Abstract
Understanding the interactions between protected areas and the surrounding landscape has become a central issue to conservation of biodiversity. The important role of protected areas in the preservation of biodiversity in tropical hotpots is widely recognized, but the role of the landscape surrounding those hotspots is poorly understood, particularly with regard to insects. In this study, we evaluated the species richness, composition, and beta diversity of Odonata assemblages inside and in the surroundings of a protected area in the Atlantic Forest hotspot. Sampling was carried out in the Private Reserve of Natural Heritage Veracel Station and its surroundings in the southern region of Bahia. Forty sites were sampled, 22 within the reserve and 18 in the surrounding areas. We found both a greater total species richness, and a greater richness with regard to the suborder Anisoptera in the surrounding areas. In addition, the species composition differed less between the sampling sites inside the protected area. Some of the species found inside the protected area did, however, make a greater contribution of the individual species to beta diversity (SCDB). Our study suggests that the surroundings of a protected area can contribute to the maintenance of regional diversity of dragonflies, but the protected areas play a vital role in supporting critically endangered species and populations of forest specialists, e.g., phytotelmatous species.
Implications for insect conservation
Our results show that the composition of the odonate species assemblages may provide a means to assess the importance of protected areas to Odonata communities. Our study also highlights the importance of PAs to the maintenance of the regional Odonata species pool, especially to forest specialist species and to threatened species.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Protected areas (PAs) play a key role in the efforts to preserve both local and global biodiversity, particularly when it comes to threatened species, habitats and ecosystems (Mittermeier et al. 2005; Ferreira et al. 2014; Azevedo-Santos et al. 2017; Peralta et al. 2019). The changing land use and the expansion of PAs have amplified the interaction between protected and unprotected areas worldwide (Watson et al. 2014) and have become central to the conservation of biodiversity and ecosystem services (Blanco et al. 2020). Therefore, it is increasingly vital to know the importance of protected areas and their surroundings for the conservation of local assemblages and for regional diversity (Blanco et al. 2020).
In Brazil, PAs cover more than 1.590 billion square kilometers, i.e., about 18.69% of the territory (MMA 2021). They are unevenly distributed across biomes, and still poorly studied with regard to their biodiversity (Oliveira et al. 2017; Fonseca and Venticinque 2018; Leal et al. 2020). The lack of data and the limited number of areas in certain regions create uncertainties about the effectiveness of PAs and the ways in which they interact with surrounding areas to preserve the country's biodiversity (Fonseca and Venticinque 2018).
Among the Brazilian forest biomes, the Atlantic Forest is considered a biodiversity hotspot (Mittermeier et al. 2011). This biome still suffers from consecutive impacts caused by land use changes linked to the cultivation of coffee, sugar cane, soy, corn and pasture areas, and also a large human population, forming the largest urban and industrial centres in the country, has caused a drastic reduction in native vegetation (Mello et al. 2020). The result is a heavily fragmented and anthropized landscape with only around 12% original vegetation (S.O.S Mata Atlântica 2018; MapBiomas 2021); most of which is located inside PAs. Another important issue to consider is that the creation of PAs in Brazil has been strongly focused on terrestrial biota and threats to them rather than freshwater systems and species (Fagundes et al. 2016; Samways 2017; Frederico et al. 2018; Reid et al. 2018; Basset and Lamarre 2019; Leal et al. 2020).
Invertebrates are often overlooked when it comes to conservation (Mammola et al. 2020), despite the fact that they constitute the vast majority of animal species, and that they are among the groups that suffer most from anthropogenic disturbances (Cardoso et al. 2020; Sundar et al. 2020). As the knowledge about the importance of these organisms to the ecosystems and the human population is still limited, they are frequently excluded or overlooked from conservation programs (Cardoso et al. 2020; Samways et al. 2020; Mammola et al. 2020). There are some rare exceptions, such as the State Wildlife Refuge Dragonflies of Serra de São José, explicitly created to protect dragonfly species in the Atlantic Forest (Bede et al. 2015), but there are very few protected areas designed to preserve aquatic insects in biodiversity hotpots around the world (Sundar et al. 2020). Current studies have shown that the PAs are not very efficient in protecting the biodiversity of freshwater (Fagundes et al. 2016; Frederico et al. 2018; Leal et al. 2020). However, few studies consider invertebrates (Fagundes et al. 2016; Frederico et al. 2018; Leal et al. 2020). This causes concern, as we still do not know how important PAs and surrounding areas are to the local and regional invertebrate diversity, and how they interact with each other.
Among freshwater aquatic invertebrates, dragonflies (Odonata) have been widely used as indicators of environmental health (Oliveira-Junior and Juen 2019; Voster et al. 2020; Ribeiro et al. 2021a, b). They are dependent on both aquatic and terrestrial ecosystems, and changes in these ecosystems are likely to affect the occurrence and reproduction of adults and the development of larvae. These modifications in turn affect the capacity for thermoregulation, choice of partners, availability of oviposition sites, selecting different groups of species in modified sites (De Marco et al. 2015; Oliveira Junior et al. 2015; Valente-Neto et al. 2016; Rodrigues et al. 2018). In the neotropical region the odonates are divided into two suborders, Zygoptera and Anisoptera (De Marco et al. 2015). In general, the Zygopterans are, considered to be more sensitive than anisopterans to landscape changes (from forest to open-areas) and aquatic habitat modification (as lotic for lentic and physicochemical water changes) due to their lower dispersal capacity and greater dependence on the environment for thermoregulation and suitable habitats for oviposition and development of larvae (De Marco et al. 2015; Carvalho et al. 2018; Rodrigues et al. 2018).
