Abstract
The objectives of this research were to prepare and characterize inclusion complex of Ezetimibe (EZE) with cyclodextrins (β-cyclodextrin (β-CD) and hydroxypropyl-β-cyclodextrin (HPβ-CD)) and to study the effect of complexation on the dissolution rate of EZE, a water insoluble drug. Phase solubility curve was classified as A P -type for both cyclodextrins, indicating the 2:1 stoichiometric ratio for β-CD–EZE and HPβ-CD – EZE inclusion complexes. The inclusion complexes in the molar ratio of 2:1 (β-CD–EZE and HPβ-CD–EZE) were prepared by various methods such as kneading, coevaporation and physical mixing. The molecular behaviors of drug in all samples were characterized by fourier-transform infrared (FTIR) spectroscopy, differential scanning calorimetry (DSC) and powder X-ray diffraction (PXRD) studies. The results of these studies indicated that complex prepared by kneading and coevaporation methods showed inclusion of the EZE molecule into the cyclodextrins cavities. The highest improvement in in-vitro dissolution profiles was observed in complex prepared with hydroxypropyl-β-cyclodextrin using co-evaporation method. Mean dissolution time and similarity factor indicated significant difference between the release profiles of EZE from complexes and physical mixtures and from pure EZE.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
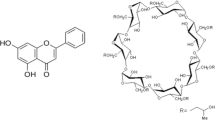
Ezetimibe (EZE) is chemically 1-(4-fluorophenyl)-3(R)-[3-(4-fluorophenyl)-3(S)-hydroxypropyl]-4(S)-(4-hydroxyphenyl)-2-azetidinone. Its structural formula is shown below.
EZE reduces blood cholesterol by inhibiting the absorption of cholesterol by the small intestine. Due to its very high hydrophobic character, EZE exhibits highly erratic and very low dissolution profile in gastrointestinal fluids. Together with permeability, the solubility and/or dissolution rate of a drug are key determinants of its oral bioavailability. It is generally considered that compounds with very low aqueous solubility will show dissolution rate-limited absorption and hence poor absorption, distribution and target organ delivery [1]. Improvement of aqueous solubility in such a case is a valuable goal to improve therapeutic efficacy.
Cyclodextrins (CDs) form a group of structurally related oligosaccharides with cylinder-shaped cavities that have the capacity to form inclusion complexes with many drugs by taking a whole drug molecule, or a part of it, into the cavity [2, 3]. CDs first came to the fore in marketed products as drug delivery technologies that enabled the development of various prostaglandins [4].
β-cyclodextrin (β-CD) has ideal dimensions to complex a range of commonly used drugs. Hydroxypropyl-β-cyclodextrin (HPβ-CD), a chemical derivative of β-CD, similarly improves the aqueous solubility of many drugs, but it is more hydrophilic than the β-CD, forms a less stable complex with cholesterol, and is therefore less toxic [5]. HPβ-CD is more water-soluble than the parent molecule and has hydroxypropylester groups attached to the hydroxyl groups in position 2. Inclusion complex of Rofecoxib/ HPβ-CD (1:1 molar ratio) has been prepared using kneading method with a subsequent improvement in dissolution due to amorphization [6]. Many other drugs such as ibuprofen, tolbutamide, ganciclovir, nimesulide, itraconazole etc. have been tested for CDs inclusion to enhance solubility [7, 8, 9, 10].
In this study, an attempt was made to compare the similarity between in vitro dissolution profiles of EZE from complexes, physical mixture and pure EZE. Dissolution profiles can be compared by calculating similarity factor (f 2). The method was first reported by Moore and Flanner [11]. A value of 100% for the similarity factor (f 2) suggests that the test and reference profiles are identical. Values between 50 and 100 indicate that the dissolution profiles are similar whilst smaller values imply an increase in dissimilarity between release profiles [11].
Mean dissolution time (MDT) reflects the time for the drug to dissolve and is the first statistical moment for the cumulative dissolution process that provides an accurate drug release rate [12]. It is accurate expression for drug release rate. A higher MDT value indicates greater drug retarding ability [13].
The objective of the present study was to prepare inclusion complexes of EZE with β-CD and HPβ-CD using various methods such as kneading, coevaporation and physical mixing to improve its aqueous solubility and dissolution rate. The study was further aimed to characterizations of prepared inclusion complexes by different methods such as fourier-transform infrared (FTIR) spectroscopy, differential scanning calorimetry (DSC) and powder X-ray diffraction (PXRD) studies.
