Zn2+, Pb2+, Hg2+, Ag+) to the solution of receptor 1a, no selective fluorescence enhancement was observed for any metal ion (Fig. 3).
The fluorescence titrations of compound 1b were then performed with Cu2+ ions (Fig. 4). The addition of increasing amounts of Cu2+ ions to the solution of receptor 1b showed a 10-fold fluorescence enhancement in the anthracenyl triplet of receptor 1b, centered at 437 nm (Fig. 4). This is due to the fact that when Cu2+ ions are added to the receptor 1b, the lone pair of electrons on nitrogen gets involved in the coordination with the Cu2+ ion. This leads to the decrease in the electron density on nitrogen atom as a result of which the electron transfer from the nitrogen to the photoexcited anthracene moiety is suppressed and hence allows the fluorescence emission. Similar reports exist for imine based fluorogenic receptors, where the signaling mechanism is through photoinduced electron transfer (PET) in particular, for Cu2+ ions [22, 53, 61, 62]. Fitting the changes in fluorescence spectra of compound 1b with Cu2+ ions, using the nonlinear regression analysis program SPECFIT [66] gave good fit and demonstrated that 1:1 stoichiometry (Host: Guest) is the most stable species in the solution withbinding constant log β11 = 4.83 (M−1). Similarly, fitting the changes in fluorescence spectra of compounds 1a–b with various other metal ions, using the nonlinear regression analysis program SPECFIT [66] gave good fit with 1:1 (Host: Guest) stoichiometry. The stability constants determined for both receptors 1a–b with various metal ions are summarized in Table 1. These values indicate that Cu2+ preferentially binds to compound 1b though other metal ions also bind with 1b but with lower binding constants. For compound 1a, there is no preferential binding for any metal ion.
In order to determine the stoichiometry of the 1b–Cu2+ complex, the method of continuous variation (Job’s plot) was also used. The total concentration of the receptor 1b and Cu2+ was kept constant (2.5 × 10−5 M), with a continuous variable molar fraction of guest ([Cu2+]/[1b] + [Cu2+]). Figure 5 shows the Job’s plot of compound 1b with Cu2+ at 437 nm. The 1b–Cu2+ complex concentration approaches a maximum when the molar fraction of Cu2+ is 0.5, which means 1b and Cu2+ formed a 1:1 (Host: Guest) complex.
Further, to test the practical applicability of compound 1b as a Cu2+-selective fluorescence sensor, competitive experiments were carried out in the presence of Cu2+ ions at 1 × 10−3 M mixed with Li+, Na+, K+, Cd2+, Ni2+, Zn2+, Pb2+,Hg2+, and Ag+ at 1 × 10−3 M, and as shown in Fig. 6, no significant variation in the fluorescence was found by comparison with that without the other metal ions besides Cu2+ ion except Ni2+, Zn2+, Hg2+, and Pb2+ which slightly interfere leading to a quenching of fluorescence due to the 1b–Cu2+ complex.
Conclusions
Thus, we have synthesized new calix[4]arene based receptors 1a and 1b by simple condesation of 5,11,17,23-Tetra-tert-butyl-25,27-bis(2-aminoethoxy)-26,28-dipropoxy-calix[4]arene of cone and 1,3-alternate conformation with 9-anthraldehyde, respectively. Receptor 1b selectively recognizes copper among the different metal ions tested. A colour change from colourless to light yellow was observed by naked eye when receptor 1b was treated with copper ions.
Experimental
General methods and instrumentation
All reagents were purchased from Aldrich and used without further purification. CH3CN was dried over P2O5 and with K2CO3 and kept over molecular sieves overnight before use. Fluorescence spectra were recorded on SHIMADZU RF-5301 spectrofluorimeter. UV–vis Spectra were recorded on SHIMADZU UV-2450 spectrophotometer, with a quartz cuvette (path length: 1.0 cm). The cell holder was thermostatted at 25 °C. 1H and 13C NMR spectra were recorded on JEOL-FT NMR-AL 300 MHz spectrophotometer using CDCl3 as solvent and TMS as internal standards. Solutions of compound 1a–b and various metal perchlorates (Li+, Na+, K+, Ni2+, Cd2+, Cu2+, Zn2+, Pb2+, Hg2+, Ag+) for UV–vis and fluorescence studies were prepared in CH2Cl2 and CH3CN AR grade. All spectrophotometric titration curves were fitted with SPECFIT\32 software.
