Abstract
Two ionic clathrate hydrates with different structures are formed in the binary system tetrabutylammonium fluoride–water, namely tetragonal structure-I hydrate (TS-I) (n-С4H9)4NF · 32.8H2O, and cubic superstructure-I hydrate (CSS-I) (n-С4H9)4NF · 29.7H2O. The heats of fusion (ΔHf) of these polyhydrates were measured calorimetrically with differential scanning calorimeter. For TS-I polyhydrate ΔHf = (204.8 ± 2.3) kJ/mol hydrate, for CSS-I hydrate ΔHf = (177.5 ± 3.1) kJ/mol polyhydrate. The change of water molecules energy state in the water lattices of TS-I and CSS-I polyhydrates are discussed.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
The polyhydrates of peralkylammonium salts (ionic clathrate hydrates according to G. Jeffrey’s classification [1]) are inclusion compounds that have both hydrophilic and hydrophobic hydration. Depending on the charge, an anion substitutes one or several water molecules in the water host lattice to form H-bonds (hydrophilic hydration). A cation is also incorporated into cavities of the water-anion framework displacing water molecule with nitrogen atom, so that distances between the cation atoms and water framework atoms are no less than a sum of their van der Waals radii (hydrophobic hydration).
Tetraisoamyl and tetrabutyl moieties are the most potent in clathrate formation among tetraalkyl derivatives. An interesting feature of these compounds is that within a narrow range of concentrations, the same salt may form several ionic clathrate hydrates with different structures, albeit with similar stoichiometry and thermal stability [2]. Occurrence of peralkylammonium salt–water system with a single polyhydrate is rather exceptional. This fact becomes obvious only upon thorough investigation of T, X-phase diagrams of peralkylammonium–water binary systems coupled with crystal structure research and sometimes—together with examination of isotherms of corresponding ternary systems. Importantly, examination of phase diagrams is needed both for elucidation of number of polyhydrates and their compositions as well as synthesis of crystals with a definite structure. A very illustrative example of this is tetraisoamylammonium fluoride–water system for which the only polyhydrate (i-C5H11)4NF · 38H2O (1), orthorhombic, Pbmm, a = 12.08 Å, b = 21.61 Å, c = 12.82 Å [3] was known for a long time. It was not until Т, Х-phase diagram of (i-C5H11)4NF–H2O system was carefully examined, that the existence of additional two polyhydrates (i-C5H11)4NF · 32.7H2O (2) and (i-C5H11)4NF · 27H2O (3) in this system became clear. The existence of the latter was confirmed by single crystal X-Ray diffraction analysis: (2) is tetragonal, P42/m, a = 23.729 Å, c = 12.466 Å; (3) is tetragonal, I41/a, a = 16.894 Å, c = 17.111 Å [4]. Noticeably, the monotonous change of equilibrium concentration of liquid phase results in abrupt change of the solid phase composition due to formation of new framework structure (first-order phase transition).
The purpose of the present study was to estimate the thermodynamic properties of different ionic clathrate hydrates of the same tetraalkylammonium salt depending on their structure and stoichiometry. We used differential scanning calorimetry to measure the melting enthalpies of ionic clathrate hydrates, crystallizing in (n-С4H9)4NF–H2O binary system. This binary system is one of a few similar for which the phase diagram and structures of compounds studied in detail [5–9]. Because of that, it is possible to achieve a good result in synthesis of distinct polyhydrates formed in this system and as well as accurately correlate the experimentally measured melting enthalpies with structure and stoichiometry of different polyhydrates present in this binary system.
