Abstract
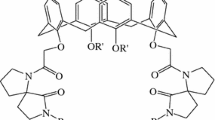
Two novel chromogenic cone calix[4]dibenzothiacrown ethers 3 and 4 in which nitrophenylazo groups attached at the phenyl ring of dibenzothiacrown unit were described. The extraction properties of 3 and 4 toward different transition metal ions have been studied using conductometric technique and found to exhibit Cu2+ and Hg2+ selectivity with very high stability constants range from log K assoc = 5.19 to log K assoc = 8.72.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Recognition and sensing of heavy and transition metal ions via artificial receptors are of current interest in supramolecular chemistry because of their significant importance in chemical, biological and environmental assays. Calixarenes are ideal building blocks for the development of chromogenic receptors in molecular recognition of ionic species of chemical and biological interest [1]. The incorporation of an appropriate sensory group into the calixarene having a preorganized substrate binding site results in a tailored chromogenic receptor. A variety of chromogenic calixarenes bearing nitrophenol, nitrophenylazophenol, indoaniline, indophenol and azophenol have been synthesized and show high selectivity in recognition for cations and organic molecules [2–10]. Most of the research on calix[4]arenes as chromogenic ionophores involves the modification of the lower rim with metal chelating groups such as acids, amides, esters, ketones or crown ethers [11–18]. Several chromogenic calix[4]crown ethers have been synthesized by incorporating the chromogenic moiety mostly into the upper rim or rarely into the periphery of crown loops.
In our recent work, we synthesized a series of calixdibenzothiacrown ethers 1–2 and studied their complexation properties with some transition metal ions [19]. As a continue to this work, we report herein the synthesis of new chromogenic ionophores 3 and 4 based on incorporating of p-nitrophenylazo chromogenic groups at the benzene ring of the crown loop and their cation binding ability for the transition metal ions (Scheme 1).
Experimental
Melting points are uncorrected. 1H and 13C NMR spectra were recorded on Brucker Ac-200 and Ac-50 MHz spectrometer, respectively. In all cases, samples were dissolved in CDCl3 using TMS as internal standard unless otherwise noted. The mass spectrometers that used for obtaining the mass spectra is the Agilent 1100 series LCMSD, model # G1946A. All materials were analytical grade and used without further purification. For conductivity experiments, acetonitrile (HPLC grade, GCC, assay 99.8%) was dried over calcium hydride and then double-distilled fractionally to give anhydrous solvent (<3 × 10−7 S cm−1). The following salts were obtained from the suppliers indicated: AgNO3 (GCC, 99%), Hg(ClO4)2·xH2O (BDH, 98%), Cu(ClO4)2·6H2O (Aldrich), Zn(ClO4)2·6H2O (Aldrich), Cd(ClO4)2 (Aldrich), Pb(ClO4)2·3H2O (Aldrich), Co(ClO4)2·6H2O (Alfa).
Chromatographic separations were performed on silica gel columns (60–120 mesh, CDH). Thin layer chromatography (TLC) was carried out using silica gel GF254 (Fluka). Unless otherwise noted, all reactions were carried out under dry nitrogen. The description of the conductometer and the details of the conductance measurements have been given previously [20]. Spectrophotometric measurements were carried out using Varian/Cary 2390 spectrophotometer connected to a thermostating unit (Haak Mess-Technik Gmbh U. Co. Type F3).
Synthesis
5-(4-nitrophenylazo)-2-hydroxybenzylalcohol (7)
To a 500-mL E-flask, p-nitroaniline (9.83 g, 0.071 mol) was added which contained a solution of 1:1 THF:H2O (150 ml) and NaNO2 (5.02 g, 0.072 mol). To the solution, which was being stirred at 0 °C, HCl (8.55 mL, 12 M), was slowly added. This solution was added drop wise to 500-mL single neck R.B.F which contained 2-hydroxybenzyl alcohol 6 (8.0 g, 0.065 mol) in a solution of 1:1 THF:H2O (150 mL) at 0 °C. The final solution was stirred at 0 °C for 45 min. The resulting dark red solution was extracted with diethyl ether (500 mL). The organic layers were dried over anhydrous MgSO4, filtered and the filtrate reduced to give dark red solid. The crude product was treated with CHCl3 or diethyl ether, filtered and washed with CHCl3 to give dark red-brown solid 7 (5.6 g, 32%); mp 165–166 °C; 1H NMR δH (CDCl3): 5.30 (d, J = 6 Hz, 2H), 7.40 (d, J = 6 Hz, 2H), 7.75 (d, J = 4 Hz, 1H), 7.90 (br, 1H), 7.98 (d, J = 8 Hz, 2H), 8.12, (s, 1H), 8.38 (d, J = 8 Hz, 2H); 13C NMR δc (acetone-d 6): 60.1, 116.1, 124.0, 124.3, 126.0, 126.1, 130.1, 147.6, 150.0, 157.1, 161.0; MS m/z: 273 (M+, 27), 208 (4), 149 (26), 123 (54), 105 (88).