Human activities causing loss of forested habitats increase the local extinction risk dragonflies considered forest specialists and facilitate the colonization of generalist species (Rodrigues et al. 2016, 2018; Carvalho et al. 2018; Oliveira-Junior and Juen 2019), which lead to drastic changes in the local diversity. In the Atlantic Forest, the majority of areas surrounding PAs are anthropized landscapes where the natural forest cover is reduced, and the PAs are likely to be the only refuges available to some forest specialist species, particularly zygopterans (Ribeiro et al. 2021a, b).
Among the metrics used to assess biodiversity changes of Odonata, the richness, abundance and composition have been widely used to assess physical changes in channels and loss of surrounding vegetation in aquatic ecosystems. (Oliveira-Junior and Juen 2019; Ribeiro et al. 2021a, b). Another tool to detect diversity patterns are beta diversity analysis, also used to measure the impacts caused on the diversity between environments (Quintana et al. 2017; Altermatt and Holyoak 2012). Recently, Legendre and De Cáceres (2013) developed a single-number estimate of beta diversity through the calculation of the total variance of the species composition. This method allows the assessment of two other measures: species contributions to beta diversity (SCDB) and local contributions to beta diversity (LCBD) (Legendre and De Cáceres 2013). And can be used to understand drivers of beta diversity (e.g., Landeiro et al. 2018) which may indicate site’s conservation value (Legendre and De Cáceres 2013).
Based on this general expectation, we evaluated how the communities changed comparing a protected area and its surroundings on the Odonata assemblages in the Atlantic Forest. The structure (composition and richness) of the Odonata assemblages in streams within and around the PA was assessed. In addition, we assessed the contribution of each site to beta diversity (LCBD) and species contribution to beta diversity (SCDB) in streams within and outside the PA. We also checked if any of the species present could be regarded as bioindicators of pristine forest areas.
As a first prediction, we expected to find a higher richness of zygopterans at the sites within the PA, and a higher richness of anisopterans in the surrounding areas due to the PA maintaining more forested areas and greater physical integrity of the channels when compared to the surrounding streams. (De Marco et al. 2015; Rodrigues et al. 2016, 2018; Oliveira-Junior and Juen 2019; Ribeiro et al. 2021a, b). As the second prediction we expected a difference in the composition of the species assemblages, with the protected areas maintaining a greater diversity of Zygoptera and the surrounding areas a greater diversity of Anisoptera. As the third prediction, we expected that the PA maintain important sites (LCBD) for the regional beta diversity as well as maintaining species that contribute to the increase of regional beta diversity (SCDB); the PA housing the majority of species regarded as forest specialists, i.e. species dependent on forested areas for their survival (Carvalho et al. 2018). As the fourth prediction we expected that the PA may harbour a selected group of species that might be considered bioindicators of preserved areas (Rodrigues et al. 2019).
Materials and methods
Study area
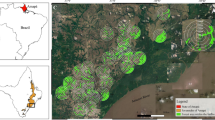
The study was carried out in and around the Private Reserve of Natural Heritage (RPPN) Veracel Station, located in the southern part of the state of Bahia, between the municipalities of Santa Cruz Cabrália and Porto Seguro (16°23ʹ27ʺ S and 39°10ʹ28ʺ W). The RPPN Veracel Station has an area of 6069 ha. It is considered the largest private reserve in North-Eastern Brazil, and the second largest in the Atlantic Forest Biome. The area maintains primary vegetation with high biological diversity (RPPN 2016). The region’s climate is Af according to the Koppen classification, characterized by average annual temperature of 18 °C or higher, with very little annual temperature variation. And with significant precipitation, average rainfall of at least 60 mm each month, and high humidity.
All the streams, both within and outside the PA, were considered small and narrow (estimated from first to second order). The streams inside the PAs had the width varying between 0.78 cm the 5.01 cm (mean 2.76 cm and sd 1.35 cm) and depth varying between 0.08 cm the 0.35 cm (mean 0.28 cm and sd 0.12 cm). And the surrounding streams with width varying between 0.74 cm the 5.40 cm (mean 2.65 cm and sd 1.28 cm) of and depth varying between 0.12 cm the 0.63 cm (mean 0.19 cm and sd 0.15 cm). The streams sampled inside the RPPN were surrounded by a more closed and well-preserved canopy, formed by primary vegetation and without evidence of visual pollution, erosion or anthropogenic physical changes along the stream channel. The areas surrounding the reserve were composed of pasture and agriculture matrices with settlement areas and large rural properties. The streams generally did not have uniform riparian vegetation, and a larger proportion of the sites were more open to direct sunlight. At some sampling sites, the stream channel was interrupted by constructed dams for watering animals and/or other use within the property. We also observed a small amount of domestic waste and plastic bags.
Sampling sites
Sampling was carried out at 40 sites, 22 within the Veracel Station Conservation Unit and 18 sites surrounding the RPPN. Each site was sampled twice (September 2018 and February 2019), i.e., during both the rainiest and the driest season, in order to obtain a more comprehensive representation of the annual odonate assemblages (Fig. 1).
