Abstract
A new polystyrene based membrane electrode of methyl substituted 6,7:13,14-dibenzo-2,4,9,11-tetraphenyl-1,5,8,12-tetraazacyclotetradeca-1,4,6,8,11,13-hexaene (I) with sodium tetraphenylborate (NaTPB) and dibutyl phthalate (DBP) as anion excluder and plasticizing agent was prepared and investigated as Hg (II)-selective electrode. The electrode exhibits a Nernstian response for Hg (II) ions over a wide concentration range of 1.0 × 10−1–8.9 × 10−6 M with a slope of 30 ± 1 mV per decade concentration. It has a response time of 10 s and can be used for at least 4 months without any divergence in potentials. The membrane works satisfactorily in a partially non-aqueous medium up to a maximum 30% (v/v) content of methanol and ethanol. The proposed sensor revealed good selectivity over a wide variety of other cations including alkali, alkaline earth, heavy and transition metal ions and could be used in a pH range of 2.5–5.0. Normal interferents like Ag+, Cd2+ and Pb2+ low interfere in the working of the electrode. The electrode was successfully used in the direct determination of Hg2+ in aqueous solution.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
The determination of mercury is important, due to its toxicity even in low concentration. Over exposure to mercury results in ill effects on the human body systems nervous systems and other body systems. Mercury exposure is still an occupational hazard for people in many industries and in environment. The need for mercury ion determination in clinical analysis and environmental monitoring has led to a number of methods for the measurement of this analyte. The available method for low-level determination of mercury and other heavy metals in solution include AAS, but it involves expensive instrumentation and sample pretreatment, that is time consuming and inconvenient. Ion-selective electrodes have been convenient tools for measuring ion activity or concentration in various fields of chemical analysis. Potentiometric sensors have been used for the measurement of a wide variety of different ions directly in complex biological and environmental samples [1, 2]. In recent years macrocyclic ligands have been widely used as suitable neutral carriers for constructing ion-selective electrodes for various metals ions [3–6].
The use of ion-selective electrode is simple and even allows in vivo measurements. A variety of potential ion-carriers have been used in the construction of mercury (II) selective electrodes. The development of a selective electrode for mercury has been a subject of investigation to analytical chemists. So far, it has not been possible to have a good electrode for this ion and efforts in this direction are called for. To improve the analytical selectivity, it is essential to search carrier compounds that would react with mercury at high selectivity levels. Many organic and inorganic compounds have been tested as ionophore in producing ISEs, including Schiff’s bases [7], amide derivatives [8] oxamides [9], macrocycle [10] crown ethers [11–15], salicyldehyde thiosemicarbazone [16], p-tert-butyl calix-4-crowns with imine units [17], calixarene derivative [18] mercapto compounds [19, 20] and acyclic neutral carriers [21–27]. Availability of improved highly selective materials, have opened up new channel for developing specific sensors.
In recent years, we have used macrocyclic ligands as neutral carriers in preparation of new ion-selective electrodes for some transition and heavy metal ions [28–33]. There is a lack of efficient commercial mercury (II) ion-selective electrodes and even quite sparse literature reports on such electrodes. Efforts were initiated by us to develop selective electrodes for Hg2+ ions using macrocyclic ligands as sensor material. The most important requirement for an ionophore to act as good electroactive materials in membranes is its ability to act as a selective extractant, or to form strong complexes, preferentially with only target metal ions. In this paper, we report the electroanalytical applicability of a methyl substituted 6,7:13,14-dibenzo-2,4,9,11-tetraphenyl-1,5,8,12-tetraazacyclotetradeca-1,4,6,8,11,13-hexaene as an excellent neutral carrier in construction of mercury ion-sensor.
The result presented in this paper show that the sensor developed for Hg (II) ion using the above system as electroactive phase in polystyrene matrix has a wide concentration range and a fast response time with reproducible results.
Experimental
Reagent and materials
Analytical grade reagents and chemical were used. Double distilled water was used for the preparation of solutions of metal salts of different concentration by diluting stock standard solutions (0.1 M). Sodium tetraphenyl borate (NaTPB) from BDH (U.K.), dibutylphthalate (DBP) from Reidel (India), dioctylpthalate (DOP), dibutylbutylphosphonate (DBBP) and chloronaphthalane (CN) were purchased from Merck and used as received. Polystyrene was obtained from G.S.C. (India) and mercury (II) was used as mercuric chloride for the studies of the membrane sensor.