5-(4-nitrophenylazo)-2-(2-bromoethoxy)benzylalcohol (8)
In a 250 mL one-necked R. B. flask equipped with a magnetic stirrer bar and a reflux condenser, compound 7 (2.0 g, 7.2 mmol), 1, 2-dibromoethane (13.6 g, 72 mmol) and anhydrous K2CO3 (1.0 g, 7.2 mmol) were mixed with anhydrous CH3CN (150 mL). The mixture was refluxed for 24 h and then cooled to room temperature, filtered and the solid was washed with CH3CN. The filtrate was evaporated to dryness to give crude dark brown solid which was washed with diethyl ether to give 8 (2.55 g, 92%); mp 75–76 °C; 1H NMR δH (CDCl3): 3.76 (t, J = 4 Hz, 2H), 4.45 (t, J = 4 Hz, 2H ), 4.83 (d, J = 6 Hz, 2H), 7.0 (d, J = 6 Hz, 1H), 7.98 (br, 1H), 8.0 (s, 1H), 8.10 (d, J = 8 Hz, 2H), 8.40 (d, J = 8 Hz, 2H); 13C NMR δc(CDCl3): 29.2, 61.9, 66.2, 110.8, 122.3, 122.4, 122.7, 124.6; MS m/z: 271 (M+-CH2CH2Br, 37), 149 (72), 121 (100), 93 (41), 75 (24), 65 (71).
25, 27-O-Bis[2-hydroxymethyl-5-(4-nitrophenylazo)phenoxyethyl]-p-tert-butyl calix[4]arene (9)
In a 500 mL one-necked flask equipped with a magnetic stirrer bar and a reflux condenser, compound 8 (4.2 g, 11.1 mmol), p-tert-butylcalix[4]arene (3.60 g, 5.55 mmol), and anhydrous K2CO3 (4.6 g, 5.55 mmol) were mixed with anhydrous CH3CN (400 mL) and refluxed for 6 days. The mixture was filtered and the solid was washed with CH3CN. The combined filtrate was evaporated to dryness. The crude product was purified by flash chromatography using ethyl acetate:hexane (3:7) to give 9 as red solid (3.65 g, 53%); mp 123–125 °C 1H NMR δH(CDCl3): 1.11 (s, 18H, t-But), 1.26 (s, 18H, t-But), 3.42 (d, J = 16 Hz, 4H, ArCH 2 Ar), 4.33 (d, J = 16 Hz, 4H, ArCH 2 Ar), 4.30 + 4.50 (br, 8H, OCH 2 CH 2 O), 4.70 (d, J = 4 Hz, 4H, CH 2 OH), 4.96 (t, J = 2 Hz, CH2 OH), 6.68 (d, J = 4 Hz, 2H), 7.00 (s, 4H), 7.06 (s, 4H), 7.63 (d, J = 3 Hz, 2H), 7.94 (s, 2H), 7.98 (d, J = 10 Hz, 4H), 8.35 (d, J = 10 Hz, 4H), 8.70 (s, 2H, OH); 13C NMR δc(CDCl3): 31.0, 31.5, 32.0, 34.0, 34.1, 60.8, 67.5, 74.6, 111.0, 123.1, 124.8, 125.5, 127.0, 127.6, 128.0, 131.0, 133.2, 143.0, 146.6, 148.2, 148.3, 149.2, 156.0, 160.4; +APCI HRMS calcd for C74H82N6O12 (M+1)+ 1247.6, found 1247.5.