Experimental
Tetrabutylammonium fluoride was prepared as previously described [8]. The crystals of cubic and tetragonal polyhydrates of tetrabutylammonium fluoride were grown in accordance with the data on T, X-phase diagram of (n-С4H9)4NF–H2O binary system (Fig. 1). After a short time at room temperature polyhedral crystals were formed in ∼40% wt.% salt solution (Fig. 2). In ∼15 ÷ 20% wt.% salt solutions under the same conditions elongated prisms were obtained (Fig. 3). The crystals were isolated from the solution and dried shortly between two sheets of filter paper. A part of crystals was further placed into the flasks for an analytical determination of composition, whereas the other part was sealed up in 0.1 mL steel pans for measurement of enthalpies of fusion. Samples’ weight was in the range of 0.03–0.09 g. The concentration of the tetrabutylammonium fluoride was determined by potentiometric titration with sodium tetraphenylborate solution using ion-selective electrode. The polyhydrates’ stoichiometry established as described above is consistent with the following compositions: (n-C4H9)4NF.(32.4 ± 0.4)H2O (elongated prisms from ∼15 ÷ 20% wt.% solutions) and (n-C4H9)4NF(28.9 ± 0.3)H2O (crystals of polyhedral shape from ∼40% wt.% solutions). The differential scanning calorimeter DSC-111 (Setaram) was used for determination of enthalpies of fusion. An empty 0.1 mL steel pan was used as reference. The enthalpy changes per unit time were measured by heating the samples at the rate of 0.5 °C per minute. The observed heats of fusion were standardized by electric calibration suggested by manufacturer.
Results and discussion
Based on T, X-phase diagram of (n-C4H9)4NF–H2O binary system and chemical analysis of the isolated polyhydrates it was previously demonstrated that within the clathrate formation region two polyhydrates are crystallized: (n-C4H9)4NF · 32.3H2O (m.p. 27.2 °C) and (n-C4H9)4NF · 28.6 H2O (m.p. 27.4 °C) [5, 6].Footnote 1 X-ray structure analysis of the first compound demonstrated the (n-C4H9)4NF · 32.8H2O stoichiometry; it was tetragonal, of space group P42/m, a = 23.52 Å, c = 12.30 Å [7]. It was related to the tetragonal structure-I (TS-I). The structure of the second compound was determined as cubic, of space group I4̄3d, a = 24.375 Å with the adjusted stoichiometry (n-C4H9)4NF · 29.7H2O [8]. Its idealized water framework was isostructural with cubic structure-I (CS-I) of gas hydrates but has an eight-fold unit cell, and thus represented a cubic superstructure-I (CSS-I).
Using differential scanning calorimetry we measured heats of fusion of the above-mentioned ionic clathrate hydrates. The measured values and some characteristics of the tetrabutylammonium fluoride ionic clathrate hydrates are summarized in Table 1. The enthalpies of fusion were calculated using the hydrate numbers obtained from crystallographic and analytical data.
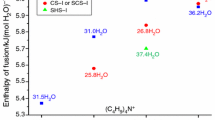
Melting enthalpies for the tetrabutylammonium fluorides polyhydrates were reported previously by H. Nakayama [10] ((n-C4H9)4NF · 30H2O, 184 ± 4 kJ/mol at 25 °C) and Z. Lindenbaum [11] ((n-C4H9)4NF30.22H2O, 197.3 kJ/mol at 25 °C). However, it is impossible to compare directly these data with the ΔH values measured in this study as the authors of [10] and [11] did not take into account the possibility of the presence of two polyhydrates in the studied system. Only one polyhydrate was found upon examination of a phase diagram of (n-C4H9)4NF–H2O system in [12] (although earlier studies of the same group identified this hydrate as (n-C4H9)4NF · 28 H2O [13]). The phase diagram of (n-C4H9)4 NF–H2O binary system demonstrates that there is a metastable extension of the liquidus line of the CSS-I polyhydrate in the stability region of the TS-I polyhydrate (Fig. 1, dotted line) and a metastable extension of the liquidus line of the TS-I polyhydrate in the stability region of the CSS-I polyhydrate (Fig. 1, dashed line). According to our experience, tetraalkylammonium polyhydrates can exist in metastable state for a substantial amount of time. Moreover, it is not unusual that more than one hydrate phase crystallizes in the same T, X-conditions. Special procedures are often required to get rid of the metastable phase (e.g., repeated heating of the solution for partial melting of the crystals followed by cooling). In the studies mentioned above the authors discuss the sole polyhydrate with 30 water molecules in this system. Under described experimental conditions, however, a mixture of tetragonal and cubic forms of polyhydrates can be formed. These phases, as we have shown here, have different values of ΔHf.