Synthesis of receptor 1a
To a stirred solution of diamine 2 (409 mg, 0.50 mmol) in ethanol (25 mL) was added a solution of 9-anthraldehyde (227 mg, 1.10 mmol) in ethanol (10 mL). The reaction mixture was refluxed for 6 h to separate a solid, which was filtered, washed with ethanol and recrystallised from dichloromethane/methanol. Yield (412 mg, 52%), mp 178 °C; IR νmax (KBr, cm−1) 1,632 (C=N); 1H NMR (CDCl3, 300 MHz) δ (ppm): 0.92 (s, 18H, C(CH3)3), 1.09 (t, J = 7.5 Hz, 6H, CH3), 1.32 (s, 18H, C(CH3)3), 2.15–2.20 (m, 4H, CH2), 3.25 (d, J = 12.6 Hz, 4H, ArCH2Ar), 3.82 (t, J = 7.5 Hz, 4H, OCH2), 4.58–4.67 (m, 8H, OCH2, ArCH2Ar), 4.82 (t, J = 8.1 Hz, 4H, NCH2), 6.60 (s, 4H, ArH), 7.08–7.12 (m, 6H, ArH), 7.21–7.25 (m, 6H, ArH), 7.78 (d, J = 8.7 Hz, 4H, ArH), 8.21 (s, 2H, CH=N), 8.35 (d, J = 8.7 Hz, 4H, ArH), 9.56 (s, 2H, ArH); 13C NMR (75 MHz, CDCl3) : 10.9 (CH3), 23.9 (CH2), 31.3 (C(CH3)3), 31.4 (ArCH2Ar), 31.7 (C(CH3)3), 33.7 (C(CH3)3), 34.1 (C(CH3)3), 62.5 (OCH2), 74.0 (OCH2), 77.6 (NCH2), 122.2 (ArC), 124.5 (ArC), 124.7 (ArC), 125.5 (ArC), 126.2 (ArC), 127.0 (ArC), 128.1 (ArC), 130.8 (ArC), 132.5 (ArC), 135.2 (ArC), 140.0 (ArC), 145.0 (ArC), 154.3 (ArCO), 157.2 (ArCO), 160.9 (CH=N); FAB-MS (m/z) 1196 (M + 1)+; EA Calcd for C84H94N2O4: C, 84.38; H, 7.92; N, 2.34%. Found: C, 84.29; H, 7.62; N, 2.45%.
Synthesis of receptor 1b
To a stirred solution of diamine 4 (204, 0.25 mmol) in CH2Cl2 (5 mL)/MeOH (5 mL) was added a solution of 9-anthraldehyde (114 mg, 0.55 mmol) in methanol (5 mL). The reaction mixture was refluxed for 6 h to separate a solid, which was filtered and washed with ethanol. The yellow solid obtained was further recrystallized from dichloromethane/methanol. Yield (245 mg, 82%), mp 263 °C; IR νmax (KBr, cm−1) 1624 (C=N); 1H NMR (CDCl3, 300 MHz) δ (ppm): 0.68 (t, J = 7.5 Hz, 6H, CH3), 1.05–1.13 (m, 4H, CH2), 1.31 (s, 18H, C(CH3)3), 1.37 (s, 18H, C(CH3)3), 3.41 (t, J = 7.5 Hz, 4H, OCH2), 3.67 (t, J = 7.5 Hz, 4H, OCH2), 3.90–4.05 (m, 12H, NCH2, ArCH2Ar), 7.05 (s, 4H, ArH), 7.26 (s, 4H, ArH), 7.34–7.45 (m, 8H, ArH), 7.99 (d, J = 7.8 Hz, 4H, ArH), 8.41 (d, J = 7.8 Hz, 4H, ArH), 8.48 (s, 2H, CH=N), 9.44 (s, 2H, ArH); 13C NMR (75 MHz, CDCl3) : 10.1 (CH3), 22.4 (CH2), 31.6 (C(CH3)3), 32.0 (C(CH3)3) 34.0 (C(CH3)3), 34.2 (C(CH3)3), 39.2 (ArCH2Ar), 61.6 (OCH2), 69.0 (OCH2), 71.7 (NCH2), 124.7 (ArC), 125.2 (ArC), 125.8 (ArC), 125.9 (ArC), 126.7 (ArC), 128.2 (ArC), 128.8 (ArC), 129.9 (ArC), 131.3 (ArC), 133.4 (ArC), 144.0 (ArC), 144.1 (ArC), 154.3 (ArCO), 154.9 (ArCO), 161.1 (CH=N); FAB-MS (m/z) 1196 (M + 1)+; EA Calcd for C84H94N2O4: C, 84.38; H, 7.92; N, 2.34%. Found: C, 83.78; H, 7.59; N, 2.42%.