25, 27-O-Bis[2-chloromethyl-5-(4-nitrophenylazo)phenoxyethyl]-p-tert-butylcalix[4] arene (10)
To a solution of 9 (1.0 g, 0.8 mmol) in anhydrous CH2Cl2 (100 mL) was added freshly distilled SOCl2 (0.49 mL, 6.4 mmol) at room temperature. The reaction was stirred for 6 h and then cold water was added. The organic layer was separated and washed with water, dried and evaporated to give 10 as dark red solid (0.95 g, 92%). Sample was further purified for analysis by TLC using ethyl acetate:hexane (3:7); mp 118–120 °C (decomp.); 1H NMR δH(CDCl3):1.05 (s, 18H, t-But), 1.30 (s, 18H, t-But), 3.20 (d, J = 18 Hz, 4H, ArCH 2 Ar), 4.55–4.28 (m, 12H, OCH 2 CH 2 O and ArCH 2 Ar), 4.72 (s, 4H, CH2Cl), 6.86 (s, 4H), 7.00 (J = 3 Hz, 2H), 7.04 (s, 4H), 7.50 (s, 2H), 8.10–7.88 (m, 6H), 8.31 (d, J = 10 Hz, 4H); 13C NMR δc(CDCl3): 30.5, 31.4, 34.0, 41.5, 67.9, 73.6, 111.5, 123.5, 124.5, 124.7, 125.1, 125.6, 126.0, 127.5, 128.0, 132.6, 142.1, 146.5, 147.8, 148.6, 149.5, 150.5, 155.6, 159.0; +APCI HRMS calcd for C74H80Cl2N6O10 (M+1)+ 1283.5, found 1283.4.
General procedure for the synthesis of Azocalix[4]dibenzothiacrown Ethers 3 and 4
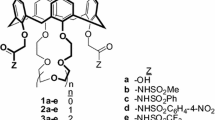
Macrocycles 3 and 4 were synthesized under high dilution conditions. In a 250 mL two-necked flask equipped with a magnetic stirrer bar and a reflux condenser and a gas line to maintain a nitrogen atmosphere, anhydrous K2CO3 (0.11 g, 0.39 mmol) was suspended in anhydrous CH3CN (100 mL). To this well-stirred solution at reflux temperature was added a solution containing dithiol [HS(CH2CH2S)nH, n = 1, 2] and dichloride 10 (0.39 mmol) in anhydrous CH3CN (100 mL) dropwise over a period of 10–12 h. The reaction mixture was further refluxed with stirring for another 12 h. The reaction mixture was filtered and the filtrate was evaporated. The residue was dissolved in CHCl3 (100 mL) and washed with H2O. The organic layer was dried over anhydrous MgSO4 and then evaporated. The purification was carried out as indicated by the entries below for the separate compounds.
Azocalix[4]dibenzothiacrown ethers (3)
The crude product was purified by column chromatography using ethyl acetate/hexane (3:7) as eluent to give 3 as dark red solid (0.11 g, 20%); mp 165–167 °C, (decomp); 1H NMR δH(CDCl3): 0.95 (s, 18H, t-But), 1.30 (s, 18H, t-But), 2.68 (s, 4H), 3.32 (d, J =14 Hz, 4H, ArCH 2 Ar), 4.01 (s, 4H),4.56–4.30 (br,12H), 6.76 (s, 4H), 6.95 (s, 2H), 8.03–7.85 (br, 6H), 8.30 (d, J = 6 Hz, 4H); 13C NMR δc(CDCl3): 30.0, 31.1, 31.5, 31.8, 32.0, 34.1, 67.1, 74.1, 111.2, 123.7, 125.0, 125.2, 126.0, 128.1, 129.8, 132.8, 141.8, 147.0, 147.6, 148.2, 149.8, 150.6, 155.8, 159.8; +APCI HRMS calcd for C76H84N6O10S2 (M+1)+1305.5, found 1305.3.
