Abstract
Cyclodextrins (CD) and calixarenes are complexing agents that have been successfully used as pharmaceutical drug carriers, to improve the bioavailability of medicines. The aim of this work was to investigate the complexation of the local anesthetic tetracaine 1 with β-cyclodextrin 2, as well as with p-sulphonic acid calix[6]arene 3. 1H NMR experiments were carried out in D2O, i.e., with the charged tetracaine species 1. HR-DOSY 1H NMR allowed determination of the fraction of complexed population (%p bound = 55% and 70%) and the apparent association constants (K a = 1358 and 3889 M−1), respectively, for 1/2 and 1/3. These results confirm that a strong association takes place between 1 and 2, while the 1/3 complex is even more stable, due to the negatively charged sulphonic groups of 3. Studies conducted at pH 10 revealed that the association of the uncharged form of 1 with 3 is considerably weaker, while that with 2 increased significantly (K a = 6597 M−1), protecting the anesthetic against alkaline hydrolysis. 1H-ROESY 1D NMR experiments allowed determination of the host-guest relative positions, revealing that the proposed topologies for the 1/2 and 1/3 complexes were quite different. The complexation of 1 with either 2 or 3 is being investigated in view of its potential use in new therapeutic formulations, designed to increase the bioavailability and/or to decrease the systemic toxicity of tetracaine, in anesthesia procedures.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Tetracaine belongs to the aminoester family of local anesthetics (LA) an important class of nonciceptive agents whose action involves blockage of nervous impulse transmission. It is believed that both the cationic and the uncharged species of LA—that coexist at physiologic pH—bind to the Na+ channels of the nerve membranes, stabilizing its inactivated state and thus blocking the initiation and propagation of nervous impulses. However, LA show a relatively short duration of action (1–4 h) and may have adverse side effects such as cardiac and CNS toxicity, accompanied sometimes by allergic reactions [1].
The use of cyclodextrins (CD), calixarenes, liposomes and polymers as drug carrier systems has become an increasingly successful method to improve the potency of many therapeutic molecules whose bioavailability is threatened by problems such as limited (water/membrane) solubility, low chemical stability, fast serum clearance, etc. [2].
Among the available complexing agents, CD are the most widely used in drug formulations [3], while calixarenes belong to a new class of cyclooligomers, formed via phenol-formaldehyde reactions [4]. Calixarenes and CD share common features since they are macrocyclic molecules with a repeating unit, and both are cage-like molecules with a hydrophobic cavity. The natural cyclodextrins (α-, β- and γ-CD) have inner-cavity diameters of 5.7, 7.8, and 9.5 Å, respectively [2a]. In contrast, the inner cavity diameters of calix[4]arene, calix[6]arene, and calix[8]arene are 3.0, 7.6, and 11.7 Å, respectively [5]. Thus, the inner-cavity diameter of calix[6]arene is comparable to that of β-CD. Just like micelles, CD and sulphonate-calix[n]arenes can provide hydrophobic environments to guest molecules (through the glycoside and benzene rings, respectively), and hydrophilic outer surfaces (OH and SO −3 , respectively) [6]. However, there is a significant difference imposed by the oligosaccharide units of CD and the phenol units of calixarenes: whereas CD are quite rigid molecules, calixarenes are highly flexible molecules, possessing the ability to undergo complete ring inversion [7].
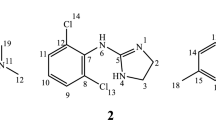
The characterization of the delivery systems formed by complexation of charged tetracaine 1 in β-cyclodextrins 2 or p-sulphonic acid calix[6]arene 3 (Scheme 1) will provide important information to optimize their future performance, which requires a better knowledge of their molecular properties [8]. Two pieces of information are crucial for the characterization of these complexes: the formation/dissociation constant and the relative positioning of the carrier/guest inclusion complex, both of which can be obtained from PGSE (pulsed field gradient spin-echo) [9] and NOE (nuclear Overhauser effect) [10] experiments, respectively. Such NMR techniques have been successfully applied to other supramolecular host-guest structures [11]. The aim of the present paper is to describe tetracaine 1 complexation with 2 and 3 (Scheme 1), by application of PGSE and NOE NMR methodologies.

Results and discussion
Sulphonate-calix[n]arenes have been used for the encapsulation of commercial steroids, furosemide, nifedipine and niclosamine [12] and the literature contains plenty of examples of CD based pharmaceutical formulations [2a, 13], most of them involving LA [14].