Let us consider a hypothetical reaction of the crystalline cubic phase polyhydrate with water at 27.7 °C that results in formation of crystalline tetragonal phase (the following calculations, including the heats of fusion, are based on the crystallographic hydrate numbers):
The enthalpy of this reaction is ΔH1. This value can be estimated using the data from this study and the literature. The following scheme can be used:

where ΔHf2 and ΔHf3 are our experimental values of heat of fusion for cubic polyhydrate and heat of crystallization for tetragonal polyhydrate, correspondingly; Φ L—heat of dilution. Heats of dilution of (n-C4H9)4NF solutions at 25 °C are reported in [14] and apparent molal heat content are evaluated by equation
where m is the concentration of solution in mol/kg of water, Φ L is expressed as calories per mole. This Eq. 2 is valid for m in the range of 0.1135 to 1.837 mol/kg. Extrapolating Φ L for higher concentrations (composition of (n-C4H9)4NF · 29.7H2O corresponding to m = 1.869 mol/kg water, slightly higher than the upper limit for Eq. 2), yielded 2.6 kJ for dilution process (n-C4H9)4NF29.7H2O(liq.) → (n-C4H9)4NF32.8 H2O(liq.). Although the discussed process takes place at 27.7 °C that is higher than the temperature for which the Eq. 2 is valid (25 °C), the resulting error is negligible. Thus ΔH1 = ΔHf2 − Φ L − ΔHf3 = −29.9 kJ/mol.
The data in Table 1 show that the enthalpy of fusion per one mole water is higher for TS-I than for CSS-I polyhydrate. Thus we conclude that the water molecules are bound stronger in the framework of tetragonal hydrate than in the framework of cubic hydrate. Noticeably, both values are somewhat lower than the hypothetical melting enthalpy for ice Ih at 27.7 °C (6.98 kJ/mol [15]). In order to quantitatively estimate difference between the energy states of water molecules in the frameworks of these polyhydrates, ΔH1 of the reaction (1) may be calculated by an alternative approach. The essential contribution to ΔH1 value is made by the heat of crystallization of 3.1 moles of liquid water to form the water framework of tetragonal structure and equals 3.1 × 6.23 = 19.3 kJ (6.23 kJ/mol water is the value obtained for the heat of fusion of TS-I polyhydrate in this study, see Table 1). Thus, simplified, one can assume that the difference −29.9−(−19.3) = −10.6 kJ for reaction (1) is related to the change in energy state of water molecules in the water lattices between TS-I and CSS-I polyhydrates in (n-C4H9)4NF–H2O binary system.
Since there is some discrepancy in polyhydrate compositions found by chemical analysis and XRD method (possibly a loss of some quantity of water may occur upon sampling), all calculations described above were repeated using hydrate numbers obtained by chemical analysis. The following values were obtained: ΔH1 = 31.7 kJ/mol, the heat of crystallization of 3.5 moles of water—21.9 kJ and the difference in energy state of water molecules in the water lattices of TS-I and CSS-I polyhydrates comes to 9.8 kJ per mole hydrate, which is similar to the previous result of 10.6 kJ.
The evaluated difference in energy state of water molecules in the water lattices of TS-I and CSS-I polyhydrates (9.8 ÷ 10.6 kJ per mole hydrate) could result from a combination of (a) difference in the energy of the idealized water frameworks of these two hydrates, (b) distortions introduced upon the guest inclusions, (c) difference in the guest–guest and (d) host–guest interactions. Presently, it is impossible to discriminate between contributions of these factors. Undoubtedly, a small difference in water framework energy of different hydrates results in formation of several phases that differ in structure but are similar in composition and melting temperature. Thermodynamical characterization of these phases, based on the full and reliable information on the phase diagram of the corresponding systems (the actual number of clathrate phases, their composition and melting temperature) together with the structural analysis of the forming hydrates will allow to get quantitative correlation between the compositions, structural and energy characteristics of the hydrate frameworks. These data can become useful for the analysis of the water states in biological macromolecules, thermodynamical modelling of the clathrate hydrates and beyond.