Azocalix[4]dibenzothiacrown ethers (4)
The crude product was purified by TLC using ethyl acetate/hexane (3:7) as eluent to give 4 as red solid (0.12 g, 23%); mp 152–155 °C (decomp); 1H NMR δH(CDCl3): 0.92 (s, 18H, t-But), 1.31 (s, 18H, t-But), 2.59 (br, 8H), 3.35 (d, J =12 Hz, 4H, ArCH 2 Ar), 3.95 (s, 4H), 4.48 (br,12H), 6.76 (s, 4H), 6.95 (s, 2H), 7.02 (d, J =3 Hz, 2H), 7.10 (s, 4H), 8.02–7.82 (m, 6H), 8.32 (d, d, J = 4 Hz, 4H); 13C NMR δc(CDCl3): 30.1, 31.2, 31.3, 32.0, 32.6, 34.0, 67.5, 74.5, 111.2, 123.2, 124.9, 125.0, 125.1, 125.2, 125.4, 128.0, 128.9 132.5, 141.9, 147.2, 147.5, 148.5, 149.6, 150.5, 156.0, 159.8; +APCI HRMS calcd for C78H88N6O10S3 (M+1)+1365.5, found 1365.4.
Results and discussion
Synthesis
Azocalix[4]dibenzothiacrown ethers 3 and 4 were synthesized using a method almost similar to that used in the preparation of 1–2 (Scheme 2) [19]. Salicylaldehyde was envisioned as being suitable precursor to condense with p-nitrodiazonium salt. However all attempts to synthesize the p-nitroazoaldehyde 5 were failed. This can be ascribed to the deactivating effect of the formyl group which deactivate the coupling reaction of the arenedizonium salt with the aromatic ring. Fortunately, condensation of 2-hydroxybenzylalcohol 6 with the diazonium salt produced the target compound 7 as dark orange solid in good yield which shorting the route by one step. Alkylation of compound 7 with an excess of 1, 2-dibromoethane in presence of two equivalents of anhydrous K2CO3 in refluxing anhydrous CH3CN afforded, after washing the crude product with diethyl ether, compound 8 in 92% yield as dark brown solid. Compound 9 was prepared by condensation of p-tert-butylcalix[4]arene with two equivalents of compound 8 in refluxing anhydrous CH3CN containing two equivalents of anhydrous K2CO3. Compound 9 was formed as red solid in 53% after purifying the crude product by flash chromatography using ethylacetate:hexane (3:7). Treatment of 9 with fresh distilled SOCl2 at room temperature in anhydrous CH2Cl2 for 6 h produced compound 10 as dark red solid in 92% yield. Base mediated coupling of equimolar amounts of ethane 1, 2-dithiol or bis(mercaptoethyl)sulfide and 10 afforded crude products of 3 and 4. Each of the resulting reaction products was purified by column chromatography to give 3 and 4 as orange solid in 20 and 23% yield, respectively. Compounds 3 and 4 were fully characterized and established to be in cone conformation by the presence of two sets of doublet signals in their 1H NMR spectra at δ 3.29 and 4.30 ppm and at δ 3.31 and 4.45 ppm, respectively due to the methylene bridge protons as well as by presence of two singlet signals due to the tert-butyl groups at δ 0.96 and 1.30 ppm, and at δ 0.93 and 1.31 ppm respectively.
Complexation studies
UV-vis spectral titrations
Upon addition of acetonitrile solution of Cu2+ or Hg2+ to acetonitrile solutions of either hosts 3 or 4, no distinct color change was observed. But UV-vis spectra of hosts 3 or 4 in presence of various concentrations of Cu2+ are shown in Figs. 1 and 2, respectively as typical example.
Although no presence of significant changes in the position of the absorption maxima, it can be seen that the absorbance in both figures increased continually upon addition of Cu2+. This change in absorption intensity indicates presence of a complexation taking place between Cu2+ and either hosts 3 or 4. Furthermore, it is noteworthy that the presence of isosbestic point at about 410 nm implies that two species, the host and host-Cu2+ complex, are present in equilibrium. Upon increasing equivalent numbers of Cu2+ (up to 15 equiv in Fig. 1 and up to 20 equiv. in Fig. 2), changes in the UV-vis spectra were only minor for both compounds 3 and 4 which imply that the complexes reached the saturation limit. Therefore, addition of greater than 15 or 20 equiv. of Cu2+ would not give any further significant spectral changes. The presence of the complexation between 3 or 4 and either Cu2+ or Hg2+ was confirmed also by a conductometric titration experiments as shown below.