The association of 1 with 2 or 3 was first evaluated by complexation-induced hydrogen chemical shifts (Δδ) in the 1/2 and 1/3 complexes, relative to free 1. Interestingly, chemical shift differences in the 1H NMR spectra of 1—free or in 1/2 complex—were mainly observed for H-3 hydrogens (Table 1). Complexation between 1 and 3 induced large shielding effects in all the hydrogens of 1, mainly H-3′, H-4′ and H-6′ (Δδ 0.43, 0.72 and 0.63, respectively, Table 1), indicating interactions between the ammonium group of 1 with the SO −3 group of 3 [15].
Diffusion-ordered spectroscopy (DOSY) NMR experiments were pivotal to demonstrate that 1 and 2 or 1 and 3 form stable complexes. It serves also to distinguish compounds or complexes by their differences in diffusion coefficients [9a]. The diffusion coefficients of pure 1, 2 and 3 (D 1 = 5.58 × 10−10, D 2 = 3.27 × 10−10 and D 3 = 3.05 × 10−10 m2 s−1, respectively) were first obtained (Table 2). In the presence of 2 or 3, compound 1 showed a significant reduction of the diffusion rate (D 1/2 = 4.24 × 10−10 and D 1/3 = 3.70 × 10−10 m2 s−1, respectively) (Table 2, representative Figs. 1 and 2), indicating that 1 formed host-guest complexes with either 2 or 3. Moreover, the diffusion rate values (Table 2) of 1 in the 1/3 complex are in agreement with the assumption of a strong association. Taking into account that we are dealing with a rapidly equilibrating system, both the chemical shifts and the diffusion coefficients are weight-averaged NMR values between free and bound 1 species. From these diffusion coefficients and applying a well established methodology [16], we have calculated the complexed population (%p bound) and apparent binding constants (K a) of the complexes. In D2O %p bound was found to be 55% and 70% for 1/2 and 1/3, respectively. The values of K a (1358 M−1 and 3889 M−1 for 1/2 and 1/3, respectively) confirmed that a stronger association took place between 1 and 3 than between 1 and 2, due to the negatively charged sulphonic groups of calix[6]arene.
At pH 8.2 there is an equilibrium between the protonated and non protonated form of 1 [17]. Studies conducted at pH 10.0 revealed an increase in the association between uncharged tetracaine 1 and 2 (K a = 6597 M−1). The association constant between uncharged 1 and 3 was not determined due to the alkaline hydrolysis of the ester bond of 1 (t 1/2 < 0.1 h). Fortunately alkaline hydrolysis was prevented (t1/2 > 16 h) by the strong association of 1 and 2 at pH 10.0, allowing the DOSY experiments to be carried out.
We further established the stoichiometry for the 1/2 and 1/3 complexes, using the Job plot method [18]. The plots obtained from the NMR analysis indicated the predominant formation of 1:1 complexes, both between 1 and 2 and with 1 and 3 (Figures not shown).
To gain more insight into the topological aspects of these two complexes (1/2 and 1/3) we have performed 1H-ROESY NMR experiments, which are usually suited to measure NOEs in complexes with ωτc close to 1 [19]. Specific ROE signals were observed between H-2 and H-3 of 1 with H-3 (enhancement of 0.46% and 0.45%, respectively) and H-5 (1.01% and 0.15% of signal enhancement respectively) of β-CD 2. We therefore suggest that the aromatic moiety of tetracaine 1 was inside the CD cavity. We have also addressed the association issue however no dipolar interaction between the H-1, H-2 and H-4 of the β-CD 2 was observed with the tetracaine 1, thus the 1/2 complex’ topology was proposed and is depicted in Fig. 3.
The signal enhancements in 1/3 (ROE) between H-6′ of the tetracaine 1 and H-3 (1.27% signal enhancement) of the p-sulphonic acid calix[6]arene 3 indicate that the ammonium group of 1 can be included in the cavity of 3, as suggested in Fig. 4. The lack of more ROE signal is probably due to the conformational behavior of the p-sulphonic acid calix[6]arene 3 which will be better investigated in the near future.
Conclusions
The combined use of PGSE and ROE techniques has helped the determination of the host-guest structure, kinetic stability and degree of guest encapsulation in solution, for the 1/2 and 1/3 complexes.