Specimen collection, curation and identification
All adult Odonata specimens found were captured through active netting. For each point, a 100-m stretch along the water-line was sampled on both banks for 90 min with two collectors. The sampling was conducted on rain-free days at the time of peak odonate activity, from 9 am to 4 pm (Calvão et al. 2018). The collected specimens were placed in entomological envelopes and plastic boxes and later placed in freezers to be sacrificed. The adults were taken to the Laboratory of Aquatic Organisms (LOA) at the State University of Santa Cruz for further identification to the lowest possible taxonomic level by means of identification keys (Garrison et al. 2006; Lencione 2005, 2006, 2017). All specimens collected are deposited in the Zoological collection of Aquatic Organism Laboratory—LOA in the Santa Cruz State University, Ilhéus, Bahia, Brazil.
Statistical analyses
Species richness was calculated both for the total odonate community and separately for the suborders Zygoptera and Anisoptera. The data was analysed using a generalized linear model (GLM), using a log-linear model, with Poisson distribution (Gotelli and Ellison 2011). For this analysis, the richness (species numbers) was used as a response variable, and the two treatments (within and surrounding the RPPN) as predictor variables. A principal coordinates analysis (PCOA) of the abundance data was conducted to summarize the species composition inside and around the protected area, respectively, using the Bray–Curtis dissimilarity index (Legendre and Legendre 1998). This grouping was analysed through a permutational analysis of variance (PERMANOVA) (Anderson 2001), that was performed with 9999 replications (Anderson and Walsh 2013), to test for significant differences in species composition.
To evaluate the relation of beta diversity with the sampling sites in and around the APs, we calculated the total beta diversity (BDtotal) and we consider two measures: The local contribution to beta diversity (LCBD), to assess each site's contribution to beta diversity, and the contribution of the individual species to beta diversity (SCBD), to assess the contribution of each species to beta diversity (Legendre and De Cáceres 2013). We first applied Hellinger transformation to the community composition and then estimated BDtotal as the unbiased total sum of squares of the species composition data (Legendre and De Cáceres 2013). The relative contribution of each sampling unit to the beta diversity (LCBD) was estimated by the total variation in the species composition data (Legendre and De Cáceres 2013). To determine which taxa contributed the most to beta diversity patterns in streams within and around the RPPN, we calculated the SCBD. The species with SCBD values higher than the mean of all taxa are the species that make high contributions to beta diversity patterns (Legendre and De Cáceres 2013). The association of the local assemblies with beta diversity may indicate sites that contribute disproportionately to the regional species pool relative to species richness, and this may be particularly useful for identifying keystone sites (Legendre and De Cáceres 2013; Ruhí et al. 2017, Valente-Neto et al. 2020).
The indicator species analysis (IndVal) was calculated as proposed by De Cáceres et al. (2010). This analysis classify each specie showing the probability of them belong to a specific group of sites, in our case within the PA and its surroundings. The IndVal also enables us to evaluate two components for species considered to be indicators. Component A, also called specificity, is the probability that an indicator species belongs to one of the selected groups. Component B, also called fidelity, determines the probability for each species to be found either inside the PA or in the surroundings (De Cáceres et al. 2010). The analyses were performed using the statistical program R (R Core Team 2020) and the package indicspecies (De Caceres and Legendre 2009).
Results
We collected 722 specimens of 47 species belonging to 31 genera and eight families. Six of the species were only encountered within the PA, 29 species occurred only in the surrounding areas, and 14 species were found in both areas (Appendix 1). The species Aceratobasis cornicauda, Leptagrion macrurum, Leptagrion acutum, Forcepsioneura sancta, Heliocharis amazona and Phyllogomphoides sp. were found only at sampling sites within the protected area. The most abundant Zygoptera species were Heteragrion aurantiacum, Hetaerina rosea, Argia modesta with 202, 79 and 71 specimens, respectively. The most abundant species of Anisoptera were Erythrodiplax fusca and Erythrodiplax paraguayensis with 30 and 12 individuals, respectively (Appendix 1). Most species were represented by fewer than 10 specimens.
The total Odonata richness showed a significant difference in species number between the sites collected inside and outside the RPPN (AIC = 168.8, df = 36, p = 0.003), with an average of 4.57 species inside and 7.63 species outside the RPPN; the model explaining 21% of the variation in total richness in relation to areas, inside and outside the RPPN. The suborder Anisoptera also showed significant differences (AIC = 137.7, df = 36, p = p < 0.0001) between the sampled areas, with an average of one species inside and four species outside the RPPN. The model explained 31% of the variation in Anisoptera richness in relation to areas, inside and outside the RPPN. There was no significant difference in the richness of zygopterans between areas (p = 0.91), with an average of 3.5 species for the sites within the RPPN and 4.1 species in the sampling sites outside it (Fig. 2).
With regard to species composition, the assemblages found within and outside the PA differed significantly from each other (PERMANOVA test, p = 0.001 and R = 0.5018) (Fig. 3). The assemblages within the protected area were more similar to each other, suggesting less variation in their species composition. The compositions of the assemblages outside the RPPN were much more varied, indicating a greater variation in species composition between the sites outside the RPPN (Fig. 3). The species composition of the three sites outside the protected area (F01, FO3 and F07) was very similar to that of the sites within the protected area (Fig. 3).
Graph of the PCOa analysis showing the similarity in composition of the adult Odonata assemblages between the areas inside and outside the RPPN. Black dots denote areas within the protected area, and in grey dots denote the surrounding areas. Site codes shown in Appendix 2
In relation to LCBD, eight sites had a significant contribution to the local beta diversity (p < 0.05; LCBD ≥ 0.04). They were all situated outside the protected area (Appendix 2). When we estimated the SCDB, 24 species showed higher contribution values to beta diversity regionally. Of these, six species were associated with streams located within the RPPN (H. amazona, L. macrurum, L. acutum, H. aurantiacum, H. longipes, E. cannacrioides). The first three were species found exclusively within the preserved area (Appendix 1).