Materials and methods
Materials
HPβ-CD and β-CD were a generous gift from Roquette Frères, France. EZE (molecular weight = 409) was received as a gift sample from Zydus Cadila, (Ahmedabad, India). Directly compressible lactose, maize starch, sodium starch glycollate, colloidal silicon dioxide, magnesium stearate and sodium lauryl sulfate were received as gift samples from Maan Pharmaceuticals Ltd., (Ahmedabad, India). All chemicals and solvents used in this study were of analytical reagent grade. Freshly distilled water was used throughout the work.
Phase solubility study
Phase-solubility studies were performed according to the method reported by Higuchi and Connors [14]. EZE, in constant amounts (5 mg) that exceeded its solubility, were transferred to screw capped vials containing 25 ml of aqueous solution of β-CD (molecular weight = 1135) or HPβ-CD (molecular weight = 1500) in various molar concentrations (2.0, 4.0, 6.0, 8.0, 10.0, 12.0 and 14.0 mM/L, each for β-CD and HPβ-CD). The contents were stirred on electromagnetic stirrer (Remi, India) for 48 h at 37 °C ± 0.1 °C and 400 rpm (this duration was previously tested to be sufficient to reach equilibrium). After reaching equilibrium, samples were filtered through a 0.22 μm membrane filter, suitably diluted and analyzed spectrophotometrically for drug content at the wavelength of 233 nm using spectrophotometer (Shimazdu-1601, UV/Vis spectrophotometer, Shimadzu Corp, Kyoto, Japan). Solubility studies were performed in triplicate.
Preparation of inclusion complexes
Complexes of β-CD and HPβ-CD with EZE were prepared in the molar ratio of 2:1 (on the basis of phase solubility study) by different methods like physical mixture, coevaporation, and kneading. For ease in discussion, the samples are designated with different abbreviations shown in Table 1.
Physical mixture
Physical mixture (PM) of CDs and EZE were prepared by simply mixing powders with a spatula for 15 min.
Coevaporation method
For preparation of complexes by coevaporation method, methanol and water were used as solvents. The required quantities of EZE and CDs were dissolved in same quantities (5 ml) of methanol and water, respectively. Both the solutions were mixed and solvents were evaporated by controlled heating at 45–50 °C. The resultant solids were pulverized and then sieved through 120 #.
Kneading method
For preparation of complexes by kneading method, the required quantities of CDs and distilled water were mixed together in a mortar so as to obtain a homogeneous paste. EZE was then added slowly; while grinding, a small quantity of methanol was added to assist the dissolution of EZE. The mixtures were then ground for 1 h. During this process, an appropriate quantity of water was added to the mixture in order to maintain a suitable consistency. The pastes were dried in oven at 45–50 °C for 24 h. The dried complexes were pulverized and then sieved through 120 #.
Drug content
The samples of complexes and physical mixtures were assayed for EZE content by dissolving a specific amount of the complexes in methanol and analyzing for the EZE content spectrophotometrically at 233 nm on spectrophotometer (U.V. visible spectrophotometer, Shimazdu-1601).
Characterizations of complexes
Fourier transform infrared (FTIR) spectroscopic analysis
FTIR spectrums of moisture free powdered samples of EZE, CDs, its PMs and complexes with β-CD and HPβ-CD were obtained using a FTIR spectrometer (FTIR-8300, Shimadzu Co., Kyoto, Japan) by potassium bromide (KBr) pellet method.
Powder X-ray diffraction (PXRD) analysis
The physical state of EZE in the various preparations was evaluated by poder X-ray diffraction study. Powder X-ray diffraction patterns of all samples were determined using Phillips PW 3710 scanner, IW 1830 generator with a CuK α anode at 40 kV and 30 mA and at a scan rate of 1° min−1 from 2θ range from 1° to 40°.
Differential scanning calorimetry (DSC) analysis
DSC scans of all powdered samples were recorded using DSC-Shimadzu 60 with TDA trend line software. The samples (6–7 mg) were accurately weighed in crimped aluminum pans and heated from 50 °C to 300 °C, at a scanning rate of 10 °C /min under air flow (100 ml/min).