Conductometric Titration:
In present complexation study, the conductometric method is used to evaluate both the stability constants of the complexes and the stoichiometry of the ligand:metal ratio.
According to the strength of complexation between the ligand and the metal cation, plot of molar conductance Λm (S cm2 mol-1) against [L]T/[M+]T show three different patterns for strong (two straight lines with a sharp break at the reaction stoichiometry), moderate (broad break in the curvature so the composition of the complex is determined by extrapolating the lines prior to and after the end-point of the titration), and weak (slight or nonexisting changes in the slope) complexes. Among the cations investigated in acetonitrile (Cu2+, Hg2+, Zn2+, Cd2+, Pb2+, Ag+), the complexation of Cu2+ with 3 and 4 led to titration curves which are the result of a combination of two straight lines intersecting at the reaction stoichiometry of 1:2, while for Hg2+ the intersecting of the two straight lines occurs at the reaction stoichiometry of 1:1 as shown in Figs. 3 and 4.
These finding indicate the formation of highly stable complexes between 3 or 4 and Cu2+ and Hg2+ cations in acetonitrile.
It is obvious, from Figs. 3 and 4, addition of ligands 3 or 4 to solutions of Cu2+ or Hg2+ results in a decrease in molar conductivity of the resulting solutions. This indicates that the resulting complexes are less mobile than free solvated Cu2+ or Hg2+. On the other hand, as shown in Fig. 5, addition of ligand 4 to solutions of Zn2+, Cd2+, Pb2+ or Ag+ results in an increase in molar conductivity of the resulting solutions. This indicates that the resulting complexes are more mobile than free solvated metal cations alone.
No detectible changes in molar conductances were found when a solution of 3 was added to metal solutions of Zn2+, Cd2+, Pb2+ or Ag+ (Figure not shown). This conclude that ligand 3 is more selective than ligand 4 toward the metal ions under investigation. The stability constants of the 3-M2+ and 4-M2+ complexes at 25 °C were calculated from variation of molar conductance as a function of [L]T/[M]T molar ratio using a nonlinear least-square program “simplex” reported elsewhere [21]. The stability constants (log K assoc ) for the complexes are listed in Table 1. As shown in Table 1, it is obvious that the stability constants of 4-Cu2+ and 4-Hg2+ complexes are higher than those of 3. This is may due to the larger cavity size of crown loop of 4 than the crown loop of 3 which match the diameter of both Cu2+ and Hg2+ metal cations.
References
(a) Hayashita, T., Takagi, M.: Comprehensive Supramolecular Chemistry, In: Gokel, G.W. (ed.), Elsevier: Oxford, 1996. (b) Vicens, J., Böhmer, V.: Calixarenes: A Versatile class of Macrocyclic Compounds, Eds.; Kluwer: Dordrecht 1991.
(a) Deligöz, H.: Azocalixarenes: synthesis, characterization, complexation, extraction, absorption properties and thermal behaviours. J. Incl. Phenom. Macrocyclic Chem. 55, 197–218 (2006) and references therein. (b) Ludwig, R., Dzung, N.T.K.: Calixarene-based molecules for cation recognition. Sensors. 2, 397–416 (2002) and references therein.
Shimizu, H., Iwamoto K., Fujimoto K., Shinkai S.: Chromogenic calix[4]arene. Chem. Lett. 2147–2150 (1991).
Yamamoto, H., Ueda K., Sandanayake K.R.A.S., Shinkai S.: Molecular design of chromogenic calix[4]crowns which show very high na+selectivity. Chem. Lett. 497 (1995).
King, M.A., Moore C.P., Sandanayake K.R.A.S., Sutherland I.O.: A highly selective chromoionophore for potassium based upon a bridged calix[4]arene. J. Chem Soc. Chem. Commun. 582–584 (1992).
Gordon, J.L., Böhmer, V., Vogt, W.: A calixarene-based chromoionophore for the larger alkali metals. Tetrahedron Lett. 36, 2445–2448 (1995).
McCarrick, M., Wu, B., Harris, S.J., Diamond, D., Barrett, G., McKervey, M.A.: Novel chromogenic ligands for lithium and sodium based on calix[4]arene tetraesters. J. Chem Soc. Chem. Commun. 1287–1289 (1992).