Of the forty-nine species collected, seven qualified as indicators of either protected or non-protected areas. Three species were considered to be indicators of streams within the PA and four were indicative of the areas surrounding the PA (Table 1). The values of the specificity (A) showed a variation from 0.71 to 1.0, and the selected species displayed a high specificity to the sampling sites (inside and outside the PA, respectively). Heteragrion aurantiacum, Hetaerina longipes and Helicharis amazona were almost exclusively collected at the sampling sites within the PA and Ischnura capreolus, Telebasis corallina, Erythrodiplax fusca and Erythrodiplax paraguayensis at the sites outside the PA. Regarding the fidelity (B), the values ranged from 0.26 to 0.84, emphasizing that Heteragrion aurantiacum (B = 0.84) was present at most of the sites within the PA. All other species had a lower fidelity, meaning a low number of records within the sites sampled inside the RPPN as well as in the surrounding areas (Table 1).
Discussion
Our results highlight the importance of protected areas in the preservation of Odonata species that are forest specialists sensitive to change in natural environments, i.e., species dependent on pristine aquatic environments (Carvalho et al. 2018; Calvão et al. 2018; Rodrigues et al. 2018). Two examples from our study were the phytothelmatous species Leptagrion macrurum and L. acutum, which were found exclusively within the PA. L. acutum is categorized as critically endangered in the Red List of endangered species in Brazil (IUCN 2014, ICMBio 2018, Ribeiro et al. 2021b). Undisturbed sites inside the PA are therefore critical to the conservation of such threatened species.
According to our first prediction a higher species richness of the suborder Anisoptera was found in the surrounding area, compared to within the protected area. This relationship has been observed in other studies on Odonata in areas experiencing urbanization impacts (Monteiro Junior et al. 2013; Rodrigues et al. 2019), as well as in areas of intensive livestock grazing and agriculture (Carvalho et al. 2018; Calvão et al. 2018). It can be attributed to different degrees of change in the aquatic environments and their surrounding habitats, e.g., loss of riparian vegetation, siltation reducing the flow in channels from lotic to lentic, and others. These changes facilitate colonization of Anisoptera species that are usually more generalist and/or adapted to lentic and altered environments (Juen and De Marco 2012; Monteiro-Junior et al. 2013; Calvão et al. 2018; Rodrigues et al. 2018; Carvalho et al. 2018; Rodrigues et al. 2019), although no lentic habitats were present in our study area.
Contrary to our first prediction, the species diversity of adult zygopterans was similar among the sites sampled within the RPPN. Changes in the surrounding areas lead to the loss of riparian vegetation and changes in the flow of channels from lotic to lentic due antropic impacts. These changes may attract species adapted to these more lentic habitats. For example, Acanthagrion cuyabae, Acanthagrion gracille, Telagrion longum, Telebasis corallina, Neoneura sylvatica, Nehalennia minuta, Lestes forficula and Lestes tricolor were only collected outside the PA in this study. All of them are commonly found in lentic environments or open areas (Monteiro Junior et al. 2013; Rodrigues et al. 2016; Vilela and Ferreira 2016).
Corroborating our second prediction, the assemblages found within and outside the PA were different, with regard to species composition, the assemblages within the PA did not vary as much as the assemblages in the surrounding areas, indicating that protected areas maintain a more stable community than unprotected areas. Unprotected areas are subject to disturbances of varying magnitude (as flow modification, siltation, loss of water quality, change in physicochemical variables and flooding), enabling species with different eco-physiological requirements to colonize thiscertain sites (De Marco et al. 2015; Rodrigues et al. 2016, 2018). Previous studies on Odonata have already shown that species composition is altered as a result of changes in natural environments, possibly due to the introduction of one or more generalist species that may out-compete the specialist species. This pattern seems to be more evident in Zygoptera (Juen and De Marco 2012; Monteiro-Junior et al. 2013; Rodrigues et al. 2016, 2018; Carvalho et al. 2018; Rodrigues et al. 2019), and our study points in the same direction.
Corroborating in part our third prediction, our results show that sites within the RPPN have low LCDB values when compared to sites surrounding the RPPN. This is closely linked to the greater species richness found in the areas surrounding the RPPN. However, the SCDB analysis did, demonstrate that some of the species with a very high SCDB value, e.g., E. cannacrioides, H. amazona, L. acutum, L. macrurum, occurred almost exclusively in streams within the RPPN. The species L. acutum, L. macrurum are phytotelmatous, and tightly linked to forested areas (Carvalho et al. 2018; Rodrigues et al. 2019; Furieri et al. 2020; Ribeiro et al. 2021a, b). The species with high SCDB values which were only collected in streams surrounding the RPPN, e.g., Diastatops obscura, E. fusca, E. paraguayensis, E. umbrata, I. capreolus and T. corallina, are either open area specialists or habitat generalists (Rodrigues et al. 2018, 2019; Carvalho et al. 2018). Another relevant finding is that even though the difference in Zygoptera diversity between the protected area and its surroundings was not statistically significant, five of the six species contributing most to SCDB and registered within the RPPN belong to the suborder Zygoptera, emphasizing that PAs often harbour some exclusive species, especially from this suborder.
