Abstract
Low-energy conformations of β-cyclodextrin under anhydrous conditions in the gas phase were investigated by DFT calculations. In these conformations, two homodromic hydrogen bond rings are formed with very short hydrogen bonds at the narrow side of the cyclodextrin ring and a second one at the wider side. These hypothetical conformations are not comparable to those conformations, which have been studied experimentally, forming inclusion complexes with small and medium-sized guest molecules, but their energy is significantly lower than the open conformations (ΔE = 10 kcal/mol).
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Hydrogen bonding is very important for the structure of cyclodextrin and cyclodextrin complexes. Particularly, crystal structures of β-CD exhibit a number of O-H··O hydrogen bonds between the three different hydroxyl groups and water molecules, which were investigated by X-ray crystallography and by neutron diffraction [1–6]. In addition to intermolecular hydrogen bonds, intramolecular hydrogen bonds are formed between the secondary hydroxyl groups of the glucose units to the adjacent units. These intramolecular hydrogen bonds stabilize the macrocyclic conformation of β-CD, increase the rigidity of the molecule and lead in this special case to a reduced solubility of the compound in comparison to other CDs, with a smaller or larger number of glucose units, and also to substituted derivatives.
The application of ab initio and DFT calculations was limited in the past by the size of the molecular system. Numerous theoretical studies were performed on the structure of CDs and CD complexes, based on semiempirical quantum chemical methods [7]. Accurate molecular calculations were performed only for smaller systems like glucose dimers [8–11]. For the structure of CDs only a few papers were published, comparing empirical, semiempirical and low-level ab initio methods (HF/3-21G), concluding that Molecular Mechanics is the most convenient method to describe structural features of CDs, followed by the ab initio method [12]. Some recent investigations were performed using DFT methods [13].
In the present paper, we describe the gas phase structures of β-CD as obtained from ab initio (HF/3-21G and HF/6-31G(d,p)) as well as DFT (B3LYP/6-31G(d,p)) calculations, in order to get some insight into the intramolecular hydrogen bond network of this molecule.
Methods of calculation
C7 symmetry was forced by building up the Z-matrix starting from the oxygen-oxygen distances at the more narrow rim of the β-CD moiety. HF/3-21G and HF/6-31G(d,p), as well as B3LYP/6-31G(d,p) were used for full geometry optimisation. The calculations were performed, using the program package GAUSSIAN03 [14] on the Schrödinger III cluster of the University of Vienna. Scanning of the oxygen-oxygen distance was done in order to obtain the structures of minimum energies.
Results and discussion
X-ray crystals of CDs and CD complexes are grown from aqueous solution and also most of the equilibrium constants and the related thermodynamic parameters were obtained from solution of pure water or water co-solvent mixtures. Only a very few examples are known for CD-guest complexation in non-aqueous conditions [15, 16].
In order to investigate the conformational behaviour of CDs, we have analysed the gas phase conformations of β-CD under symmetric conditions (C7) by a systematic scan of the oxygen-oxygen distance of the O6 hydroxyl groups. Three conformational minima were found.
The energies of the minimum conformations are given in Table 1, together with selected structural parameters.
The lowest energy minimum (HF/3-21G and B3LYP/6-31G(d,p)) was found for a rather short O6-O6 distance, a second less pronounced at a slightly elongated distance, a third minimum at a distance around 6.5 Å, which is similar to the experimentally (X-ray crystallographic) determined structure.
Although all methods used describe a very similar geometry, the energy differences between the obtained minima vary drastically. Even the energy ranking is described differently by HF/6-31G(d,p), whereas HF/3-21G and B3LYP/6-31G(d,p) calculations lead to the same ranking. The reason for these discrepancies is that hydrogen bonding superimposed by quite subtle steric interactions determines the geometry of the molecule. A higher-level DFT method is therefore necessary to describe the geometries sufficiently well, and this method will be used for further considerations.
