Abstract
Central-west and south of Mexico are particularly rich in oak species, coexisting from two to six species and generating gradients of oak diversity that could potentially affect community structure of canopy arthropods. We evaluated changes in canopy arthropod diversity of the Quercus laurina along a gradient of oak diversity in the central-west portion of the Trans-Mexican Volcanic Belt. Five study sites that represent an oak diversity gradient and, where Q. laurina is also widespread were selected. At each site, five mature trees of Q. laurina were randomly selected to collect canopy arthropods using fogging techniques. We assessed the effects of local climate variables on the oak community and the canopy arthropod diversity. We collected 7479 arthropods representing 1154 morphospecies grouped into 15 different orders. Differences in arthropod diversity, abundance and guild composition between oak communities were detected. The general pattern showed a highest richness and abundance of arthropods in Tequila volcano, which represented the site with the greatest diversity of oaks. A significant positive relationship between arthropod abundance and oak species diversity was found. Arthropod guild composition differed along the gradient of oak diversity. Local environmental variables were related with oak community, but did not explained canopy arthropod diversity. At the local scale, plant species richness is the main factor that determines the canopy arthropod abundance and richness of Q. laurina, affecting the structure and composition of arthropod guilds. We highlight the importance of conserving oak species because they represent key elements of temperate forest that harbor high arthropod diversity.
Implications for insect conservation
Our findings confirm that at the local scale, plant species richness is the main factor that determines the arthropod diversity associated with the canopy of Quercus laurina, affecting trophic levels and the composition of arthropod guilds. Therefore, the oak species diversity in temperate forests is a key factor to harbor and preserving the diversity of canopy arthropods in temperate ecosystems.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Tree canopies perhaps maintain the largest portion of arthropod diversity, which in total with terrestrial arthropods vary from 5 to 12 million species (Stork et al. 2008; Hamilton et al. 2013), of which 20 to 25% are canopy exclusive (Stork 2018). The canopy habitat provides food resources, shelter and sites for reproduction, oviposition and development for diverse arthropod guilds, such as herbivores (including chewing, sucking, gall-inducing and leaf-mining groups), predators, parasitoids and detritivores, among others (Ulyshen 2011; Maldonado-López et al. 2018).
Different factors could be associated with arthropod diversity on tree canopies. Environmental factors such as temperature, humidity, light incidence and wind speed, as well as aspects of the structure and composition of plant communities, including species richness, plant density and age, have been proposed as drivers of canopy arthropod diversity at different spatial scales (Tal et al. 2008; Ulyshen 2011). Additionally, the physical structure of tree canopies, comprising forest height, vertical foliage complexity and the abundance of tree cavities and other structural aspects that may enhance habitat heterogeneity, has been proposed as one of the main factors that explain arthropod diversity (Basset et al. 2003; Ulyshen 2011).
At the local scale, plant diversity is a determinant factor that influences canopy arthropod diversity (Moreira et al. 2016). For example, the diversity of herbivorous insects increases from communities with few plant species to more diverse communities (Cuevas-Reyes et al. 2004; Ulyshen et al. 2011; Hertzog 2017). This pattern is due to the fact that a more diverse plant community represents a higher number of resources and niches to be used and colonized by herbivorous arthropod species (Cuevas-Reyes et al. 2004; Schmidl et al. 2008). Similarly, a greater diversity of resources provided by a high herbivore diversity present in highly diverse plant communities increases the diversity of predators and other trophic levels, affecting the whole community structure of arthropods (Randlkofer et al. 2010; Haddad et al. 2011; Moreira et al. 2016;). Therefore, the structure of plant communities can be a key factor that determines arthropod diversity along ecological gradients (Haddad et al. 2011).
Two possible hypothesis that are not mutually exclusive can explain these patterns. The first is based on “the natural enemy hypothesis” (Root 1973) that proposes that the diversity of predators and parasitoids (natural enemies) increases in communities with a great diversity of plants because these represent sites with more potential prey species and microenvironments, stressing the importance of “top-down effects” (Wilby and Thomas 2002; Sobek et al. 2009). This hypothesis implies an indirect mechanism in the interaction between plants and herbivorous insects, according to which the presence of their natural enemies such as spiders, ants, wasps, ground-beetles, etc., exerts a top-down control on herbivore abundance (Pearce et al. 2003). For example, Sobek et al. (2009) showed an increase in herbivorous insects and predator arthropod diversity as the diversity of trees increases in a temperate forest. The second, called “the resource specialization hypothesis” (Novotny et al. 2002) predicts a greater abundance of specialized arthropods in communities with a greater diversity of plants, with herbivore insect diversity being modulated by the diversity and quality of plants, thus giving preponderance to bottom-up effects (Cook-Patton et al. 2011; Leroux and Loreau 2015).