Notes
Yet another hydrate (n-C4H9)4NF · 5.5H2O, monoclinic, was found in the low water content region [9]. In this compound tetrabutylammonium cations form the host channel framework, whereas the chains of small paired water-anion cavities are guests.
References
Jeffrey, G.A.: Hydrate inclusion compounds. In: MacNicol, D.D., Toda, F., Bishop, R. (eds.) Comprehensive Supramolecular Chemistry, vol. 6, chap. 23, pp. 757–788. Pergamon, Oxford (1996)
Aladko, L.S., Dyadin, Yu.A., Rodionova, T.V., Terekhova, I.S.: Effect of size and shape of cations and anions on clathrate formation in the system: halogenides of quaternary ammonium bases and water. J. Mol. Liq. 106(2–3), 229–238 (2003)
Feil, D., Jeffrey, G.A.: The polyhedral clathrate hydrates. Part 2. Structure of the hydrate of tetra iso-amyl ammonium fluoride. J. Chem. Phys. 35, 1863–1873 (1961)
Lipkowski, J., Suwinska, K., Rodionova, T.V., Udachin, K.A., Dyadin, Yu.A.: Phase and X-ray study of clathrate formation in the tetraisoamylammonium fluoride – water system. J. Incl. Phenom. Mol. Rec. Chem. 17, 137–148 (1994)
Dyadin, Yu.A., Terekhova, I.S., Polyanskaya, T.M., Aladko, L.S.: Clathrate hydrates of tetrabutylammonium fluoride and oxalate. J. Struct. Chem. 17, 566–571 (1977) (translated from Russian Zh. Struct. Khim. 17, 655 (1976))
Dyadin, Yu.A., Udachin, K.A.: Clathrate formation in water – peralkylonium salts system. J. Incl. Phen. 2, 61–72 (1984)
McMullan, R.K., Bonamico, M., Jeffrey, G.A.: Polyhedral clathrate hydrates. V. Structure of the tetra-n-butyl ammonium fluoride hydrate. J. Chem. Phys. 39, 3295–3310 (1963)
Komarov, V.Yu., Rodionova, T.V., Terekhova, I.S., Kuratieva, N.V.: The cubic superstructure-I of tetrabutylammonium fluoride (C4H9)4NF.29.7 H2O clathrate hydrate. J. Incl. Phenom. 59, 11–15 (2007)
Udachin, K.A., Lipkowski, J.: Water-fluorine chains in (n-Bu)4NF.5.5H2O hydrate. J. Supramol. Chem. 2, 449–451 (2002)
Nakayama, H.: Hydrates of organic compounds. VI. Heats of fusion and of solution of quaternary ammonium halide clathrate hydrates. Bull. Chem. Soc. Jpn. 55, 389–393 (1982)
Narten, A.H., Lindenbaum, S.: Diffraction pattern and structure of the system tetra-n-butylammonium fluoride – water at 25oC. J. Chem. Phys. 51, 1108–1114 (1969)
Nakayama, H.: Solid-liquid and liquid – liquid phase equilibria in the symmetrical tetraalkylammonium halide – water systems. Bull. Chem. Soc. Jpn. 54, 3717–3722 (1981)
Nakayama, H., Watanabe, K.: Hydrates of organic compounds. II. The effect of alkyl groups on the formation of quaternary ammonium fluoride hydrates. Bull. Chem. Soc. Jpn. 49, 1254–1256 (1976)
Lindenbaum, S.: Heats of dilution at 25o of aqueous solution of the bolaform electrolyte [Bu3N-(CH2)8-NBu3]X2. J. Chem. Phys. 73, 4334–4337 (1969)
Eisenberg, D., Kauzmann, W.: Structure and Properties of Water. Oxford Univ. Press, London (1969)
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Rodionova, T.V., Manakov, A.Y., Stenin, Y.G. et al. The heats of fusion of tetrabutylammonium fluoride ionic clathrate hydrates. J Incl Phenom Macrocycl Chem 61, 107–111 (2008). https://doi.org/10.1007/s10847-007-9401-5
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10847-007-9401-5