UV–Vis and fluorescence studies
The stock solution of ligands 1a–b and metal perchlorates were prepared in CH2Cl2/CH3CN (1:1, v/v). The concentrations of the solutions were maintained at 1 × 10−5 M. The fluorescence experiments were carried out on a SHIMADZU RF-5301 PC. The samples were excited at 350 nm in a 1.0 cm quartz cell. The slit width was set at 3 nm (Excitation and Emission). Absorption spectra of the solutions were measured on Shimadzu UV-2450PC in the range of 250–600 nm with a slit width of 1.0 nm. Job’s plot experiment was carried out using fluorescence, by keeping the total concentration [1b] + [Cu 2+] = 2.5 × 10−5 M.
References
Bissell, R.A., de Silva, A.P., Gunaratne, H.Q.N., Lynch, P.L.M., Maguire, G.E.M., Sandanayake, K.R.A.S.: Molecular fluorescent signalling with ‘fluor–spacer–receptor’ systems: approaches to sensing and switching devices via supramolecular photophysics. Chem. Soc. Rev. 21, 187–195 (1992)
de Silva, A.P., Gunaratne, H.Q.N., Gunnlaugsson, T., Huxley, A.J.M., McCoy, C.P., Rademacher, J.T., Rice, T.E.: Signaling recognition events with fluorescent sensors and switches. Chem. Rev. 97, 1515–1566 (1997)
Prodi, L., Bolletta, F., Montalti, M., Zaccheroni, N.: Luminescent chemosensors for transition metal ions. Coord. Chem. Rev. 205, 59–83 (2000)
de Silva, A.P., Fox, D.B., Huxley, A.J.M., Moody, T.S.: Combining luminescence, coordination and electron transfer for signalling purposes. Coord. Chem. Rev. 205, 41–57 (2000)
Desvergne, J.P., Czarnik, A.W., (eds.): Chemosensors for Ions and Molecular Recognition, vol. 492. NATO ASI Series; Kluwer Academic: Dordrecht, The Netherlands (1997)
Valeur, B., Leray, I.: Design principles of fluorescent molecular sensors for cation recognition. Coord. Chem. Rev. 205, 3–40 (2000)
Fabbrizzi, L., Poggi, A.: Sensors and switches from supramolecular chemistry. Chem. Soc. Rev. 24, 197–202 (1995)
Linder, M.C., Hazegh-Azam, M.: Copper biochemistry and molecular biology. Am. J. Clin. Nutr. 63, 797S–811S (1996)
Uauy, R., Olivares, M., Gonzalez, M.: Essentiality of copper in humans. Am. J. Clin. Nutr. 67, 952S–959S (1998)
Harris, Z.L., Gitlin, J.D.: Genetics and molecular basics for molecular toxicity. Am. J. Clin. Nutr. 63, 836S–845S (1996)
Scheinberg, I.H., Sternlieb, I.: Wilson disease and idiopathic copper toxicosis. Am. J. Clin. Nutr. 63, 842S–845S (1996)
Shnek, D.R., Pack, D.W., Arnold, F.H., Sasaki, D.Y.: Metal-induced dispersion of lipid aggregates: A simple, selective, and sensitive fluorescent metal ion sensor. Angew. Chem. Int. Ed. Engl. 34, 905–907 (1995)
Torrado, A., Walkup, G.K., Imperiali, B.: Exploiting polypeptide motifs for the design of selective Cu(II) ion chemosensors. J. Am. Chem. Soc. 120, 609–610 (1998)
Grandini, P., Mancin, F., Tecilla, P., Scrimin, P., Tonellato, U.: Exploiting the self-assembly strategy for the design of selective CuII ion chemosensors. Angew. Chem. Int. Ed. Engl. 38, 3061–3064 (1999)
Klein, G., Kaufmann, D., Schürch, S., Reymond, J.-L.: A fluorescent metal sensor based on macrocyclic chelation. Chem. Commun. 561–562 (2001)
Zheng, Y., Huo, Q., Kele, P., Andreopoulos, F.M., Pham, S.M., Leblanc, R.M.: A new fluorescent chemosensor for copper ions based on tripeptide Glycyl-Histidyl-Lysine (GHK). Org. Lett. 3, 3277–3280 (2001)