Synthesis of macrocycle (I)
Methanolic solution of 1,3-diphenyl-1,3-propanedione (0.02 mol, 4.48 mL) was added to methanolic solution of 3,4–diaminotoluene (0.02 mol, 2.44 mL). The resulting mixture was refluxed for 10 h with constant stirring after which it was reduced to half of its original volume by heating and then cooled at room temperature. Precipitate thus obtained was washed thoroughly with methanol, dried in vacuum, and recrystallised to get 6,7:13,14-dibenzo-2,4,9,11-tetraphenyl-1,5,8,12-tetraazacyclotetradeca-1,4,6,8,11,13-hexaene (I). Yield: 75%, m.p.185 °C. Elemental analysis: [C44H36N4–calculated (%): C, 85.2; H, 5.8; N, 9.0 found (%): C, 85.6; H, 5.9; N, 8.5]. The NMR studies of the ligand gave singlets at δ 1.44 (6H, methyl protons), 2.30–2.86 (4H, methylene protons) and 6.80–7.30 (26H, aromatic protons) (Fig. 1).
Electrode preparation
A number of membranes [34–36] were prepared. Membranes of adequate strength, which gave reproducibility and stable potential with a fast response time and which did not develop cracks on prolonged use were prepared. To meet these requirements, compositions in varying ratio of macrocycle to polystyrene were tested (Table 1). The mixture was heated to 80 °C (The softening point of polystyrene) under a pressure of ca. 6000–6500 psi in a dye kept in a metallurgical specimen mount press. Membranes were fabricated under optimum conditions of temperature and pressure, which were established after lengthy preliminary investigations. Membranes prepared in this way were quite stable, cream in color and did not show any dispersion in water and in other electrolyte solution.
The membranes were also investigated under a microscope to observe the surface cracks and homogeneity. Suitable membrane were then exposed to electrochemical examination and only membranes which gave reproducibility and stable potentials with a fast response time were selected for further studies. Membrane to membrane (and batch to batch) reproducibility was assured by carefully controlling the condition of fabrication. The membranes (2.5 cm diameter and 0.5 cm thick) were affixed to one end of a small Pyrex glass tube with araldite, while the other remained open.
Potential measurements
The membranes were equilibrated with 1.0 M Hg (II) chloride solution for 6 days and the potential across the membrane was measured by setting up the following cell assembly: Hg/Hg2Cl2 | KCl (satd.) | 1.0 M HgCl2 || membrane || test solution | Hg/Hg2Cl2 | KCl (satd.)
Results and discussion
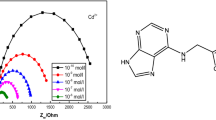
Due to the solubility of the ligand in water and the presence of four donating nitrogen atoms in its structure, it was expected to act as a suitable ion carrier in the polystyrene matrix with respect to metal ions of proper size and charge. The potential response of the membrane was determined as a function of activity not only of Hg2+ but many other ions and the result obtained are shown in Fig. 2. It is seen from figure that the electrode has a better response Nernstian linearity and detection limit for Hg2+ than for other metal ions. We therefore studied in detail the properties of the macrocyclic ligand doped membrane for mercury(II) ion.
The sensitivity and selectivity obtained for a given ionophore depend significantly on the membrane composition and the properties of the solvent mediator employed as well as the polystyrene/plasticizer ratio used [37–42]. It is expected that the solvent mediators play a key role in determining the ion selective characteristics. Therefore, various solvent mediators, viz. DBP, DOP, DBBP and CN were used in preparation of ion-selective electrodes (Table 1). Among the various membranes prepared using the ion-active phase (I), the DBP plasticizer was found to give the best sensitivity and widest linear range. The effect of relative amount of ionophore on the response function of membrane was investigated. NaTPB was also added to the membrane components for better results. The results indicate the best sensitivity and linear range are obtained for membrane number 3 with composition (w/w) 6:1:1:1 (I:polystyrene:DBP:NaTPB).