Wettability and Dissolution Studies
Wettability study was performed using open tubes containing a EZE, CDs, its PMs and complexes with β-CD and HPβ-CD were placed with their lower capillary ends dipped into colored water (0.01% eosin in water). The upward migration of the colored front was registered as a function of time.
Dissolution studies of EZE in powder form, its PMs and complexes with β-CD and HPβ-CD were performed to evaluate in vitro drug release profile. Dissolution studies were carried out using USP dissolution apparatus type II with 500 ml dissolution medium at 37 °C ± 0.5 °C and 50 rpm for 4 h. Phosphate buffer (pH 7.8) containing 1% w/v of sodium lauryl sulfate (SLS) was used as a dissolution mediums. At fixed time intervals 5 ml aliquot was withdrawn, filtered, suitably diluted and assayed for EZE content by measuring the absorbance at 233 nm using spectrophotometer. Equal volume of fresh medium prewarmed at the same temperature was replaced in to the dissolution medium to maintain constant volume throughout the test. Dissolution studies were performed in six replicates, and calculated mean values of cumulative drug release were used while plotting the release curves. MDT and f 2 values were calculated to compare dissolution rate of EZE from pure drug, its PM and complexes with β-CD and HPβ-CD. Preliminary tests demonstrated that there was no change in the λmax of EZE due to the presence of CDs dissolved in the dissolution medium.
Formulation studies
Formulation excipients were selected on the basis of preliminary tests which demonstrated no interference of these excipients with the λmax of EZE. Tablets containing 10 mg of EZE were made by direct compression using different formulation excipients like directly compressible lactose, colloidal silicon dioxide, and magnesium stearate. Tablets containing complexes prepared by kneading method equivalent to 10 mg EZE were made similarly but using less quantity of lactose. The blend was compressed on an eight-station single rotary machine (Cadmach, India) using round-shaped, flat punches to obtain tablets of 3–5 kg/cm2 hardness and 4–5 mm thickness. The tablets were studied in six replicates for release profile of drug using the same methodology as described in in vitro dissolution studies.
Statistical analysis:
Model independent mathematical approach proposed by Moore and Flanner [11] for calculating a similarity factor f 2 was used for comparison between dissolution profiles of different samples. The similarity factor f 2 is a measure of similarity in the percentage dissolution between two dissolution curves and is defined by following equation:
where n is the number of withdrawal points, R t is the percentage dissolved of reference at the time point t and T t is the percentage dissolved of test at the time point t.
A value of 100% for the similarity factor (f 2) suggests that the test and reference profiles are identical. Values between 50 and 100 indicate that the dissolution profiles are similar whilst smaller values imply an increase in dissimilarity between release profiles.
In order to understand extent of improvement in dissolution rate of EZE from its complexes and physical mixture, the obtained dissolution data of pure EZE, it’s PM and complexes with CDs were fitted into equation
Here, i is dissolution sample number, n is number of dissolution times, t mid is time at the midpoint between times t i and t i−1, and ΔM is the amount of EZE dissolved (μg) between times t i and t i−1. MDT reflects the time for the drug to dissolve and is the first statistical moment for the cumulative dissolution process that provides an accurate drug release rate. It is accurate expression for drug release rate. A higher MDT value indicates greater drug retarding ability.
Results and discussion
Phase solubility study
Phase solubility analysis has been among the preliminary requirements towards the optimization of the development into inclusion complexes of the drugs as it permits the evaluation of the affinity between CDs and drug molecule in water. This process has been used by many researchers for the determination of the exact molar ratios in which the drugs could make complexes with CDs [15, 16].
The phase solubility curve of EZE in the presence of CDs is shown in Fig. 1a,b. This curve indicated a linear increase in solubility of EZE with an increase in concentrations of CDs in water. Increasing amounts of CDs increased the amount of EZE going into water, improving the aqueous solubility of EZE. Solubility of EZE is increased by 17-fold and 27-fold at 37 °C at 14 mM/L concentrations of β-CD and HPβ-CD, respectively. Increased solubility may be due to improved dissolution of EZE particles in water by CDs.