Zheng, Y., Gattàs-Asfura, K.M., Konka, V., Leblanc, R.M.: A dansylated peptide for the selective detection of copper ions. Chem. Commun. 2350–2351 (2002)
Zheng, Y., Orbulescu, J., Ji, X., Andreopoulos, F.M., Pham, S.M., Leblanc, R.M.: Development of fluorescent film sensors for the detection of divalent copper. J. Am. Chem. Soc. 125, 2680–2686 (2003)
Boiocchi, M., Fabbrizzi, L., Licchelli, M., Sacchi, D., Vazquez, M., Zampa, C.: A two-channel molecular dosimeter for the optical detection of copper(II). Chem. Commun. 1812–1813 (2003)
Roy, B.C., Chandra, B., Hromas, D., Mallik, S.: Synthesis of new, pyrene-containing, metal-chelating lipids and sensing of cupric ions. Org. Lett. 5, 11–14 (2003)
Ghosh, P., Bharadwaj, P.K., Roy, J., Ghosh, S.: Transition metal (II)/(III), Eu(III), and Tb(III) ions induced molecular photonic OR gates using trianthryl cryptands of varying cavity dimension. J. Am. Chem. Soc. 119, 11903–11909 (1997)
Martinez, R., Zapata, F., Caballero, A., Espinosa, A., Tarraga, A., Molina, P.: 2-Aza-1,3-butadiene derivatives featuring an anthracene or pyrene unit: Highly selective colorimetric and fluorescent signaling of Cu2 + cation. Org. Lett. 8, 3235–3238 (2006)
Wen, Z.-C., Yang, R., He, H., Jiang, Y.-B.: A highly selective charge transfer fluoroionophore for Cu2+. Chem. Commun. 106–108 (2006)
Gutsche, C.D.: Calixarenes. Royal Society of Chemistry, Cambridge, UK (1989)
Casnati, A., Ungaro, R., Asfari, Z., Vicens, J.: Crown ethers derived from calix[4]arenes. In: Asfari, Z., Bohmer, V., Harrowfield, J., Vicens, J. (eds.) Calixarenes 2001, pp. 365–384. Kluwer Academic, Dordrecht, The Netherlands (2001)
Andreetti, G.D., Ugozzoli, F., Ungaro, R., Pochini, A.: Inclusion of ions and neutral molecules in calixarenes. In: Atwood, J.L., Davies, J.E.D., MacNicol, D.D. (eds.) Inclusion Compounds, vol. 4, pp. 64–125. Oxford University Press: New York (1991)
Bohmer, V.: Calixarenes, Macrocycles with (almost) unlimited possibilities. Angew. Chem. Int. Ed. Engl. 34, 713–745 (1995)
Schimidtchen, F.P., Berger, M.: Artificial organic host molecules for anions. Chem. Rev. 97, 1609–1646 (1997)
Gale, P.A.: Anion receptor chemistry: highlights from 1999. Coord. Chem. Rev. 213, 79–128 (2001)
Gale, P.A.: Anion and ion-pair receptor chemistry: highlights from 2000 and 2001. Coord. Chem. Rev. 240, 191–221 (2003)
Beer, P.D., Gale, P.A.: Anion recognition and sensing: the state of the art and future perspectives. Angew. Chem. Int. Ed. 40, 486–516 (2001)
Kim, J.S., Quang, D.T.: Calixarene derived fluorescent probes. Chem. Rev. 107, 3780–3799 (2007)
Souchon, V., Leray, I., Valeur, B.: Selective detection of cesium by a water-soluble fluorescent molecular sensor based on a calix[4]arene-bis(crown-6-ether). Chem. Commun. 4224–4226 (2006)
Malval, J.-P., Leray, I., Valeur, B.: A highly selective fluorescent molecular sensor for potassium based on a calix[4]bisazacrown bearing boron-dipyrromethene fluorophores. New J. Chem. 29, 1089–1094 (2005)
Jin, T.: A new Na+ sensor based on intramolecular fluorescence energy transfer derived from calix[4]arene. Chem. Commun. 2491–2492 (1999)
Leray, I., O’Reilly, F., Jiwan, J.-L. H., Soumillion, J.-P., Valeur, B.: A new calix[4]arene-based fluorescent sensor for sodium ion. Chem. Commun. 795–796 (1999)