The complexation of 1 with either 2 or 3 has been investigated in view of its potential use for the preparation of new therapeutic formulations, to increase the bioavailability and to decrease the systemic toxicity of tetracaine 1 in anesthesia procedures. In water, we determined a strong association between 1 and 2. The interaction between 1 and 3 was found to be even more stable, due to the binding of the ammonium group of 1 and the SO −3 group of 3. This was confirmed by experiments performed at pH 10.0, which revealed that the association between 1 and 3 decreased in relation to that in D2O, while that of 1 and 2 increased (K a = 6597 M−1), preventing the alkaline hydrolysis of 1.
The proposed topologies of the 1/2 and 1/3 complexes were established using ROESY 1D and they are intrinsically distinct. In 1/2, 1 is almost totally inserted into the cyclodextrin 2 cavity while in 1/3 it is located at the sulphonic rim so that we can say that 1/2 complexation is governed by hydrophobic interactions while complexation of 1/3 is governed by ion pair interactions. A topic of major interest in the development of drug delivery system is to understand the specific features that determine the interaction between host-guest molecules and the study of the molecular aspects involved in it is continuing in our laboratory.
Experimental
Chemicals and reagents
Tetracaine 1 (99%), β-CD 2 (99%), and D2O (99.75%) were purchased from Aldrich, Acros Organics and Merck, respectively. All other reagents were of analytical grade. p-sulphonic acid calix[6]arene 3 was synthesized in our laboratory following literature procedures [20].
Preparation of solid inclusion complexes
Inclusion complexes (1/2 or 1/3) with 1:1 molar ratios were prepared by shaking appropriate amounts of 1 and 2 or 3, e.g. 2 mmol l−1, in deionized water at room temperature (298 ± 1 K) for 1 h. Kinetic experiments revealed that equilibrium was reached after 40 min incubation (data not shown).
After reaching equilibrium, the solution was freeze-dried in a Labconco Freeze-dry system (Freezone 4.5) and stored at 253 K until further use.
NMR spectroscopy
All experiments were performed at 298 K in D2O. For the experiments with uncharged 1, the pH value of the solutions was adjusted by addition of 0.02 mol l−1 carbonate buffer, prepared in D2O.
Routine 1D 1H experiments were acquired with an INOVA-500 Varian spectrometer operating at 499.885 MHz for 1H (64 k data points, 30° excitation pulse duration of 2.2 μs, spectral width of 6 kHz, acquisition time of 3.3 s and relaxation delay of 10 ms) in a 5 mm probe with inverse detection mode at room temperature unless stated otherwise.
NOE measurements The ROESY 1D experiments were obtained with a selective 180° and a non-selective 90° pulse, a mixing time of 0.5 s was used during the spin-lock. The selective pulses were generated by a waveform generator, which automatically attenuates the shape, power, and pulse duration to obtain the required selectivity. The subtraction of the on- and off-resonance acquisition furnished the ROESY 1D experiment. All spectra were acquired with a 5 mm inverse probe at 298 K in 5 mm tubes.
HR-DOSY experiments were carried out by carefully choosing the correct pulse sequence and gradients for the experiments. The measurements were made using: (a) 5 mm inverse probe with Z-gradient coil; (b) the GCSTESL (Gradient Compensated Stimulated Echo Spin Lock) HR-DOSY sequence; (c) amplitudes of the gradient pulses ranging from 0.000685 to 0.003427 T cm−1, where an approximately 90%–95% decrease in the resonance intensity was achieved at the largest gradient amplitudes. For all experiments, 25 different gradient amplitudes were used. The baselines of all arrayed spectra were corrected prior to processing the data. The processing program (the DOSY macro in a Varian instrument) involves the determination of the peak heights of all signals above a pre-established threshold and the fitting of the decay curve for each peak to an exponential decay. The DOSY macro was run with data transformed using fn = 64 K. Very crowded spectra were processed in sections due to the limitation of handling only 512 lines at a time. The results of the DOSY method of analysis are pseudo two-dimensional spectra with NMR chemical shifts along one axis and calculated diffusion coefficients (m2 s−1 × 10−10) along the other.
Determination the stoichiometry of complexation
Job plots have been prepared with 2 mmol l1− stock solutions of 1 and 2 or 1 and 3 [21].