The ionic strength of Hg(NO3)2 solution use to calibrate the sensors in given range was 0.3 M and all studied was carried out at pH∼4. It exhibits the best working concentration range of 8.9 × 10−6–1.0 × 10−1 M with a slope of 30.0 mV/dacade of activity. The working concentration ranges and slopes for all the membrane electrodes are given in Table 1. It is apparent from Table 1, that the membrane no. 4 with a composition 8:1:1 (I:polystyrene:NaTPB) without solvent mediator shows linearity in the concentration range 5.0 × 10−5–1.0 × 10−1 M with a slope of 28.0 mV per decade of activity. The addition of plasticizer (DBP) enhanced the working concentration range. The Fig. 3 shows the working concentration ranges and slopes for the membrane electrodes (nos. 1–4). The potentials were measured as a function of Hg2+ activity with internal solution concentration of 1.0 × 10−1 M with DBP as plasticizer. The membrane with DOP, DBBP and CN as plasticizers gave poor performance with regards to working concentration range, slope and response time. Hence membrane no.3 was chosen for further electroanalytical studies.
The static response time of the electrode was determined by measuring the time required to achieve a 90% of the steady potential. The membrane without plasticizers but with NaTPB showed a response time off 20 s while the electrodes with plasticizers as membrane ingredients achieved equilibrium response within 10–25 s over the whole concentration range. The best response time recorded was 10 s for membrane no. 10 having DBP as plasticizer. The potential generated by this membrane remained stable for more than 3 min after which it started deviating. The main factor for limited lifetime is the loss of ionophores from the membrane while contacting with aqueous solution. Sufficient lipophilicity of ionophore and plasticizer ensures stable potentials and long lifetime [43, 44]. The lifetime of the electrodes was worked out by performing calibrations periodically with standard solutions and calculating the response, slope over the range 1.0 × 10−6–1.0 × 10−1 HgCl2 solution. The experimental result show that the lifetime of the present electrode was over a period of four months without significant change in potentials. During this period, the detection limit and the slope of the electrode remained almost constant. Subsequently the electrochemical behavior of the electrode gradually deteriorated which may be due to ageing of the polymer, plasticizers and ionophore. Whenever a drift in potential was observed, membranes were re-equilibrated with 0.5 M Hg2+ solution for 2 days.
The effect of pH for electrode was examined by use of 1.0 × 10−2 and 1.0 × 10−3 M mercury nitrate solutions. The pH was adjusted by introducing small drops of nitric acid (0.1 M) and sodium hydroxide (0.1 M). The influence of the pH response on the membrane electrode is shown in Fig. 4. As it can be seen, the potentials were found to stay constant from 2.5 to 5.0, beyond which the potential changes considerably. Under more acidic conditions, the ligand may be protonated and thereby losing its capacity to form a complex with the target metal ion. When the pH is neutral the cation [HgOH]+ react with ligand. The drift of potential values >5.0 are attributed due to the formation of mercury(II) hydroxide.
The functioning of the electrode was also investigated in partially non-aqueous medium using methanol–water, ethanol–water and acetone–water mixtures. It was observed that the sensor could tolerate up to 30% (v/v) and slope remains unaltered. However, above 30% non-aqueous content the slope decreases appreciably because of the membrane decomposes due to leaching of ionophore from matrix. The working concentration range was reduced and potential showed a drift.