Leray, I., Asfari, Z., Vicens, J., Valeur, B.: Synthesis and binding properties of calix[4]biscrown-based fluorescent molecular sensors for cesium or potassium ions. J. Chem. Soc., Perkin Trans. 2, 1429–1434 (2002)
Kim, H.J., Kim, J.S.: BODIPY appended cone-calix[4]arene: selective fluorescence changes upon Ca2+ binding. Tetrahedron Lett. 47, 7051–7055 (2006)
Bodenant, B., Weil, T., Businelli-Pourcel, M., Fages, F., Barbe, B., Pianet, I., Laguerre, M.: Synthesis and solution structure analysis of a bispyrenyl bishydroxamate calix[4]arene-based receptor, a fluorescent chemosensor for Cu2+ and Ni2+ metal ions. J. Org. Chem. 64, 7034–7039 (1999)
Ji, H.-F., Brown, G.M., Debestani, R.: Calix[4]arene-based Cs+ selective optical sensor. Chem. Commun. 609–610 (1999)
Cha, N.R., Moon, S.Y., Chang, S.-K.: New ON–OFF type Ca2+-selective fluoroionophore having boron–dipyrromethene fluorophores. Tetrahedron Lett. 44, 8265–8268 (2003)
Kim, Y.H., Cha, N.R., Chang, S.-K.: A new fluorogenic benzothiazolyl ionophore based upon calix[4]arene-crown-5 ether for calcium determination in aqueous media. Tetrahedron Lett. 43, 3883–3886 (2002)
Talanova, G.G., Roper, E.D., Buie, N.M., Gorbunova, M.G., Bartsch, R.A., Talanov, V.S.: Novel fluorogenic calix[4]arene-bis(crown-6-ether) for selective recognition of thallium(I). Chem. Commun. 5673–5675 (2005)
Chen, Q.Y., Chen, C.F.: A new Hg2+-selective fluorescent sensor based on a dansyl amide-armed calix[4]-aza-crown. Tetrahedron Lett. 46, 165–168 (2005)
Talanova, G.G., Elkarim, N.S.A., Talanov, V.S., Bartsch, R.A.: A calixarene-based fluorogenic reagent for selective mercury(II) recognition. Anal. Chem. 71, 3106–3109 (1999)
Metiver, R., Leray, I., Lebeau, B., Valeur, B.: A mesoporous silica functionalized by a covalently bound calixarene-based fluoroionophore for selective optical sensing of mercury(II) in water. J. Mater. Chem. 15, 2965–2973 (2005)
Metiver, R., Leray, I., Valeur, B.: Lead and mercury sensing by calixarene-based fluoroionophores bearing two or four dansyl fluorophores. Chem. Eur. J. 10, 4480–4490 (2004)
Metivier, R., Leray, I., Valeur, B.: A highly sensitive and selective fluorescent molecular sensor for Pb(II) based on a calix[4]arene bearing four dansyl groups. Chem. Commun. 996–997 (2003)
Higuchi, Y., Narita, M., Niimi, T., Ogawa, N., Hamada, F., Kumagai, H., Iki, N., Miyano, S., Kabuto, C.: Fluorescent chemosensor for metal cations based on thiacalix[4]arenes modified with dansyl moieties at the lower rim. Tetrahedron 56, 4659–4666 (2000)
Narita, M., Higuchi, Y., Hamada, F., Kumagai, H.: Metal sensor of water soluble dansyl-modified thiacalix[4]arenes. Tetrahedron Lett. 39, 8687–8690 (1998)
Dessingou, J., Joseph, R., Rao, C.P.: A direct fluorescence-on chemo-sensor for selective recognition of Zn(II) by a lower rim 1,3-di-derivative of calix[4]arene possessing bis-{N-(2-hydroxynaphthyl-1-methylimine)} pendants. Tetrahedron Lett. 46, 7967–7971 (2005)
Cao, Y.-D., Zheng, Q.Y., Chen, C.F., Huang, Z.T.: A new fluorescent chemosensor for transition metal cations and on/off molecular switch controlled by pH. Tetrahedron Lett. 44, 4751–4755 (2003)
Liu, H., Xu, Y., Li, B., Yin, G., Xu, Z.: A new highly selective calix[4]arene-based fluorescent probe for Ca2+. Chem. Phys. Lett. 345, 395–399 (2001)