In accordance with to our fourth prediction, among the species tested as bioindicators, three species (H. aurantiacum, H. longipes and H. amazona) showed a preference for forested areas. The adults of these species favour more shaded places with a greater environmental integrity between the surroundings and the stream channel (Garisson et al. 2010, Ferreira-Puriquetti and De Marco 2002; Borges et al. 2019). Some studies have shown that their larvae are found in un-altered permanent streams (Garrison et al. 2010). Among the species suggested as indicators of the areas surrounding the PA, T. corallina and I. capreolus are generally associated with more open and lentic environments (Rodrigues et al. 2016, 2019).
To conclude, our results show that the composition of the odonate species assemblages may provide a means to assess the importance of protected areas to Odonata communities. Streams located within the RPPN harboured species with a high SCDB value and were good candidates to be keystone communities, contributing to the regional species pool. Our study highlights the importance of PAs to the maintenance of the regional species pool, to forest specialist species and to threatened species. Management strategies are needed to maintain the disproportionally great contribution of sites located within protected areas to the regional species pool. We particularly call attention to the conservation of the protected areas to provide the ecological conditions required by forest specialists, such as big trees, high humid conditions, stable ambient temperature, greater integrity of habitats and availability of sites for reproduction and development of larvae. In fact, the conservation of these sites inside protected areas play a vital role not only in the preservation of forest specialist dragonflies, but also many other taxonomic groups with similar habitat requirements (Fernández et al. 2004, 2012; Gray et al. 2016). These sites also provide other ecological services, such as carbon storage, maintenance of soil and water quality and decomposition among others (DeFries et al. 2010; Palomo et al. 2014; Cumming 2016; Maestre-Andrés et al. 2016). The conservation strategies for dragonflies in the Atlantic Forest could therefore be linked with comprehensive strategies for forest conservation and restoration. Assisting in the conservation of the Atlantic Forest’s biodiversity.
Data availability
The data and material are available.
Code availability
Not applicable.
References
Anderson MJ (2001) A new method for non-parametric multivariate analysis of variance. Astral Ecol 26:32–42
Anderson MJ, Walsh DCI (2013) PERMANOVA, ANOSIM, and the Mantel test in the face of heterogeneous dispersions: What null hypothesis are you testing? Ecol Monogr 83:557–574
Altermatt F, Holyoak M (2012) Spatial clustering of habitat structure effects patterns of community composition and diversity. Ecol 93(5):1125–1133. https://doi.org/10.1890/11-1190.1
Azevedo-Santos VM, Fearnside PM, Oliveira CS, Padial AA, Pelicice FM, Lima-Junior DP, Vitule JRS (2017) Removing the abyss between conservation science and policy decision in Brazil. Biodivers Conserv 26:1745–1752. https://doi.org/10.1007/s10531-017-1316-x
Basset Y, Lamarre GPA (2019) Toward a world that values insects. Conserv 364:1230–1231. https://doi.org/10.1126/science.aaw7071
Bede LC, Machado ABM, Piper W, Souza MM (2015) Odonata of the Serra de São José - Brazil’s first Wildlife Reserve aimed at the conservation of dragonflies. Not Odonatologica 8(117–155):3
Blanco J, Bellón B, Fabricius C et al (2020) Interface processes between protected and unprotected areas: A global review and ways forward. Glob Change Biol 26:1138–1154. https://doi.org/10.1111/gcb.14865
Borges LR, Barbosa MS, Carneiro MAA, Vilela DS, Santos JC (2019) Dragonflies and damselflies (Insecta: Odonata) from a Cerrado area at Triângulo Mineiro. Biota Neotrop, Minas Gerais, Brazil. https://doi.org/10.1590/1676-0611-bn-2018-0609
Calvão LB, Juen L, Oliveira-Junior JMB, Batista JD, De Marco PJ (2018) Land use modifies Odonata diversity in streams of the Brazilian Cerrado. J Insect Conserv 22:675–685. https://doi.org/10.1007/s10841-018-0093-5
Cardoso P, Parton PS, Berkhofer K, Chichorro F, Deacon C, Fartmann T, Fukushina CS, Gaigher R, Habel JC, Hallmann CA, Hell MJ, Hachkerch A, Kwak ML, Mammola S, Noruga JA, Orjinger AB, Pedraza F, Pryke JS, Vorster C, Samways MJ (2020) Scientists’ Warning to humanity on insict extinctions. Biol Conserv. https://doi.org/10.1016/j.biocon.2020.108426
Carvalho FG, Roque FO, Barbosa L, Montag LFA, Juen L (2018) Oil palm plantation is not a suitable environment for most forest specialist species of Odonata in Amazonia. Anim Conserv 21:526–533. https://doi.org/10.1111/acv.12427
Cumming GS (2016) The relevance and resilience of protected areas in the Anthropocene. Anthropocene 13:46–56. https://doi.org/10.1016/j.ancene.2016.03.003