Kubo, Y., Hamaguchi, S., Niimi, A., Yoshida, K., Tokita, S.J.: Synthesis of a 1,3-bis(indoaniline)-derived calix[4]arene as an optical sensor for calcium ion. J. Chem. Soc. Chem. Commun. 305–307 (1993).
Kubo, Y., Maeda, S., Tokita, S., Kubo, M.: Colorimetric chiral recognition by a molecular sensor. Nature 382, 522–524 (1996).
Chawla, H.M., Srinivas, K.: Molecular diagnostics: synthesis of new chromogenic calix[8]arenes as potential reagents for detection of amines. J. Chem. Soc. Chem. Commun. 2593–2594 (1994).
Van der Veen, N.J., Rgberink, R.J.M., Engbersen, J.F.J., Van Veggel, F.J.C.M., Reinhoudt, D.N.: Conformationally flexible calix[4]arene chromoionophores: optical transduction of soft metal ion complexation by cation–π interactions. Chem. Commun. 681–682 (1999).
Hatem, H., Isabelle, D.-B., Christian, D., Claude, B., Noëlle, E., Monique, P., Roger, L.: Synthesis, conformations and extraction properties of new chromogenic calix[4]arene amide derivatives. Eur. J. Org. Chem. 2002, 4202–4210 (2002).
Hyun, J.K., Jong, S.K.: BODIPY appended cone-calix[4]arene: selective fluorescence changes upon Ca2+ binding. Tetrahedron Lett. 47, 7051–7055 (2006).
Mine, S.A., Deligöz, H.: The synthesis of ester and ketone derivatives of azocalix[4]arene containing chromogenic groups. J. Incl. Phenom. Macrocyclic Chem. 55, 223–228 (2006).
Lukesh B., Mohindra C., Tania F., Natarajan V.: Synthesis and evaluation of 5,11-bis-(2-thioallylphenylazo)-25,26,27,28-tetrtahydroxycalix[4]arene and 5,11,17,23-tetrakis-(2-thiohexadecylphenylazo)-25,26,27,28-tetrahydroxycalix[4]arene as chromogenic receptors of Hg(II) and Pd(II) ions. Arkivoc 200–210 (2005).
Seoung, H.L., Tony, Y.K., Jaejung, K., Ji, Y.L., Jong, S.K.: Regioselective complexation of metal ion in chromogenic calix[4]biscrowns. J. Org. Chem. 69, 2902–2905 (2004).
Jung, K.C., Su, H.K., Juyoung, Y., Keum-Hyeung, L., Richard, A.B., Jong, S.K.: A PCT-based, pyrene-armed calix[4]crown fluoroionophore. J. Org. Chem. 71, 8011–8015 (2006).
Hiu-Suet, L., Sung-Kong, Y., Man-Chung Wong, K., Nianyong, Z., Vivian Wing-Wah, Y.: Selective luminescence chemosensing of potassium ions based on a novel platinum(II) alkynylcalix[4]crown-5 complex. Organometallics, 25, 3537–3540 (2006).
Ashram, M.: Synthesis of calix[4]crowns containing soft donor atoms and a study of their metal–cation binding properties: highly selective receptors for Cu2+. J. Chem. Soc., Perkin Trans. 2, 1662–1668 (2002).
Ashram, M.H.: Conductance and thermodynamic study of the complexation of ethyl p-tert-butylcalix[4]arene tetra acetate with alkali metal and silver ions in various solvents. J. Incl. Phenom. Macrocyclic Chem. 42, 25–31 (2002).
Nedler , J.A., Mead, R.: A simplex method for function minimization. Comput J. 7, 308–313 (1965) Tawarah, K., Mizyed, S.: A thermodynamic study of the association of alkali metal cations with dicyclohexano-18-crown-6. J. Incl. Phenom. 6, 555–564 (1988).
Acknowledgements
Ms Linda Winsor at the Memorial University of Newfoundland, Canada, is thanked for recording the mass spectra reported herein.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Ashram, M. Synthesis and extraction properties of new chromogenic azo-calix[4]dibenzothiacrown ethers. J Incl Phenom Macrocycl Chem 59, 315–321 (2007). https://doi.org/10.1007/s10847-007-9330-3
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10847-007-9330-3