An indication of the process of transfer of EZE from pure water to aqueous solution of CDs was obtained from the values of Gibbs free energy change. The Gibbs free energy of transfer (ΔG tr °) of EZE from pure water to aqueous solutions of CDs was calculated using equation [17]
where So/Ss = the ratio of molar solubility of EZE in aqueous solution of CDs to that of the pure water. The obtained values of ΔG tr° are shown in Table 2. This data provided the information regarding the increased solubility of EZE in the presence of CDs. Negative ΔG tr° values indicate favorable conditions. ΔGtr° values were all negative for CDs at various concentrations, indicating the spontaneous nature of EZE solubilization, and it decreased with an increase in its concentration, demonstrating that the reaction became more favorable as the concentration of CDs increased. These values also indicated that the extent of improvement in solubility was more with HPβ-CD as compared to β-CD.
Stoichiometric ratio at which optimum complexation occurs was confirmed by phase solubility analysis. The phase solubility plot showed an A P type solubility curve for both CDs, which indicated formation of 2:1 β-CD – EZE and HPβ-CD – EZE inclusion complex. The stability constants (Ks) for the complexes at 37 °C, assuming a 2:1 stoichiometry, calculated from the slope of the initial straight portion of the phase solubility diagram were 772 M−1 for β-CD: EZE and 1316 M−1 for HPβ-CD: EZE, which indicated a suitable and stable complex formation. It is reported that cyclodextrin-drug complexes with the values of Ks in the range of 200–5000 M−1 show improved dissolution properties and hence better bioavailability [14].
Drug content
The drug content of the PMB, PMH, KNB, COEB, KNH and COEH (see Table 1) were found out to be 93.31% (±10.23), 94.89% (±9.83), 98.46% (±6.17), 98.56% (±5.82), 98.12% (±4.07), and 99.02% (±5.02), respectively, which indicate content uniformity of EZE in its complex form.
Characterization of complexes
Fourier transform Infrared (FT-IR) Spectroscopic analysis
Fourier transform infrared spectroscopy (FT-IR) has been used to assess the interaction between β-CD and guest molecules in the solid state. The chemical interaction between the drug and the carrier often leads to identifiable changes in the infrared (IR) profile of complexes. However, some of the changes are very subtle requiring careful interpretation of the spectrum [18].
The FT-IR spectras of PMB (C), KNB (D), COEB (E), PMH (G), KNH (H) and COEH (I) were compared with spectrum of β-CD (B), HPβ-CD (F) and EZE (A) (Fig. 2a,b). The spectrum of pure EZE presented characteristic peaks at 3261.4 cm−1 (Broad, intermolecular hydrogen bonded, O-H strech), 2912.31 cm−1 (Aromatic C–H sterch), 1893.97 cm−1 (C = O strech of lactone ring), 1712.67 cm−1 (C = O strech), 1600.81 cm−1 (ring C–C sterch), 1400.22 and 1448.44 cm−1 (C–N strech), 1353.94 cm−1 (in plane O-H bend), 1271, 1217, and 1159.14 cm−1 (C–F strech), 1159.14, 1064.63, and 1101.28 cm−1 (C–O strech of secondary alcohol), 1006.77 cm−1 (ring breathing of cyclobutanes), 937.34 (ring vibration of alkyl cyclobutanes), and 831.26 cm−1 (ring vibration due to para-disubstituted benzene), respectively. The FT-IR spectrums of the β-CD and HPβ-CD are characterized by intense bands at 3300–3500 cm−1due to O–H stretching vibrations. The vibration of the –CH and CH2 groups appears in the 2800–3000 cm−1 region. The presence or absence of characteristic peaks associated with specific structural groups of the drug molecule was noted. Any sign of interaction would be reflected by changes in the characteristic peaks of EZE, depending on the extent of interaction.
The FT-IR spectras of PMB, KNB, COEB, PMH, KNH and COEH showed no peaks other than those of CDs and EZE. These results indicate absence of well defined chemical interaction between CDs and EZE during coevaporation, kneading, and mixing. The FT-IR spectras of COEB and COEH showed more similarity to FT-IR spectras of β-CD and HPβ-CD, respectively. FT-IR spectras of these two samples showed the absence of most characteristic peak of EZE at 1712.67 cm−1 (C = O strech) and 1893.97 cm−1 (C = O strech of lactone ring), which were present in other samples, indicating inclusion of EZE in CDs cavity in these samples. The FT-IR spectras of PMB, KNB, PMH, and KNH were equivalent to the addition spectrum of CDs and EZE. Although it could be expected to have hydrogen bonding between the hydrogen atom of the OH group present at the interior cavities of CDs and the oxygen atom of lactone ring present in the drug, this could not be demonstrated.