De Caceres M, Legendre P (2009) Associations between species and groups of sites: indices and statistical inference. Ecology 90(12):3566–3574. https://doi.org/10.1890/08-1823.1
De Cáceres M, Legendre P, Moretti M (2010) Improving indicator species analysis by combining groups of sites. Oikos 119(10):1674–1684
De Marco PJ, Batista JD, Cabetle HSR (2015) Community assembly of adult odonates in tropical streams: an ecophysiological hypothesis. PlosOne 4:1–17. https://doi.org/10.1371/journal.pone.0123023
DeFries R, Karanth KK, Pareeth S (2010) Interactions between protected areas and their surroundings in human-dominated tropical landscapes. Biol Conserv 143(12):2870–2880. https://doi.org/10.1016/j.biocon.2010.02.010
Fagundes CK, Vogt RC, De Marco PJ (2016) Testing the efficiency of protected areas in the Amazon for conserving freshwater turtles. Diversity Distrib 22:123–135. https://doi.org/10.1111/ddi.12396
Fernández JD, Gómez JM (2012) Advantages and drawbacks of living in protected areas: The case of the threatened Erysimum popovii (Brassicaceae) in SE Iberian Peninsula. Biodivers Conserv 21(10):2539–2554. https://doi.org/10.1007/s10531-012-0316-0
Fernández-Juricic E, Vaca R, Schroeder N (2004) Spatial and temporal responses of forest birds to human approaches in a protected area and implications for two management strategies. Biol Conserv 117(4):407–416. https://doi.org/10.1016/j.biocon.2003.02.001
Ferreira J, Aragão LEOC, Barlow J, Barreto P, Berenguer E, Bustamante M, Gardner TA, Lees AC, Lima A, Louzada J, Pardine R, Parry L, Peris CA, Pompeu PS, Tabarelli M, Zuanon J (2014) Brazil’s environmental leadership at risk. Science 346:706–707. https://doi.org/10.1126/science.1260194
Ferreira-Peruquetti PS, Marco De, PJr, (2002) Efeito da alteração Ambiental sobre comunidades de Odonata em riachos de Mata Atlântica em Minas Gerais. Brasil Revta Bras Zoo 19(2):317–327
Fonseca CR, Venticinque EM (2018) Biodiversity conservation gaps in Brazil: A role for systematic conservation planning. Perspect Ecol Conserv 16:61–67. https://doi.org/10.1016/j.pecon.2018.03.001
Frederico RG, Zuanon J, De Marco PJ (2018) Amazon protected areas and its ability to protect stream-dwelling fish fauna. Biol Conserv 219:12–19. https://doi.org/10.1016/j.biocon.2017.12.032
Furieri KS, Fraga FB, Tribull C, Colombo WD (2020) Description of two females of Leptagrion Selys (Odonata: Coenagrionidae). Zootaxa 4821(2):343–352. https://doi.org/10.11646/zootaxa.4821.2.6
Garrison RW, Ellenrieder NV, Louton JA (2006) Dragonfly genera of the New World: An illustrated and annotated key to the Anisoptera. The Johns Hopkins University Press, Baltimore
Garrison RW, Von Ellenrieder N, Louton JA (2010) Damselfly Genera of the New World: an illustrated and annotated key to the Zygoptera. Baltimore: The John Hopkins University: 490
Gotelli NJ & Ellison AM Princípios de estatística em ecologia. Porto Alegre: Artmed, 2011.
Gray CL, Hill SLL, Newbold T, Hudson LN, Börger L, Contu S, Hoskins AJ, Ferrier S, Purvis A, Scharlemann JPW (2016) Local biodiversity is higher inside than outside terrestrial protected areas worldwide. Commun, Nat. https://doi.org/10.1038/ncomms12306
ICMBio/MMA (2018) Livro Vermelho da Fauna Brasileira Ameaçada de Extinção: Volume VII Invertebrados, 1ed. Brasília
IUCN (2014) IUCN red list categories and criteria. Version 3.1, 2nd ed.; IUCN Species Survival Commission: Gland, Switzerland; Cambridge, MA, USA
Juen L, De Marco PJ (2012) Dragonfly endemism in the Brazilian Amazon: competing hypotheses for biogeographical patterns. Biodivers Conserv 21:3507–3521. https://doi.org/10.1007/s10531-012-0377-0
Landeiro VL, Franz B, Heino J, Siqueira T, Bini LM (2018) Species-poor and low-lying sites are more ecologically unique in a hyperdiverse Amazon region: Evidence from multiple taxonomic groups. Divers Distrib. https://doi.org/10.1111/ddi.12734
Leal CJ, Lennox GD, Ferraz SFB, Ferreira J, Gardner TA, Thomson JR, Berenguer E, Lees AC, Hughes RM, MacNally R, Aragão LEOC, Brito JG, Castello L, Garrett RD, Hamada N, Juen L, Leitão RP, Louzada J, Morello TF, Moura NG, Nessimian JL, Oliveira-Junior JMB, Oliveira VHF, Oliveira VC, Parry L, Pompeu OS, Solar RRC, Zuanon J, Barlow J (2020) Integrated terrestrial-freshwater planning doubles conservation of tropical aquatic species. Science 6512:117–121. https://doi.org/10.1126/science.aba7580
Legendre P, De Ca´ceres M, (2013) Beta diversity as the variance of community data: dissimilarity coefficients and partitioning. Ecol Lett 16:951–963. https://doi.org/10.1111/ele.12141
Legendre P, Legendre L (1998) Numerical Ecology, 2ed. Elsevier, Amsterdam, The Netherlands
Lencion, FAA (2006) Damselflies of Brazil: an illustrated identification guide. Vol. 2. All Print Editora– São Paulo, 146 pp.
Lencioni FAA (2005) Damselflies of Brazil: An Illustrated Identification Guide – 1 – Non Coenagrionidae Families. 1ª Edição, São Paulo, All Print Editora.
Lencioni FAA (2017) Damselflies of Brazil - an illustrated identification guide - Southeast region. Vol. 1. E-Book, Brasil, 412pp.