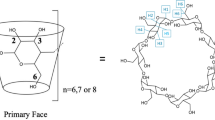
The geometries of the three symmetric conformations are depicted in Fig. 1. The top view shows that the lowest energy minimum (A), which is 10 kcal/mol more stable than the second one (C), possesses a very narrow rim at the upper side of the β-CD ring. Both other conformations are somewhat more open, and differ in the arrangement of the glucose units. Some selected structural parameters are given in Table 2 including the numbering of the atoms for one glucose unit in the adjacent scheme. Scheme 1
Table 2 is divided into four groups of structural parameters. In the first three rows, the distances between oxygen atoms are reported, which are involved in hydrogen bonding contacts at least in one conformation. Strong hydrogen bonds exist in conformation A within the ring built up from oxygens 6 (O6). This distance is very close to those found for hydrogen bond networks in open chains and cyclic polymers of water and alcohols [17]. Also the valence angle of the hydrogen bond is rather close to the optimal value of 180°. A second hydrogen bond system occurs between oxygens O2 and O3. These interactions are somewhat weaker, as the heavy atom distance is longer and also the valence angle is less favourable. The shortest distance exhibits conformation C. The third distance (O2-O3) describes a very weak hydrogen bond contact for all conformations. The orientation of the hydroxyl groups is identical for all conformations except for the dihedral angle O6-C6-C5-C4 (third group in the table).
Surprisingly small changes occur at the bonds connecting the glucose rings together, only the above-mentioned orientation of the -CH2OH group changes completely. Remarkably, some distortions of the glucose rings can be observed for the different conformations.
Because of the co-operativity of the homodromic hydrogen bonds at the upper and the lower side of the β-CD ring, four conformations of A with different energies are possible as a consequence of the chirality of the glucose rings. For conformation A these energies are given in Table 3.
The top view of the molecule (Fig. 1) indicates, that the strong hydrogen bonds at the smaller rim of β-CD are oriented counter clockwise, whereas the more extended ring is oriented clockwise. This conformation shows the lowest conformational energy (A1, reference in Table 3). Flipping of the orientation of the hydrogen bonds at the larger ring has almost no influence on the energy and enhances the energy value only slightly (conformation A3). Reorientation of the ring of the strong hydrogen bonds leads to a significant increase of the energy (2.15 kcal mol−1 for the clockwise orientation of the larger ring (A2) and 2.30 kcal mol−1) for the counterclockwise one (A4).
Discussion
Three gas phase conformations of β-CD were found with C7 symmetry, which differ significantly in their energy, obtained from B3LYP/6-31G(d,p) calculations. The lowest-energy conformation is characterized by a surprisingly tight ring at one side of the CDs ring. This shape is not comparable to the structures found experimentally. A ring of homodromic hydrogen bonds was found in this conformation, with hydrogen bonds very close to ideal hydrogen bridge geometries obtained in open chains of cyclic clusters of water or alcohols. The chirality of the glucose units of β-CD induces energy differences between geometries with different orientation of the co-operativity of hydrogen bonds.
References
Chacko, K.K., Saenger, W.: Topography of cyclodextrin inclusion complexes. 15. crystal and molecular structure of the cyclohexaamylose-7.57 water complex, form III. four- and six-membered circular hydrogen bonds. J. Am. Chem. Soc. 103, 1708–1715 (1981).
Betzel, C., Saenger, W., Hingerty, B.E., Brown, G.M.: Circular and flip-flop hydrogen bonding in β-cyclodextrin undecahydrate: a neutron diffraction study? J. Am. Chem. Soc. 106, 7545–7557 (1984).
Zabel, V., Saenger, W., Mason, S.A.: Neutron diffraction study of the hydrogen bonding in β-cyclodextrin undecahydrate at 120 K: from dynamic flip-flops to static homodromic chains. J. Am. Chem. Soc. 108, 3664–3673 (1986).
Steiner, T., Mason, S.A., Saenger, W.: Disordered guest and water molecules. Three-center and flip-flop 0–H…0 hydrogen bonds in crystalline, βb-cyclodextrin ethanol octahydrate at T = 295 K: a neutron and X-ray diffraction study? J. Am. Chem. Soc. 113, 5676–5687 (1991).
Steiner, T., Koellner, G.: Crystalline β-cyclodextrin hydrate at various humidities: fast, continuous, and reversible dehydration studied by X-ray diffraction. J. Am. Chem. Soc. 116, 5122–5128 (1994).
Saenger, W., Jacob, J., Gessler, K., Steiner, T., Hoffmann, D., Sanbe, H., Koizumi, K., Smith, S.M., Takaha, T.: Structures of the common cyclodextrins and their larger analogues-beyond the doughnut. Chem. Rev. 98, 1787–1802 (1998).