Powder X-ray diffraction (PXRD) analysis
Powder X-ray diffraction spectroscopy (PXRD) has been used to assess the degree of crystallinity of the given sample. When complexes of drug and polymer are formed, the overall numbers of crystalline structure are reduced and more the number of amorphous structures are increased. So the final product sample shows less number as well as less intensity of peaks. This shows that overall crystallinity of complexes is decreased and due to more amorphous nature, the solubility is increased.
The PXRD spectras of all the samples are shown in Fig. 3a,b. EZE showed major peak at 2θ values of 7.9, 13.89, 15.81, 17.22, 18.66, 19.39, 20.64, 21.80, 22.91, 23.42, 24.52 and 26.32. β-CD showed major peaks at 2θ values of 4.39, 8.87, 10.55, 12.40, 15.29, 19.46, 21.03, 22.56, 27.01, 31.81 and 34.64. Due to amorphousness of HPβ-CD, no major peaks were observed in spectra of HPβ-CD. The decrease in degree of crystallinity means improvement in amorphousness of the samples. Degree of crystallinity was decreased to maximum extent in case of COEH. Complexes prepared using HPβ-CD showed more decrease in degree of crystallinity (only one or two peaks related to EZE) as compared to that of β-CD. In case of complexes with β-CD too, COEB showed maximum decrease in degree of crystallinity. Hence, from this discussion, it can be confirmed that coevaporation method was the best method for the preparation of complexes.
Differential scanning calorimetry (DSC) analysis
Differential scanning calorimetry enables the quantitative detection of all processes in which energy is required or produced (i.e., endothermic or exothermic phase transformations). The thermograms of all samples are presented in Fig. 4a,b. The EZE showed a melting peak at 166.47 °C (Δ H = −210.60 mJ/g). In the thermogram of the β-CD and HPβ-CD peak between 90 °C–120 °C was due to loss of water from CDs molecules.
In the thermograms of all samples, peaks due to β-CD and HPβ-CD was observed at the same position i.e. between 80 °C–120 °C. Peak of EZE at 166.47 °C was present at the same position i.e. near to 165 °C in PMB, KNB, PMH, and KNH. In case of COEH and COEB peak due to EZE is almost disappeared this may be due to trapping of EZE in the CDs cavity. This also confirmed that coevaporation method was the best method for the preparation of inclusion complexes.
Wettability and dissolution studies
The improvement in wettability of EZE by physical mixing and complexation with CDs is presented in Fig. 5. COEH and KNH showed higher wettability in water (58.6 and 49.4, respectively), as compared to plain EZE (18.3) at 45 min. Even PMs of EZE with CDs enhanced wettability of EZE in water significantly as compared to plain EZE. Thus, the results of wettability studies indicated that both CDs improved wettability of EZE in water both in complex as well as in mixture form due to its hydrophilicity.
SLS was selected as a suitable surfactant in the present dissolution studies because preliminary experiments confirmed that SLS at 0.25% w/v exhibited higher solubilization for EZE than other surfactants with no significant change at higher concentrations. Based upon these findings, dissolution of pure EZE and all other prepared systems (complexes and physical mixture) was carried out in aqueous SLS solution (0.1% w/v).
DP30 min (percent drug dissolved within 30 min), time to dissolve 50% drug (t50%) and mean dissolution time (MDT) in phosphate buffer (pH 7.8) are reported in Table 3. From this data, it is evident that onset of dissolution of pure EZE is very low in dissolution medium (17.7% within 30 min). COEH, KNH, COEB, and KNB considerably enhanced dissolution rates within 30 min compared to pure EZE, PMB and PMH. The graphical presentation of the dissolution profile of EZE from pure EZE, its PMs and complexes with β-CD and HPβ-CD in phosphate buffer (pH 7.8) over a period of 4 h is shown in Fig. 6. It is evident that the dissolution rate of pure EZE is very low in phosphate buffer (pH 7.8), about 42.4 of the drug being dissolved in 4 h. KNB, COEB, KNH and COEH significantly enhanced dissolution rate of EZE significantly (81–100 in within 4 h). Possible mechanisms of improved dissolution rates of complexes include [19] reduction of crystallite size, a solubilization effect of carrier, absence of aggregation of drug crystallites, improved wettability, dispersibility of a drug from dispersion, dissolution of the drug in the hydrophilic carrier, conversion of drug to amorphous state, and finally, the combination of the above methods.