Maestre-Andrés S, Calvet-Mir L, Van Den Bergh JCJM (2016) Sociocultural valuation of ecosystem services to improve protected area management: A multi-method approach applied to Catalonia. Spain Reg Environ Change. 16(3):717–731. https://doi.org/10.1007/s10113-015-0784-3
Mammola S, Riccardi N, Prié V, Correia R, Cardoso P, Lopes-Lima M, Sousa R (2020) Towards a taxonomically unbiasedEuropean Union biodiversity strategy for 2030. R. Soc, Proc. https://doi.org/10.1098/rspb.2020.2166
MapBiomas (2021) Relatório Anual do Desmatamento no Brasil 2020-São Paulo, Brasil, 2021–93 pp. http://alerta.mapbiomas.org
S.O.S. Mata Atlântica. (2018). Relatório anual. São Paulo - SP. Available at: <https://www.sosma.org.br/wpcontent/uploads/2019/07/RA_SOSMA_2018_DIGITAL.pdf> (accessed 08 November 2019)
Mello K, Taniwaki RH, De Paula FR, Valente RA, Randhir TO, Macedo DR, Leal CG, Rodrigues HRM (2020) Multiscale land use impacts on water quality: assessment, planning, and future perspectives in Brazil. J Environ Manage. https://doi.org/10.1016/j.jenvman.2020.110879
Ministério de Meio Ambiente (2021) Cadastro Nacional de Unidade de Conservação. Available at: <https://antigo.mma.gov.br/areas-protegidas/cadastro-nacional-de-ucs.html>. (accessed 15 December 2021).
Mittermeier RA, Fonseca GAB, Rylands AB, Brandon K (2005) Uma breve história da conservação da biodiversidade no Brasil. Megadiversidade 1(1):14–21
Mittermeier RA, Turner WR, Larsen FW, Brooks TM, Gascon C (2011) Global Biodiversity Conservation: The Critical Role of Hotspots. In: Zachos F., Habel J. (eds) Hotspots de biodiversidade. Springer, Berlim, Heidelberg Doi: https://doi.org/10.1007/978-3-642-20992-5_1
Monteiro-Júnior CS, Couceiro SRM, Hamada N, Juen L (2013) Effect of vegetation removal for road building on richness and composition of Odonata communities in Amazonia. Brazil. Int. J. Odonatol. 16:135–144. https://doi.org/10.1080/13887890.2013.764798
Oliveira U, Soares-Filho BS, Paglia AP, Brescovit AD, Carvalho CJB, Silva DP, Rezende DP, Leite FSF, Batista JAN, Barbosa JPPP, Stehmann JR, Ascher JS, Vasconcelos MF, De Marco P, Löwenberg-Neto P, Ferro VG, Santos AJ (2017) Biodiversity conservation gaps in the Brazilian protected areas. Sci Rep 7:9141. https://doi.org/10.1038/s41598-017-08707-2
Oliveira-Junior JMB, Juen L (2019) The Zygoptera/Anisoptera Ratio (Insecta: Odonata): a New Tool for Habitat Alterations Assessment in Amazonian Streams. Neotrop Entomol 48:1–9. https://doi.org/10.1007/s13744-019-00672-x
Oliveira-Junior JMB, Shimano Y, Gardner TA, Hughes RM, De Marco PJ, Juen L (2015) Neotropical dragonflies (Insecta: Odonata) as indicators of ecological condition of small in the Eastern Amazon. Austral Ecol 40:733–744. https://doi.org/10.1038/s41598-017-08707-2
Palomo I, Montes C, Martín-López B, González JA, García-Llorente M, Alcorlo P, Mora MRG (2014) Incorporating the social- ecological approach in protected areas in the Anthropocene. Bioscience 64(3):181–191. https://doi.org/10.1093/biosci/bit033
Peralta EM, Belen AE, Buenaventura GR, Cantre FGG, Espiritu KGR et al (2019) Stream benthic macroinvertebrate assemblages reveal the importance of a recently established freshwater protected area in a tropical watershed. Pac Sci 73(3):305–320. https://doi.org/10.2984/73.3.1
Quintana C, M Girardello, AS Barfod, H Balslev (2017) Diversity patterns, environmental drivers and changes in vegetation composition in dry inter-Andean valleys. J Plant Ecol 10(3):461–475. https://doi.org/10.1093/jpe/rtw036
R Core Team (2020) R: A language and environment for statistical computing. R Foundation for Statistical Computing, Vienna
Reid AJ, Carlson AK, Creed IF, Eliason EJ, Gell PA, Johnson PTJ, Kidd KA, MacCormack TJ, Olden JD, Ormerod SJ, Smol JP, Taylor WW, Tockner K, Vermaire JC, Dudgeon D, Cooke SJ (2018) Emerging threats and persistent conservation challenges for freshwater biodiversity Biol. Rev 94:849–873. https://doi.org/10.1111/brv.12480
Ribeiro C, Juen L, Rodrigues ME (2021a) The Zygoptera/Anisoptera ratio as a tool to assess anthropogenic changes in Atlantic Forest streams. Biodivers Conserv. https://doi.org/10.1007/s10531-021-02143-5
Ribeiro C, Santos LR, Rodrigues ME (2021b) New records of the Critically Endangered Leptagrion acutum Santos, 1961 (Odonata, Coenagrionidae) from southern Bahia. Brazil. Check List. 17(1):59–62. https://doi.org/10.15560/17.1.59
Rodrigues ME, Roque FO, Quintero JMO, Pena JCC, Sousa DC, De Marco PJ (2016) Nonlinear responses in damselfly community along a gradient of habitat loss in a savanna landscape. Biol Conserv 194:113–120. https://doi.org/10.1016/j.biocon.2015.12.001
Rodrigues ME, Roque FO, Ferreira RGN, Saito VS, Samways MJ (2018) Egg-laying traits reflect shifts in dragonfly assemblages in response to different amount of tropical forest cover. Insect Conserv. Diver. 11:01–10. https://doi.org/10.1111/icad.12319