The selectivity behavior is obviously one of the important characteristics of the ion-selective electrodes, determining whether reliable measurement in the target sample is possible [35, 41]. In order to assess the selectivity of the proposed Hg (II) selective electrode over other cations, the matched potential method (MPM) [45] was employed. According to MPM, the selectivity coefficient is defined as the activity ratio of the primary ion and interfering ion that gives the same potential change in a reference solution. Thus, the change in potential upon changing the primary ion activity is measured. Then the interfering ion is added to an identical reference solution until the same potential change is obtained. The selectivity coefficient, \( K^{{Pot}}_{{A,B}} \) is determined as
In this method, the specified activity of the primary ion (a Hg = 1.0 × 10−5 M) is added to reference solution (a′ Hg = 5.0 × 10−5 M) of mercury ions, and the potential is measured. In a separate experiment interfering ion concentrations (a B = 1 × 10−4–1 × 10−1 M) were added to an identical reference solution, until the measured potential matched that obtained before the addition of the primary ions. The selectivity coefficients were then given by the resulting primary to interfering ion activity ratio. The resulting selectivity coefficients are summarized in Table 2. It is clear that the proposed mercury sensor is highly selective with respect to other common cations. Potential interferents Ag+, Cd2+, and Pb2+, have low effect on the functioning of Hg (II) electrode. In Table 3, the response characteristics and selective coefficients of the membrane electrode based on proposed ligand are compared along with previously reported based on variety of different ionophores (7, 17, 23–25). The comparison of values of selectivity coefficients determined using the MPM with those obtained with SSM or FIM methods is valid only to some extent. As can be seen, the working concentration range, response time, life time, pH range and potentiometric selectivity of the proposed electrode are comparable to those other reported mercury ion-selective electrode.
The electrode was also successfully applied to the direct determination of mercury in different water samples containing added Hg2+ ion and the result are given in Table 4. As can be seen, the recovery of mercury from different water samples is almost quantitive.
Conclusion
The mercury (II) ion-selective sensor, based on the use of methyl substituted 6,7:13,14-dibenzo-2,4,9,11-tetraphenyl-1,5,8,12-tetraazacyclotetradeca-1,4,6,8,11,13-hexaene ligand exhibits a high selectivity and sensitivity to mercury(II) ions and fast potential response. The selectivity of the electrode towards Hg2+ is very good over other cations and also shows low interference from Ag+, Cd2+, and Pb2+. The functional pH range of the proposed electrode is 2.5–5.0. A comparison between the response characteristics of the proposed potentiometric sensor and those of previously reported mercury ion-selective electrode indicated that the present sensor is invariably superior.
References
Meyerhoff, M.E., Opdycke, M.N.: Ion-selective electrodes. Adv. Clin. Chem. 25, 1–47 (1986)
Moody, G.J., Saad, B.B., Thomas, J.D.R.: The development of polymer matrix membranes for ion-selective electrodes. Electrode. Rev. 10, 71–106 (1988)
Mousavi, M.F., Alizadeh, N., Shamsipur, M., Zohari, N.: A new PVC-based 1, 10-dibenzyl-1, 10-diaza-18-crown-6 selective electrode for detecting nickel (II) ion. Sens. Actuators B Chem. 66, 98–100 (2000)
Shamsipur, M., Kazemi, S.Y., Niknam, K., Sharghi, H.A.: A new PVC membrane electrode based on a Thia-substituted macrocyclic diamide for selective potentiometric determination of silver ion. Bull. Korean. Chem. Soc. 23, 53–58 (2002)