Liang, Z., Liu, Z., Jiang, L., Gao, Y.: A new fluorescent chemosensor for copper(II) and molecular switch controlled by light. Tetrahedron Lett. 48, 1629–1632 (2007)
Kumar, M., Mahajan, R.K., Sharma nee Bhalla, V., Singh, H., Sharma, N., Kaur, I.: Synthesis of new biscalix[4]arenas with imine linkages: a search for new silver-selective sensors. Tetrahedron Lett. 42, 5315–5318 (2001)
Kumar, M., Sharma nee Bhalla, V., Babu, J.N.: Synthesis and binding studies of new bis-calix[4]arenes containing aromatic and heteroaromatic units. Tetrahedron 59, 3267–3273 (2003)
Singh, N., Kumar, M., Hundal, G.: Synthesis, NMR, X-ray structural analyses and complexation studies of new Ag+ selective calix[4]arene based dipodal hosts-a co-complexation of neutral and charged species. Tetrahedron 60, 5393–5405 (2004)
Bhalla, V., Kumar, M., Katagiri, H., Hattori, T., Miyano, S.: Synthesis and binding studies of novel bisthiacalix[4]arenes with diimine linkages. Tetrahedron Lett. 46, 121–124 (2005)
Bhalla, V., Babu, J.N., Kumar, M., Hattori, T., Miyano, S.: Synthesis and binding studies of novel thiacalixpodands and bisthiacalixarenes having O, O”-dialkylated thiacalix[4]arene units of 1,3-alternate conformation. Tetrahedron Lett. 48, 1581–1585 (2007)
Kumar, M., Babu, J.N., Dhir, A., Bhalla, V.: Chromogenic sensing of Cu(II) by imino linked thiacalix[4]arene in aqueous environment. Inorg. Chem. Commun. 12, 332–335 (2009)
Kumar, A., Ali, A., Rao, C.P.: Photo-physical behavior as chemosensor properties of anthracene-anchored 1,3-di-derivatives of lower rim calix[4]arene towards divalent transition metal ions. J. Photochem. Photobio. A 177, 164–169 (2006)
Li, G.-K., Xu, Z.-X., Chen, C.-F., Huang, Z.-T.: A highly efficient and selective turn-on fluorescent sensor for Cu2+ ion based on calix[4]arene bearing four iminoquinoline subunits on the upper rim. Chem. Commun. 1774–1776 (2008)
Babu, J.N., Bhalla, V., Kumar, M., Mahajan, R.K., Puri, R.: Chloride ion recognition using thiourea/urea based receptors incorporated into a 1,3-disubstituted calix[4]arene. New J. Chem. 33, 675 (2009)
Babu, J.N., Bhalla, V., Kumar, M., Singh, H.: Selective colorimetric sensing of cyanide ions over fluoride ions by calix[4]arene containing thiourea moieties. Lett. Org. Chem. 3, 787–793 (2006)
Gutsche, C.D., Dhawan, B., Levine, J.A., No, K.H., Bauer, L.J.: Calixarenes 9: conformational isomers of the ethers and esters of calix[4]arenes. Tetrahedron 39, 409–426 (1983)
Gampp, H., Maeder, M., Meyer, C.J., Zuberbuhler, A.D.: Calculation of equilibrium constants from multiwavelength spectroscopic data—I: mathematical considerations. Talanta 32, 95–101 (1985)
Acknowledgements
We are thankful to CSIR (New Delhi, India) for the financial assistance (research project No. 01(1934)04/EMR-II). JNB is thankful to CSIR for Senior Research Fellowship. We are also thankful to DST (New Delhi, India) for FIST programme, UGC for SAP programme, Guru Nanak Dev University for laboratory facilities and CDRI, Lucknow for FAB mass spectra.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Kumar, M., Nagendra Babu, J. & Bhalla, V. Fluorescent chemosensor for Cu2+ ion based on iminoanthryl appended calix[4]arene. J Incl Phenom Macrocycl Chem 66, 139–145 (2010). https://doi.org/10.1007/s10847-009-9670-2
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10847-009-9670-2