The dissolution rate of EZE from PMB and PMH was higher (70–75) than that of pure EZE (42.4) within 4 h. Physical mixing of EZE with CDs brings the drug in close contact CDs. The increased dissolution rate observed in case of PM can be attributed to several factors such as a solubilization effect of CDs, improved wettability of drug, and prevention of particle aggregation.
The MDT of EZE is 56.11 min in phosphate buffer (pH 7.8). The MDT values of EZE decreased to greater extent after preparing complex of EZE with CDs i.e. 36.72 min & 35.74 min for KNB & COEB and 30.92 min & 28.37 min for KNH & COEH. Even MDT values of PMB and PMH were sufficiently lower than pure EZE. Complexes prepared by coevaporation and kneading method, which exhibited good dissolution profile and lower MDT values, were used for the formulation studies.
Calculated f 2 values are presented in Table 4. From this Table, it is evident that the release profile of KNH and COEH is highly different from pure EZE (f 2 values 31.25 and 28.55). Even release profiles of pure EZE from COEB, KNB PMB, and PMH are also significantly different from pure EZE in dissolution medium.
Formulation studies
The complexes prepared by kneading and coevaporation method (KNB, COEB, KNH and COEH) were studied for physical properties to judge its tableting ability. In general, compressibility index values up to 15% and angle of repose between 25° and 30° results in good to excellent flow properties. % compressibility, angle of repose for complexes and physical properties of tablets made using these complexes are shown in Table 5. These values indicated good compressibility and flow properties, making these samples suitable for tableting.
The tablets prepared using complexes showed faster and reproducible release as compared to the tablets containing pure EZE and no CDs. Tablets prepared using COEH & KNH showed 73.1 and 70.0 release in 4 h with t50% of 73 min and 110 min, respectively (Fig. 7). Tablets prepared using COEB and KNB also showed better dissolution profiles as compared to tablets prepared using EZE alone. This confirmed the advantage of improved aqueous solubility of EZE in its complex form, which can be formulated as tablets with better dissolution characteristic. Release profiles of EZE from conventional tablets containing EZE alone are significantly different from tablets containing KNH and COEH as the f 2 values were 39.12 & 34.78. MDT of EZE from tablets containing KNH and COEH (40.84 & 36.94 min) were significantly lower than that of conventional tablets containing only EZE (72.34 min) (Table 3).
Conclusion
Solubility studies showed a significant, linear increase in the aqueous solubility of Ezetimibe with increasing concentrations of β-CD and HPβ-CD. At maximum studied concentration of β-CD and HPβ-CD (14 mM/L at 37 °C) resulted in 17.39-fold and 27.31-fold improvement in the saturation solubility of Ezetimibe. Inclusion complexes of Ezetimibe with β-CD and HPβ-CD were prepared successfully by coevaporation and kneading coevaporation method in a molar ratio of 2:1. This was confirmed by FTIR, PXRD and DSC studies. The highest improvement in solubility and in vitro drug release were observed in inclusion complex prepared with HPβ-CD by coevaporation method. Improvement in solubility and in vitro drug release of Ezetimibe were more with HPβ-CD as compared to β-CD. The solubility and in vitro drug release of the physical mixture, when compared to that of the complexes prepared by kneading and coevaporation method, was improved to a lesser degree. These findings are extremely important from a commercial point of view as the prepared complex removes draw back of poor dissolution profile of Ezetimibe.