References
(a) Bowman, W.C., Rand, M.J.: Textbook of Pharmacology, Blackwell, Cambridge (1990); (b) Butterworth, J.F., Strichartz G.R.: Molecular mechanisms of local anesthesia: a review. Anesthesiology 72, 711–734 (1990); (c) Courtney, K.R., Strichartz G.R. (eds.): Structural elements, which determine local anesthetics activity. In: Local Anesthetics, Handbook of Experimental Pharmacology, vol. 81, 291 chapter. 3, Springer-Verlag (1987); (d) Strichartz, G.R.: Local anesthetics. Handbook of Experimental Pharmacology, vol. 81, Springer-Verlag (1987); (e) Covino, B.G., Vassalo, H.G.: Local anesthetics: mechanisms of action and clinical use. Grune and Stratton, New York (1976); (f) de Jong, R.H.: Local anesthetics. Mosby-Year Book (1994)
(a) Davis, M.E., Brewster, M.E.: Cyclodextrin-based pharmaceutics: past, present and future. Nat. Rev. Drug Discov. 3 (12), 1023–1035, (2004); (b) Torchilin, V.P.: Recent advances with liposomes as pharmaceutical carriers. Nat. Rev. Drug Discov. 4 (2), 145–160, (2005)
Szejtli, J.: Cyclodextrins Technology. Kluwer Academic Publishers, Boston (1988)
Gutsche, C.D.: Calixarenes. Royal Society of Chemistry, Cambridge (1989)
Gutsche, C.D.: The Characterization and properties of calixarenes In: Stoddart, J.F., (ed.) Calixarenes, The Royal Society of Chemistry, Cambridge (1989)
Schuette, J.M., Ndou, T.T., Warner, I.M.: Cyclodextrin-induced asymmetry of achiral nitrogen-heterocycles. J. Phys. Chem. 96(13), 5309–5314 (1992)
Szejtli, J.: Cyclodextrins and Their Inclusion Complexes. Akademiai Kiado, Budapest (1982)
Davis, A.V., Yeh, R.M., Raymond, K.N.: Supramolecular assembly dynamics. Proc. Natl. Acad. Sci. USA 99, 4793–4796 (2002)
(a) Johnson Jr., C.S.: Diffusion ordered nuclear magnetic resonance spectroscopy: principles and applications progress in nuclear magnetic resonance. Spectroscopy 34 203–256 (1999); (b) Price, W. S.: Pulsed-field gradient nuclear magnetic resonance as a tool for studying translational diffusion: Part I. Basic Theory Concepts in Magnetic Resonance 9 (5), 299–336 (1997) (c) Price, W.S. Pulsed-field gradient nuclear magnetic resonance as a tool for studying translational diffusion: Part II. Experimental Aspects Concepts in Magnetic Resonance 10 (4), 197–237 (1998) (d) Stilbs, P. Fourier transform pulsed-gradient spin-echo studies of molecular diffusion progress in nuclear magnetic resonance. Spectroscopy 19 (1), 1–45, (1987); (e) Stejskal, E.O., Tanner, J.E.: Spin diffusion measurements - spin echoes in presence of a time-dependent field gradient. J. Chem. Phy. 42 (1), 288–292, (1965)
Neuhaus, D., Williamson, M.: The Nuclear Overhauser Effect in Structural and Conformational Analysis. Wiley-VCH, New York (2000)
(a) Cohen, Y., Avram, L., Frish, L. Diffusion NMR spectroscopy in supramolecular and combinatorial chemistry: an old parameter - new insights. Angewandte Chemie- International Edition 44 (4), 520–554, (2005); (b) Hamelin, B., Jullien, L., Derouet, C., Du Penhoat, C.H., Berthault, P.: Self-assembly of a molecular capsule driven by electrostatic interaction in aqueous solution. J Am Chem Soc. 120 (33), 8438–8447, (1998); (c) Mayzel, O., Cohen, Y.: Diffusion-coefficients of macrocyclic complexes using the PGSE NMR technique - determination of association constants. J. Chem. Soc. Chem. Comm. 16, 1901–1902, 1994; (d) Salvatella, X., Giralt, E.: NMR-based methods and strategies for drug discovery. Chem. Soc. Rev. 32 (6), 365–372, (2003); (e) Mo, H.P., Pochapsky, T.C.: Intermolecular interactions characterized by nuclear overhauser effects progress in nuclear magnetic resonance. Spectroscopy 30, 1–38, (1997); (f) WinterWerner, B., Diederich, F., Gramlich, V.: Analogs of Cinchona alkaloids incorporating a 9,9'-spirobifluorene moiety. Helv. Chim. Acta 79 (5), 1338–1360 (1996); (g) Martinborough, E., Denti, T.M., Castro, P.P., Wyman, T.B., Knobler, C.B., Diederich, F.: Chiral 1,1'-binaphthyl molecular clefts for the complexation of excitatory amino-acid derivatives. Helv Chim Acta 78 (5), 1037–1066 (1995); (h) Laverde, A. Jr., da Conceição, G.J.A., Queiroz, S.C.N., Fujiwara, F.Y., Marsaioli, A.J.: An NMR tool for cyclodextrin selection in enantiomeric resolution by highperformance liquid chromatography. Magn. Reson. Chem. 40 (7), 433–442, (2002); (i) Borges, R.B., Laverde, A. Jr., Porto, A.L.M., Marsaioli, A.J.: HRDOSY and sulfoxide enantiomeric discrimination by cyclodextrin spectroscopy. International Journal 14, 203 (2000); (j) Fernandes, S.A., Nachtigall, F.F., Lazzarotto, M., Fujiwara, F.Y., Marsaioli, A.J.: ‘Non-covalent Synthesis' of a chiral host of calix[6]arene and enantiomeric discrimination. Magn. Reson. Chem. 43 (5), 398–404, (2005); (l) Santos, L.S., Fernandes, S.A., Pilli, R.A., Marsaioli, A.J.: A novel asymmetric reduction of dihydro-beta-carboline derivatives using calix[6]arene/chiral amine as a host complex tetrahedron-asymmetry 14 (17), 2515–2519, (2003)
(a) Millership, J.S.: A preliminary investigation of the solution complexation of 4-sulphonic calix[n]arenes with testosterone. J. Incl. Phenom. Macro. 39 (3-4), 327–331, (2001); (b) Da Silva, E., Valmalle, C., Becchi, M., Cuilleron, C.Y., Coleman, A.W.: The use of electrospray mass spectrometry (es/ms) for the differential detection of some steroids as calix[n]arene sulphonate complexes. J. Incl. Phenom. Macro. 46 (1-2), 65-69, (2003); (c) Yang, W.Z., de Villiers, M.M.: The solubilization of the poorly water soluble drug nifedipine by water soluble 4-sulphonic calix[n]arenas. Eur. J. Pharm. Biopharm. 58 (3), 629–636, (2004); (d) Yang, W.Z., de Villiers, M.M.: Aqueous solubilization of furosemide by supramolecular complexation with 4-sulphonic calix[n]arenas. J Pharm. Pharmacol. 56 (6), 703–708, (2004)
(a) Martin, Del Valle E.M.: Proces. Biochem. 39, 1033 (2004); (b) Kirchmeier, M.J., Ishida, T., Chevrette, J., Allen, T.M.: Correlations between the rate of intracellular release of endocytosed liposomal doxorubicin and cytotoxicity as determined by a new assay. J. Lipos. Res. 11 (1), 15–29, (2001); (c) Veiga, F., Fernandes, C., Teixeira, F.: Oral bioavailability and hypoglycaemic activity of tolbutamide/cyclodextrin inclusion complexes. Int. J. Pharm. 202 (1–2), 165–171, (2000); (c) Dalmora, M.E., Dalmora, S.L., Oliveira, A.G.: Inclusion complex of piroxicam with beta-cyclodextrin and incorporation in cationic microemulsion. In vitro drug release and in vivo topical anti-inflammatory effect. Int. J. Pharm. 222 (1), 45–55, (2001)
(a) Dollo, G., Le Corre, P., Chevanne, F., Le Verge, R.: Inclusion complexation of amide-typed local anaesthetics with beta-cyclodextrin and its derivatives .1. physicochemical characterization. Int. J. Pharm. 131 (2), 219–228, (1996); (b) Dollo, G., Thompson, D.O., Le Corre, P., Chevanne, F., Le Verge, R.: Inclusion complexation of amide-typed local anesthetics with beta-cyclodextrin and its derivatives. iii. Biopharmaceutics of bupivacaine-SBE7-beta CD complex following percutaneous sciatic nerve administration in rabbits. Int. J. Pharm. 164 (1–2), 11–19, 1998; (c) Dollo, G., Le Corre, P., Freville, J.C., Chevanne, F., Le Verge, R.: Biopharmaceutics of local anesthetic-cyclodextrin complexes following loco-regional administration. Ann. Pharm. Fr. 58, 425 (2000); (d) Estebe, J.P., Ecoffey, C., Dollo, G., Le Corre, P., Chevanne, F., Le Verge, R.: Bupivacaine pharmacokinetics and motor blockade following epidural administration of the bupivacaine-sulphobutylether 7-beta-cyclodextrin complex in sheep. Eur. J. Anaesth. 