Abstract
The present short paper shows the progress of the calixcrowns chemistry. Calixcrowns which were first investigated for their metal complexation properties are now reaching new fields of research wider than supramolecular chemistry.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
The present paper does not intend to be a review on calixcrowns chemistry but rather shows its progress in research fields wider than supramolecular chemistry. Its subject stems its origin from the last sentence of a recent very informative review article of Salorinne and Nissinen [1]: “The chemistry of calixcrowns has not found its limits so far”. In this article the authors reviewed the synthesis and complexing properties of calixcrowns, calixbiscrowns and related compounds such as resorcinarene crowns. These macrocycles exhibit remarkable ionophoric properties toward alkali and alkaline earth metal cations, as well as, to amines and (alkyl) ammoniums [1].
Besides these studies concerning selective complexation of metal cations and their applications in supramolecular techniques, various functionalised calixarenes have been shown to have “supramolecular” properties, in a wide sense, possibly exploitable in what is the nascent ‘‘nanoworld’’ of the future as for instance metal-tunneling, molecular machines, nanotubes, immobilization of proteins and crystal-to-crystal transformation.
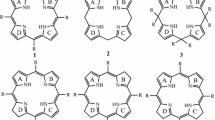
Calix[4]arenes can exist under four discrete forms: cone, partial cone, 1,2-alternate, and 1,3-alternate depending on the topology of the four aromatic rings (Scheme 1).
The calix[4]biscrowns are in the 1,3-alternate conformation which implies the formation of a square aromatic tunnel allowing the communication between the two crown-ether metal-binding sites. It was shown in several cases the oscillation of cations (alkalis and ammonium) through this π-base tunnel (Scheme 2), [2, 3].
This observation has lead to the obvious design of 1,3-alternate calix tubes as cation- [4, 5] and anion- [6, 7] conducting channels through membranes such as phospholipids bilayers or liposomes (Scheme 3).
Using the 1,3-alternate calix[4]crown unit as molecular segments it has been built calix[4]arenes nanotubes by modular synthesis with a metal shuttling through several calix crown units (Scheme 4), [8].
A calix[4]arene nanotube built with calixarenes in the 1,3-alternate conformation [6]
Filling single-walled carbon nanotubes (SWNTs) with foreign guest species is an emerging area of research [9]. In this respect, 1,3-alternate calixarene-based nanomaterials have been prepared with application in sensing, storage and fixation of NOx gases [10, 11]. Molecular machines are in vogue and scattered examples are found in literature of thermally, chemically, electrochemically and/or photochemically labile molecular systems or devices, sensors, logic gates etc. [12–14]. The globular shape of calix[4]arenebiscrowns has been exploited to prepare molecular mappemondes (globes) and gyroscopes (Scheme 5).
Representation of a calix-mappemonde [10]
They are constructed from calix[4]biscrowns-6 held in the arms of a polyether loop via 1 + 1 condensation [15]. Depending on the length of the polyether arm, a 2 + 2 dimer was also isolated, leading to a molecular mill [16]. The spinning of the calix unit can be stopped in the presence of large amounts of ammonium picrate. Dendrimers are an important family of molecules spanning applications in orientated disciplines such as material science and pharmaceutical chemistry and industry [17]. Calixdendrimers [18] some of them being constructed from calixcrowns [19] have been published (Scheme 6).
A calixdendrimer constructed with calixcrowns units [14]
Protein microarrays (protein chip technology) have recently had an explosion of interest due to the advances in proteonomics, robotics, microelectronics, and bioinformatics [20]. Calixcrowns were used to fabricate protein chips for enzyme activity assay, antibody screening and protein–protein interaction, protein–DNA interaction through non-covalent molecular interactions. It has been prepared highly sensitive microarray protein chips, ProteoChips, coated with ProLinker A and ProLinker B (Scheme 7), two novel calixcrown derivatives with a bifunctional coupling property that permit efficient immobilization of captured proteins on solid matrixes and make high-throughput analysis of protein–protein interactions possible.
The analysis of quartz crystal microbalance showed that both monoclonal antibody (mAb) and antigen (Ag) bound to the gold film of the sensor surface coated with ProLinker B and that it is useful for studies of mAb–Ag interactions. ProteoChip, aminated glass slide coated with ProLinker A, was also demonstrated to be useful for preparation of high-density array spots by using a microarrayer and for analysis of analyte Ags either by direct or sandwich methods of fluorescence immunoassay. The detection sensitivity of ProteoChip was as low as 1–10 femtogram/mL of analyte protein, useful for detection of tumor markers. ProteoChip was also useful for studies of direct protein–protein interactions as demonstrated by analysis of integrin-extracellular matrix protein interaction. These experimental results suggest that ProteoChip is a powerful tool for development of chip-based lead screening microarrays to monitor protein–protein interactions (i.e., drug target) as well as for biomarker assays which require high detection sensitivity [21–23]. The major force is the binding of the ionized amine groups (probably an ammonium group) of capture proteins with the crown moiety of the calixcrown used in the coating of the solid phases.