References
Proudfoot, S.: Factors affecting bioavailability: factors influencing drug absorption from the gastrointestinal tract. In: Aulton, M.E. (Ed.), Pharmaceutics: The Science of Dosage From Design, pp. 135–173. Churchill Livingstone London, UK (1991)
Pitha, J., Milecki. J., Fales, H., Pannell, L., Uekama, K.: Hydroxypropyl-β-cyclodextrin: preparation and characterization; Effects on solubility of drugs. Int. J. Pharm. 29: 73–82 (1986)
Duchêne, D.: Cyclodextrins and their industrial uses. Editions de Santé Paris, SS. 447–460 (1987)
Uekama, K., Otagiri, M.: Cyclodextrins in drug carrier systems. Crit. Rev. Ther. Drug Carrier Syst. 3, 1–40 (1987)
Szejtli, J.: Medicinal applications of cyclodextrins. Med. Res. Rev. 14, 353–386 (1994)
Baboota, S., Dhaliwal, M., Kohli, K.: Physicochemical characterization, in vitro dissolution behavior, and pharmacodynamic studies of rofecoxib-cyclodextrin inclusion compounds. AAPS Pharm. Sci. Tech. 6, E83–E90 (2005)
Ghorab, M.K., Adeyeye, M.C.: Elucidation of solution state complexation in wet-granulated oven-dried ibuprofen and β-cyclodextrin: FT–IR and 1H–NMR studies. Pharm. Dev. Technol., 6, 315–324 (2001)
Veiga, F.J., Fernandes, C., Carvalho, R.A., Geraldes, C.F.: Molecular modeling and 1H–NMR: ultimate tools for the investigation of tolbutamide: β-cyclodextrin and tolbutamide: hydroxypropyl-β-cyclodextrin complexes. Chem. Pharm. Bull. 49, 1251–1256 (2001)
Tirucherai, G.S., Mitra, A.K.: Effect of hydroxypropyl beta cyclodextrin complexation on aqueous solubility, stability, and corneal permeation of acyl ester prodrugs of ganciclovir. AAPS Pharm. Sci. Tech. 4, E45 (2003)
Nalluri, B.N., Chowdary, K.P.R., Murthy, K.V.R., Hayman, A.R., Becket, G.: Physicochemical characterization and dissolution properties of nimesulide-cyclodextrin binary systems. AAPS Pharm. Sci. Tech. 4, E2 (2003)
Moore, J.W., Flanner, H.: Mathematical comparison of dissolution profiles. Pharm. Tech. 20, 64–74 (1996)
Reppas, C., Nicolaides, E.: Analysis of drug dissolution data, In: Dressman, J.B., Lennernäs, H. (Eds.), Oral drug absorption prediction and assessment, pp. 229–254. Marcel Dekker, New York (2000)
Vueba, M.L., Batista de Carvalho, L.A.E., Veiga, F., Sousa, J.J., Pina, M.E.: Influence of cellulose ether polymers on ketoprofen release from hydrophilic matrix tablets. Eur. J. Pharm. Biopharm. 58, 51–59 (2004)
Higuchi, T., Connors, K.: Phase solubility techniques. Adv. Anal. Chem. Instru. 4, 17–123 (1965)
Peri, D., Wyandt, C.M., Cleary, R.W., Hickal, A.H., Jones, A.B.: Inclusion complexes of tolnaftate with β- Cyclodextrin and hydroxy-β-cyclodextrin. Drug Dev. Ind. Pharm. 20, 1401–1410 (1994)
Labenderia, J.J.T., Lopez, M.E., Penin, L.S., Jato, J.L.V.: Glibornuride-β-cyclodextrin inclusion complexes: Preparation, structural characterization, and in vitro dissolution behavior. J. Pharm. Biopharm., 39, 255–259 (1993)
Chengsheng, L., Kashappa, H.D.: Enhancement of dissolution rate of valdecoxib using solid dispersions with polyethylene glycol 4000. Drug Dev. Ind. Pharm. 1, 1–10 (2005)
Hedges, A.R.: Industrial applications of cyclodextrins. Chem. Rev. 98, 2035– 2044 (1998)
Vromans, H., Eisson, A.C., Coenraad, F.L.: Mechanism of dissolution of drug-cyclodextrin complexes. Drug Dev. Ind. Pharm. 15, 250–255 (1989)
Acknowledgements
We would like to thank Zydus Cadila, India for donating EZE and conducting PXRD studies of the samples. We are grateful to Maan Pharmaceuticals Ltd. for providing formulation excipients.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Patel, R., Bhimani, D., Patel, J. et al. Solid-state characterization and dissolution properties of ezetimibe–cyclodextrins inclusion complexes. J Incl Phenom Macrocycl Chem 60, 241–251 (2008). https://doi.org/10.1007/s10847-007-9371-7
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10847-007-9371-7