19 (4), 308–310, (2002) (e) Pinto, L.M.A., de Jesus, M.B., de Paula, E., Lino, A.C.S., Duarte, H.A., Takahata, Y.: Inclusion compounds between beta-cyclodextrin: local anesthetics a theoretical and experimental study using differential scanning calorimetry and molecular mechanics. J. Mol. Struc. Theochem 678, 63–66, (2004); (f) Pinto, L.M.A., Fraceto, L.F., Santana, M.H.A., Pertinhez, T.A., Oyama, Jr, S., de Paula, E.: Physico-chemical characterization of benzocaine-beta-cyclodextrin inclusion complexes. J. Pharmaceut. Biomed. 39 (5), 956–963, (2005); (g) Araújo, D.R., Braga, A.F.A., Fraceto, L.F., de Paula, E.: Sistemas de liberação controlada com bupivacaína racêmica (s50-r50) e mistura enantiomérica de bupivacaína (s75-r25): efeitos da complexação com ciclodextrinas no bloqueio do nervo ciático em camundongos. Revista Brasileira de Anestesiologia 55 (3), 316 (2005); (h) Araújo, D.R., Pinto, L.M.A., Braga, A.F.A., de Paula, E.: Formulações de anestésicos locais de liberação controlada: aplicações terapêuticas. Revista Brasileira de Anestesiologia 53 (5), 653–661, (2003)
Specht, A., Ziarelli, F., Bernad, P., Goeldner, M., Peng, L.: para-Sulfonated calixarenes used as synthetic receptors for complexing photolabile cholinergic ligand. Helv. Chim. Acta 88(10), 2641–2653, (2005)
(a) Rymdém, R., Carlfors, J., Stilbs, P. J.: Substrate binding to cyclodextrins in aqueous solution: a multicomponent self-diffusion study. J. Incl. Phenom. Macro. 1(2), 159, (1983); (b) Gounarides, J.S., Chen, A.D., Shapiro, M.J.: Nuclear magnetic resonance chromatography: applications of pulse field gradient diffusion NMR to mixture analysis and ligand-receptor interactions. J. Chromatogr. B -Analytical Technologies in the Biomedical and Life Sciences 725 (1), 79–90, (1999)
de Paula, E., Schreier, S.: Use of a novel method for determination of partition coefficients to compare the effect of local anesthetics on membrane structure. Biochim. Biophys. Acta 1240, 25–33, (1995)
(a) Job, P.: Formation and stability of inorganic complexes in solution. Ann. Chim. 9, 113 (1928); (b) Djedaïni, F., Lin, S.Z., Perly, B., Wouessidjewe, D.: High-field nuclear-magnetic-resonance techniques for the investigation of a beta-cyclodextrin-indomethacin inclusion complex. J. Pharm. Sci. 79 (7), 643–646, (1990); (c) Fielding, L.: Determination of association constants (k-a) from solution NMR data. Tetrahedron 56 (34), 6151–6170, (2000)
(a) Gunther, H.: NMR Spectroscopy, 2nd edn. John Wiley & Sons, Chichester, (1994); (b) Gil, V.M.S., Geraldes, C.F.G.C.: Ressonância magnética nuclear. Fundamentos, Métodos e Aplicações, Fundação Calouste Gulbenkian, Lisboa (2002)
(a) Shinkai, S., Mori, S., Tsubaki, T.: New water-soluble host molecules derived from calix[6]arene. Tetrahedron Letters 25 (46), 5315–5318 (1984); (b) Gutsche, C.D., Lin, L.G.: Calixarenes .12. The synthesis of functionalized calixarenes. Tetrahedron 42 (6), 1633–1640, (1986); (c) Gutsche, C.D., Iqbal, M.: Organic Synthesis 68, 234 (1989)
Loukas, Y.L., Vraka, V., Gregoriadis, G.: Drugs, in cyclodextrins, in liposomes: a novel approach to the chemical stability of drugs sensitive to hydrolysis. Int. J. Pharm. 162(1–2), 137–142, (1998)
Acknowledgments
The authors are indebted to FAPESP (Proc. 2005/00602-4) and CT-PETRO/CNPq (Proc. 360762/2005-0) for financial support and fellowships. The authors also thank Prof. Carol Collins for English text revision.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Fernandes, S.A., Cabeça, L.F., Marsaioli, A.J. et al. Investigation of tetracaine complexation with beta-cyclodextrins and p-sulphonic acid calix[6]arenes by nOe and PGSE NMR . J Incl Phenom Macrocycl Chem 57, 395–401 (2007). https://doi.org/10.1007/s10847-006-9224-9
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10847-006-9224-9