Panwar, A., Baniwal, S., Singh, R., Sharma, C.L., Singh, A.K.: Strontium(II)-selective electrode based on a macrocyclic ligand. Anal. Lett. 34, 2415–2428 (2001)
Singh, A.K., Panwar, A., Singh, R., Baniwal, S.A.: A new macrocyclic polystyrene-based sensor for chromium (III) ions. Anal. Bioanal. Chem. 372, 506–510 (2002)
Mashhadizadeh, M.H., Sheikhshoaie, I.: Mercury(II) ion-selective polymeric membrane sensor based on a recently synthesized Schiff base. Talanta 60, 73–80 (2003)
Siswanta, D., Kin, M., Hisamoto, H., Suzuki, K.: Novel Hg2+-Ionophores based on N-Hydroxylamide derivatives as a sensory molecule for an ion-selective electrode. Chem. Lett. 1011–1019 (1996)
Linder, E., Toth, K., Pungor, E.: Lead-selective neutral carrier based liquid membrane electrode. Anal. Chem. 56, 1127–1131 (1984)
Singh, A.K., Bhattacharjee, G., Singh, R.: Mercury(II)-selective membrane electrode using tetrathia-diazacyclotetradeca-2,9-diene as neutral carrie. Sens. Actuators B 99, 36–41 (2004)
Sheen, S., Shih, J.: Lead(II) ion-selective electrodes based on crown ethers. Analyst 117, 1691–1695 (1992)
Gupta, V.K., Chandra, S., Agarwal, S.: Mercury selective electrochemical sensor based on a double armed crown ether as ionophore. Indian J. Chem. 42, 813–818 (2003)
Fakhari, A.R., Ganjali, M.R., Shamsipur, M.: PVC-based hexathia-18-crown-6-tetraone sensor for mercury (II) ions. Anal. Chem. 69, 3693–3696 (1997)
Brozozka, Z., Pietraszkiewicz, M.: Mercury ion-selective polymeric membrane electrodes based on substituted diaza crown ethers. Electroanalysis 3, 855–858 (1991)
Javanbakht, M., Ganjali, M.R., Eshghi, H., Sharaghi, H., Shamsipur, M.: Mercury(II) ion-selective electrode based on dibenzo-diazathia-18-crown-6-dione. Electroanalysis 11, 81–89 (1999)
Mahajan, R.K., Kaur, I., Lobana, T.S.: A mercury(II) ion-selective electrode based on neutral salicylaldehyde thiosemicarbazone. Talanta 59, 101–106 (2003)
Mahajan, R.K., Kaur, R., Kaur, I., Sharma, V., Kumar, M.: Mercury(II) ion-selective electrodes based on p-tert-butyl calix[4]crowns with imine units. Anal. Sci. 20, 811–814 (2004)
Lu, J., Tong, X., He, X.: A mercury ion-selective electrode based on a calixarene derivative containing the thiazole azo group. J. Electroanal. Chem. 540, 111–117 (2003)
Bagheri, M., Mashhadizadeh, M.H., Razee, S., Momeni, A.: Hg2+-selective membrane potentiometric sensor based on a recently synthesized mercapto compound. Electroanalysis 15, 1824–1829 (2003)
Mazloom, M., Amini, M.K., Baltork, M.I.: Mercury selective membrane electrodes using 2-mercaptobenzimidazole, 2-mercaptobenzothiazole, and hexathiacyclooctadecane carriers. Sens. Actuators B 62, 80–85 (2000)
Hassan, S.S.M., Saleh, M.B., Gaber, A.A.A., Mekheimer, R.A.H., Kream, N.A.A.: Novel mercury (II) ion-selective polymeric membrane sensor based on ethyl-2-benzoyl-2-phenylcarbamoyl acetate. Talanta 53, 285–293 (2000)
Hassan, S.S.M., Mahmoud, W.H., Mohamed, A.H.K., Kelany, A.E.: Mercury(II) ion-selective polymeric membrane sensors for analysis of mercury in hazardous wastes. Anal. Sci. 22, 877–881 (2006)
Gupta, V.K., Chandra, S., Lang, H.: A highly selective mercury electrode based on a diamine donor ligand. Talanta 66, 575–580 (2005)
Singh, L.P. and Bhatnagar, J.M.: Chelating ionophores based electrochemical sensor for Hg(II) ions. J. Appl. Electro. 34, 391–396 (2004)
Perez-Martin, L., Otazo-Sanchez, E., Macedo-Miranda, G., Avila-Perez, P., Alonso Chamaro, J., Lopez-Valdivia, H.: Mercury(II) ion-selective electrode. Study of 1,3-diphenylthiourea as ionophore. Analyst 125, 1787–1790 (2000)
Ibrahim, I., Cemal, Y., Humeyra, B.: Construction and response characteristics of a sulfite/hydrogen sulfite-selective all-solid-state contact electrode based on the 4-methylpiperidinedithiocarbamate complex of mercury(II). Analyst 121, 1873–1876 (1996)