Reactions occurring in crystals are interesting because their outcome is often directed by the steering topology of the reacting atoms and functions constrained by the crystal packing [24]. Single-crystal-to-single-crystal transformations are unobvious since the atomic arrangement of the products is quite different from the one of the starting reactants. However, it seems that the presence of a coordinating metal is of a help in producing such transformations [25, 26]. It has been reported [27] the reaction of a calix[4]bisthiacrown, L (see Scheme 8), in dichloromethane with two equiv of CuI in acetonitrile at room temperature giving colorless single crystals exhibiting a 3D polymeric array of formula
The overall geometry of 1 can be described as an interconnected layer where each L is linked by a three-rungeg ladder-type unit \( \left( {{\text{Cu}}_{3} \left( {\mu_{3} - {\text{I}}} \right)\left( {\mu - {\text{I}}} \right)_{2} .} \right. \) The two Cu3I3 units bridge two L units via Cu–S bonds to form a 2-D layer. Then the adjacent 2-D layers are bridged via Cu–S bonds to form the 3-D framework. The Cu2 atom is tetrahedrally coordinated by three I atoms and one acetonitrile. No photoluminescence is observed. When single crystal 1 is heated at 175 °C, the coordinated acetonitrile molecules are completely removed to yield a desolvated colorless solid of type
The single crystallinity is maintained during the process. The overall 3-D structure of 2 is similar to that of 1. This single-crystal-to-single-crystal transformation gives rise to solvate-luminescence “off-on” behavior due to the removal of coordinated acetonitrile molecules.
Calixcrowns are one of the most widely investigated classes of cation ligands based on calixarenes. This is probably due to the binding properties they deliver toward alkali and alkaline earth metals and ammonium which can be tuned by conformational changes around the binding regions. The numerous chemical transformations they offer allow them to provide the chemists, biochemists and nanochemists with new molecular objects belonging to the field of extended supramolecular chemistry and subsequent fields such as complex matter chemistry [28] and creative advances driven by molecular changes [29, 30].
References
Salorinne, K., Nissinen, M.: Calixcrowns: synthesis and properties. J. Incl. Phenom. Macrocycl. Chem 61, 11 (2008). doi:10.1007/s10847-008-9411-y
Pulpoka, B., Vicens, J.: 1,3-Alternate calix[4]arene: the sophisticated conformer of calix[4]arene. Collect. Czech. Chem. Commun 69, 1251 (2004). doi:10.1135/cccc20041251
Koh, K.N., Araki, K., Shinkai, S., Asfari, Z., Vicens, J.: Cation binding properties of a novel 1,3-alternate calix[4]biscrown. Formation of 1:1 and 1:2 complexes and unique cation tunneling across a calix[4]arene cavity. Tetrahedron Lett 36, 6095 (1995). doi:10.1016/0040-4039(95)01212-Z
de Mendoza, J., Cuevas, F., Prados, P., Meadows, E.S., Gokel, G.W.: A synthetic cation-transporting calix[4]arene derivative active in phospholipid bilayers. Angew. Chem. Int. Ed 37, 1534 (1998). doi :10.1002/(SICI)1521-3773(19980619)37:11<1534::AID-ANIE1534>3.0.CO;2-B
Maulucci, N., De Riccardis, F., Botta, C·B., Casapullo, A., Cressina, E., Fregonese, M., et al.: Calix[4]arene-cholic acid conjugates: a new class of efficient synthetic ionophores. Chem. Commun. (Camb.) 1354 (2005) doi :10.1039/b415908j
Sidorov, V., Kotch, F.W., Abdrakhmanova, G., Mizani, R., Fettinger, J.C., Davis, J.T.: Ion channel formation from a calix[4]arene amide that binds HCl. J. Am. Chem. Soc 124, 2267 (2002). doi:10.1021/ja012338e
Sidorov, V., Kotch, F.W., Kuebler, J.L., Lam, Y.-F., Davis, J.T.: Chloride transport across lipid bilayers and transmembrane potential induction by an oligophenoxyacetamide. J. Am. Chem. Soc 125, 2840 (2003). doi:10.1021/ja029372t
Kim, S.K., Lee, J.K., Lee, S.H., Lim, M.S., Lee, S.W., Sim, W., et al.: Silver ion shuttling in the Trimer-Mimic thiacalix[4]crown tube. J. Org. Chem 69, 2877 (2004). doi:10.1021/jo035567n
Britz, D.A., Kholbystov, A.N., Porfyrakis, K., Arvadan, A., Briggs, G.A.D.: Chemical reactions inside single-walled carbon nano test-tubes. Chem. Commun. (Camb.) 37 (2005) doi:10.1039/b414247k