Liu, L., Guo, Q.X.: Use of quantum chemical methods to study cyclodextrin chemistry. J. Incl. Phenom. Macrocycl. Chem. 50, 95–103 (2004)
French, A.D., Kelterer A.M., Johnson, Dowd M.K.: B3LYP/6-31G*, RHF/6-31G* and MM3 heats of formation of disaccharide analogs. J. Mol. Struct. 556, 303–313 (2000).
Strati, G.L., Willett, J.L., Momany, F.A.: Ab initio computational study of β-cellobiose conformers using B3LYP/6-311++G**. Carbohydr. Res. 337, 1833–1849 (2002).
Strati, G.L., Willett, J.L., Momany, F.A.: A DFT/ab initio study of hydrogen bonding and conformational preference in model cellobiose analogs using B3LYP/6-311++G**. Carbohydr. Res. 337, 1851–1859 (2002).
Da Silva, C.O., Nascimento, M.A.C.: Ab initio conformational maps for disaccharides in gas phase and aqueous solution. Carbohydr. Res. 339, 113–122 (2004).
Britto, M.A.F.O., Nascimento, C.S., dos Santos, H.F.: Structural analysis of cyclodextrins: a comparative study of classical and quantum mechanical methods. Quim. Nova. 27, 882–888 (2004).
Avakyan, V.G., Nazarov, V.B., Voronezheva, N.I.: DFT and PM3 calculations of the formation enthalpies and intramolecular H-bond energies in alpha-, beta-, and gamma-cyclodextrins. Russ. J. Phys. Chem. 79, S18–S27 (2005).
Frisch, M.J, Trucks, G.W., Schlegl, H.B., Scuseria, G.E., Robb, M.A., Cheeseman, J.R., Montgomery, J.A., Jr., Vreven, T., Kudin, K.N., Burant, J.C., Millam, J.M, Iyengar, S.S., Tomasi, J., Barone, V., Mennucci, B., Cossi, M., Scalmani, G., Rega, N., Petersson, G.A., Nakatsuji, H., Hada, M., Ehara, M., Toyota, K., Fukuda, R., Hasegawa, J., Ishida, M., Nakajima, T., Honda, Y., Kitao, O., Nakai, H., Klene, M., Li, X., Knox, J.E., Hratchian, H.P., Cross, J.B., Bakken, V., Adamo, C., Jaramillo, J., Gomperts, R., Stratmann, R.E., Yazyev, O., Austin, A.J., Cammi, R., Pomelli, C., Ochterski, J.W., Ayala, P. Y., Morokuma, K., Voth, G.A., Salvador, P., Dannenberg, J.J., Zakrzewski, V.G., Dapprich, S., Daniels, A.D., Strain, M.C., Farkas, O., Malick, D.K., Rabuck, A.D., Raghavachari, K., Foresman, J.B., Ortiz, J.V., Cui, Q., Baboul, A.G., Clifford, S., Cioslowski, J., Stefanov, B.B., Liu, G., Liashenko, A., Piskorz, P., Komaromi, I., Martin, R.L., Fox, D.J., Keith, T., Al-Laham, M.A., Peng, C.Y., Nanayakkara, A., Challacombe, M., Gill, P.M.W., Johnson, B., Chen, W., Wong, M.W., Gonzales, C., Pople, J.A.: Gaussian Inc., Wallingford CT (2004).
Shannigrahi, M., Bagchi, S.: Time resolved fluorescence study of ketocyanine dye–β cyclodextrin interactions in aqueous and non-aqueous media. Chem. Phys. Letters. 403, 55–61 (2005).
Panja, S., Chowdhury, P., Chakravorti, S.: Modulation of complexation of 4(1H-pyrrole 1-yl) benzoic acid with β-cyclodextrin in aqueous and non-aqueous environments. Chem. Phys. Letters. 393, 409–415 (2004).
Karpfen, A.: Cooperative effects in hydrogen bonding. Adv. Chem. Phys. 123, 469–510 (2002).
Acknowledgements
This investigation was supported by the Hochschuljubiläumsstiftung der Stadt Wien (Project P H-778/2005). Technical assistance of Ms. Martina Ziehengraser is gratefully acknowledged.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Karpfen, A., Liedl, E., Snor, W. et al. Homodromic hydrogen bonds in low-energy conformations of single molecule cyclodextrins . J Incl Phenom Macrocycl Chem 57, 35–38 (2007). https://doi.org/10.1007/s10847-006-9166-2
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10847-006-9166-2