Rodrigues ME, Moura EB, Roque FO (2019) Dragonflies as indicators of the environmental conditions of veredas in a region of central-estern Brazil. Austral Ecol 23:969–978. https://doi.org/10.4257/oeco.2019.2304.20
RPPN Estação Veracel (2016) Plano de Manejo. Veracel Celulose, Gerência de Sustentabilidade e Conservação Internacional, Bahia, Brazil, Eunápolis
Ruhı´ AT, Datry & JL Sabo, (2017) Interpreting beta-diversity components over time to conserve metacommunities in highly dynamic ecosystems. Conserv Biol 31:1459–1468. https://doi.org/10.1111/cobi.12906
Samways MJ (2017) Reconciling ethical and scientific issues for insect conservation Foottit RG, Adler PH (Eds.), Insect Biodiversity: Science and Society (second ed), 1, Wiley-Blackwell, Oxford, UK, pp 747–766. https://doi.org/10.1002/9781118945568.ch23
Samways MJ, Barton PS, Birkhofer K, Chichorro F, Deacon C, Fartmann T, Fukushim CS, Gaigher R, Habel JC, Hallmann CA, Hill MJ, Hochkirch A, Kaila L, Kwak ML, Maes D, Mammola S, Noriega JA, Orfinger AB, Pedraza F, Pryke JS, Roque FO, Settele J, Simaika JP, Storka NE, Suhling F, Vorste C, Cardoso P (2020) Solutions for humanity on how to conserve insects. Biol Conserv. https://doi.org/10.1016/j.biocon.2020.108427
Sundar S, Heino J, Roque FO et al (2020) Conservation of freshwater macroinvertebrate biodiversity in tropical regions. Aquatic Conserv Mar Freshw Ecosyst 30:1238–1250. https://doi.org/10.1002/aqc.3326
Valente-Neto F, Roque FDO, Rodrigues ME, Juen L, Swan CM (2016) Toward a practical use of Neotropical odonates as bioindicators: testing congruence across taxonomic resolution and life stages. Ecol Ind 1:952–959. https://doi.org/10.1016/j.ecolind.2015.10.052
Valente-Neto F, da Silva FH, Covich AP, de Oliveira Roque F (2020) Streams dry and ecological uniqueness rise: environmental selection drives aquatic insect patterns in a stream network prone to intermittence. Hydrobiol 847:617–628
Vilela DS, Ferreira RG, Del-Claro K (2016) The Odonata community of a Brazilian Vereda: seasonal patterns, species diversity and rarity in a palm swamp environment. Biosci J 32:486–495
Vorster C, Samways MJ, Simaika JP, Kipping J, Clausnitzer V, Suhlingf F, Dijkstra KDB (2020) Development of a new continental-scale index for freshwater assessment based on dragonfly assemblages. Ecol Indic. https://doi.org/10.1016/j.ecolind.2019.105819
Watson JEM, Dudley N, Segan DB, Hockings M (2014) The performance and potential of protected areas. Nature 515(7525):67–73. https://doi.org/10.1038/nature13947
Acknowledgements
We thank to Coordination for the Improvement of Higher Education Personnel— CAPES for the Master’s scholarship to the student CRS (Process Number 8882.460645/2019-01), who allowed us to do this work. To the post-graduate program in Tropical Aquatic Systems—PPGSAT. The RPPN Estação Veracel for allowing research in the reserve and for support in feld activities and accommodation. We also thank the Laboratory of Aquatic Organisms—LOA for supporting our research. Our colleague Lais Rorigues for making our map and Anna Lejfelt-Sahlén kindly improved the authors' English. The Universidade Estadual de Santa Cruz—UESC and to National Council for Scientifc and Technological Development—CNPQ, for funding the research project (registered number UESC/PROPP 0220.1100.1693; registered number CNPQ 423737/2018-0). We are grateful to the CNPq, Brazil (National Council for Scientifc and Technological Development) for their research productivity grants awarded to FOR.
Funding
This research has financed from Universidade Estadual de Santa Cruz—UESC, project registered for Number 0220.1100.1693. And also, by National Council for Scientific and Technological Development—CNPQ, process Number 423737/2018–0.
Author information
Authors and Affiliations
Contributions
All the authors contributed to the development of this study. CRS and MER collected the data, identified the specimens and did the analysis. The first draft of the manuscript was written by CRS. And MER, GS and FOR assisted in the writing and discussion of all parts until the final manuscript.
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Ethics approval
Not applicable.
Consent to participate
Not applicable.
Consent for publication
Not applicable.
Research involved in humans and/or animals
Not applicable.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Appendices
Appendix 1
See Table 2.
Appendix 2
See Table 3.
Rights and permissions
About this article
Cite this article
Ribeiro, C., Rodrigues, M.E., Sahlén, G. et al. Dragonflies within and outside a protected area: a comparison revealing the role of well-preserved atlantic forests in the preservation of critically endangered, phytotelmatous species. J Insect Conserv 26, 271–282 (2022). https://doi.org/10.1007/s10841-022-00385-4
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10841-022-00385-4