Saleh M., Soliman E.M., Gaber A.A.A., Ahmed S.A.: A novel Hg(II) PVC membrane sensor based on simple ionophore ethylenediamine bis-thiophenecarboxaldehyde. Anal. Lett. 39, 659–673 (2006)
Jain, A.K., Singh, A.K., Mehtab, S., Saxena, P.: Rubeanic acid as novel carrier in construction of PVC based La(III)-selective membrane sensor. Anal. Chim. Acta. 551, 45–50 (2005)
Singh, A.K., Saxena, P., Mehtab, S., Gupta, B.: Stronium(II)-selective electrode based on a macrocyclic teraamide. Talanta 69, 521–526 (2006)
Singh, A.K., Jain, A.K., Saxena, P., Mehtab, S.: Zn(II)-selective membrane electrode based on tetraazamacrocycle [Bzo2Me2Ph2(16)eneN4]. Electroanalysis 18, 1186–1193 (2006)
Singh, A.K., Shailendra, Panwar, A., and Baniwal, S.: Chromium(III)-selective electrode based on a macrocyclic compound. Analyst 124, 521–525 (1999)
Singh, A.K., Sharma, C.L., Baniwal, S., Singh, R., Panwar, A.: Strontium(II)-selective electrode based on macrocyclic ligand. Anal. Lett. 34, 2415–2428 (2001)
Singh, A.K., Bhattacharjee, G., Singh, R.: Mercury (II)-selective membrane electrode using tetrathiadiazacyclotetradeca-2,9-diene as neutral carrier. Sens. Actuators B Chem. 99, 36–41 (2004)
Meier, P.C., Morf, W.E., Laubli, M., Simon, W.: Evaluation of the optimum composition of neutral-carrier membrane electrodes with incorporated cation exchanger sites. Anal. Chim. Acta. 156, 1–8 (1984)
Saex de Viteri, F.J., Diamond, D.: Determination and application of ionselective electrode model parameters using flow injection and simplex optimization. Analyst 199, 749–758 (1994)
Panwar, A., Baniwal, S., Sharma, C.L., Singh, A.K.: A polystyrene based membrane electrode for cadmium(II) ion. Fresenius J. Anal. Chem. 348, 768–772 (2000)
Buck, R.P., Cosofret, V.V.: Recommended procedure for calibration of ionselective electrode. Pure. Appl. Chem. 65, 1849–1858 (1993)
Lai, M.T., Shih, J.S.: Mercury(II) and silver(I) ion-selective electrodes based on dithia crown ethers. Analyst 111, 891–895 (1986)
Gupta, V.K., Jain, S., Khurana, U.: A PVC-based pentathia-15-crown-5 membrane potentiometric sensor for mercury(II). Electroanalysis 9, 478–480 (1997)
Perez, M.A., Marin, L.P., Quintana, J.C., Pedram, M.Y.: Influence of different plasticizers on the response of chemical sensors based on polymeric membranes for nitrate ion determination. Sens. Actuators B Chem. 89, 262–268 (2003)
Mazloum, M., Amini, M.K., Baltork, M.I.: Mercury selective membrane electrodes using 2-mercaptobenzimidazole, 2-mercaptobenzothiazole, and hexathiacyclooctadecane carriers. Sens. Actuators B Chem. 63, 80–85 (2000)
Hassan, S.S.M., Saleh, M.B., Gaber, A.A.A., Mehheimer, R.A.H., Kream, N.A.A.: Novel mercury (II) ion-selective polymeric membrane sensor based on ethyl-2-benzoyl-2-phenylcarbamoyl acetate. Talanta 53, 285–881 (2000)
Zhang, W., Jenny, L., Spichiger, U.E.: A comparison of neutral Mg2+-selective ionophores in solvent polymeric membranes: complex stoichiometry and lipophilicity. Anal. Sci. 16, 11–16 (2000)
Meier, P.C., Ammann, D., Morf, M.E., Simon, W.: In: Koryta, J. (Ed.) Medical and biological application of electrochemical devices, pp. 19–25. J.Wiley & Sons, New York, (1980)
Umezawa, Y., Umezawa, K., Sato, H.: Selectivity coefficients for ionselective electrode: Recommended methods for reporting values. Pure. Appl. Chem. 67, 507–518 (1995)
Acknowledgement
Authors are grateful to the Ministry of Human Resources Development, New Delhi, India for providing financial assistance to undertake this work.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Singh, A.K., Singh, R.P. & Mehtab, S. Mercury-selective membrane electrode based on methyl substituted dibenzo tetraphenyl tetraaza macrocycle. J Incl Phenom Macrocycl Chem 60, 9–15 (2008). https://doi.org/10.1007/s10847-007-9344-x
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10847-007-9344-x