Wanigasekara, E., Leontiev, A.V., Organo, V.G., Rudkevich, D.M.: Supramolecular, calixarene-based complexes that release no gas. Eur. J. Org. Chem. 2254 (2007) doi:10.1002/ejoc.200700173
Rudkevich, D.M.: Sensing and fixation of gases. Calixarenes in the nanoworld, Vicens J., Harrowfield J. (eds.) Springer, Dordrecht, The Netherlands (2007)
Easton, C.J., Lincoln, S.F., Barr, L., Onagi, H.: Molecular reactors and machines: applications, potential, and limitations. Chem. Eur. J 10, 3120 (2004). doi:10.1002/chem.200305768
Balzani, V., Credi, A., Venturi, M.: The bottom-up approach to molecular-level devices and machines. Chem. Eur. J 8, 5524 (2002). doi :10.1002/1521-3765(20021216)8:24<5524::AID-CHEM5524>3.0.CO;2-J
Asfari, Z., Vicens, J.: Molecular machines. J. Incl. Phenom 36, 103 (2000)
Asfari, Z., Naumann, C., Kaufmann, G., Vicens, J.: Molecular modelling and chemical synthesis of molecular “Mappemondes” designed from a calix[4]biscrown. Tetrahedron Lett 37, 3325 (1996). (Highlighted by Holmes A. B., Richard G.: Molecular machines. Chem. Ind. 468)
Asfari, Z., Naumann, C., Kaufmann, G., Vicens, J.: Synthesis of a molecular mill designed from a calix[4]-bis-crown. Tetrahedron Lett 39, 9007 (1998). doi:10.1016/S0040-4039(98)02066-8
Newkome, G.R., Moorefield, C.N., Vögtle, F.: Dentritic molecules. VCH, Weinheim, Germany (1996)
Cheriaa, N., Mahouachi, M., Ben Othman, A., Baklouti, L., Kim, J.S., Kim, Y., et al.: Calixdendrimers. In: Vicens, J., Harrowfield, J. (eds.) Calixarenes in the nanoworld. Springer, Dordrecht, the Netherlands (2007)
Bu, J.-H., Zheng, Q.-Y., Chen, C.-F., Huang, Z.-T.: The synthesis of calix[4]crown based dendrimer. Tetrahedron 61, 897 (2005). doi:10.1016/j.tet.2004.11.043
Zhu, H., Snyder, M.: Protein chip technology. Curr. Opin. Chem. Biol 7, 55 (2003). doi:10.1016/S1367-5931(02)00005-4
Cahill, D.J.: Protein and antibody arrays and their medicinal applications. J. Immunol. Methods 250, 81 (2001). doi:10.1016/S0022-1759(01)00325-8
Lee, Y., Lee, E.K., Cho, Y.W., Matsui, T., Kang, I.-C., Kim, T.-S., et al.: ProteoChip: a highly sensitive protein microarray prepared by a novel method of protein immobilization for application of protein-protein interaction studies. Proteonomics 3, 2289 (2003). doi:10.1002/pmic.200300541
Oh, S.W., Moon, J.D., Lim, H.J., Park, S.Y., Kim, T., Park, J.B., et al.: Calixarene derivative as a tool for highly sensitive detection and oriented immobilization of proteins in a microarray format through noncovalent molecular interaction. FASEB J 19, 1335 (2005)
Garcia-Garabay, M.A.: Engineering carbene rearrangement in crystals: from molecular information to solid-state reactivity. Acc. Chem. Res 36, 491 (2003). doi:10.1021/ar970309w
Olejnik, Z., Lis, T., Vogt, A., Wolowiec, S., Skarzewski, J.: Single-crystal-to-single-crystal transformation of the dichloromethane solvate of the Schiff base Manganese (III) complex to a solvent free material. J. Incl. Phenom. Macrocycl. Chem 38, 221 (2000). doi:10.1023/A:1008185227837
Chu, Q., Swenson, D.C., Mac Gillivray, L.R.: Single-crystal-to-single-crystal transformation mediated by argentophilic forces converts a finite metal complex into an infinite coordination network. Angew. Chem. Int. Ed 44, 3569 (2005). doi:10.1002/anie.200500400
Lee, J.Y., Lee, S.Y., Sim, W., Park, K.-M., Kim, J., Lee, S.S.: Temperature-dependent 3-d cui coordination polymers of calix[4]-bis-dithiacrown: crystal-to-crystal transformation and photoluminescence change on coordinated solvent removal. J. Am. Chem. Soc 130, 6902 (2008). doi:10.1021/ja8008693
Lehn, J.-M.: Toward self-organisation and complex matter. Science 295, 2400 (2002). doi:10.1126/science.1071063
Reinhoudt, D.N., Crego-Calama, M.: Synthesis beyond the molecule. Science 295, 2403 (2002). doi:10.1126/science.1069197
Stein, R.L.: Towards a process philosophy of chemistry. HYLE 10, 5 (2004)
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Kim, J.S., Vicens, J. Progress of calixcrowns chemistry. J Incl Phenom Macrocycl Chem 63, 189–193 (2009). https://doi.org/10.1007/s10847-008-9503-8
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10847-008-9503-8