One of the most important components of temperate forests in the Northern Hemisphere, both in terms of species number and biomass, is the genus Quercus (Fagaceae) (Cavender-Bares 2019). This genus represents a large and ecologically diverse group of woody plants with temperate origin (Hipp et al. 2020). Oaks are also known to support a great diversity of organismal groups, such as ectomycorrhizal fungi (García-Guzmán et al. 2017), epiphytic plants (Maclean et al. 2017) and canopy arthropods (Tovar-Sánchez and Oyama 2006a, b; Valencia 2015; Maldonado-López et al. 2018). Several areas in central-western and southern Mexico are particularly rich in oak species, particularly of the Lobatae (red oaks) and Quercus (white oaks) sections (Torres-Miranda et al. 2013; Rodríguez-Correa et al. 2015), where between two and up to six species can occur in sympatry at the local scale, with species of the two sections often coexisting (Cavender-Bares et al., 2018; McCauley et al. 2019). Thus, oak community diversity gradients are an excellent model to evaluate the changes in canopy arthropod diversity (Root 1973; Sobek et al. 2009). To our knowledge, the existence of this relationship has not been tested in tree canopies in temperate forests and neither along a gradient of tree diversity. Therefore, in this study we evaluated the changes in the structure and composition of canopy arthropods associated to a focal species (Quercus laurina) along a gradient of diversity along oak communities. We chose Q. laurina because it has a wide distribution in Mexico (Valencia 2004), making it feasible to find communities with varying oak species diversity where this focal species is present and abundant. The specific questions addressed were: (i) Does canopy arthropod diversity associated to Q. laurina increase in communities with a greater diversity of oak species? and (ii) How do the structure and composition of arthropod guilds vary along the oak species diversity gradient?
Materials and methods
Study species
Quercus laurina Humb et Bonpl belongs to the section Lobatae (red oaks) of the genus Quercus and is endemic species of oak for Mexico (Valencia 2004). It is a tree that grows up to 30 m, has coriaceous, lanceolate or elliptic-oblanceolate leaves, with a green and lustrous surface; the fruit is an ovoid acorn, with an average size of 15 to 20 mm long and 15 to 17 mm in diameter. It is distributed between 2440 and 3065 m.a.s.l. and occurs in mixed oak and pine-oak forests, in the Sierra Madre del Sur and the Trans-Mexican Volcanic Belt (TMBV) (Valencia 1994).
Oak diversity gradient
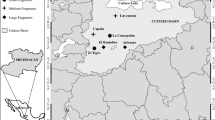
Field work was performed in five sites in the central-west portion of the Trans-Mexican Volcanic Belt in which the diversity and composition of oak communities has been previously analyzed (McCauley et al. 2019) and represent an oak diversity gradient with the presence of Q. laurina in all cases (Fig. 1). However, for this study, the oak communities were described again because it was not possible to locate precisely the same transects previously analyzed. Therefore, at each site we sampled three independent transects of 100 × 40 m with at least 500 m of separation from each other. Within each transect, we recorded the number of individuals with a diameter at breast height (DBH) ≥ 10 cm of each of the oak species present. From the summed data of the three transects per site, we calculated the oak species richness (OSR) Shannon diversity index (H′), as well as the total density of oak individuals (TDOI; trees per hectare, including all species), the density of individuals belonging to the Lobatae section (DILS) and the density of individuals belonging to the Quercus section (DIQS). All sites were almost pure oak stands (i.e. with oaks representing at least 90% of the trees), with moderate levels of anthropogenic disturbance. None of the sites was within a natural protected area.
Geographical distribution map of the study sites, which are located in the states of Michoacán and Jalisco, in the central-western zone of the Trans-Mexican Volcanic Belt (TMBV). The Tequila Volcano site is represented by the square, Carindapaz by the triangle, Indaparapeo by the circle, Los Azufres by the cross and Cerro Burro by the rhombus
Canopy arthropod diversity
Sampling of canopy arthropods was performed at the end of the rainy season (September) of 2014. At each site, five mature medium-sized trees (DBH 30–40 cm) of Q. laurina were randomly selected to collect canopy arthropods using fogging techniques (Erwin and Geraci 2009). We chose these size class of trees because larger trees are more difficult to sample with this technique. Fogging was applied in all trees in the morning between 05:00 and 6:00 A.M. Each tree was nebulized for a period of 10 min using a Swingfog SN-50 Thermal Fogger to disperse a mix of synergized pyrethrins (30 g/l) and piperonyl butoxide (150 g/l). We placed 8 funnel-shaped trays of 1m2 under the crown of each fogged tree, and after 50 min, we performed the first arthropod collection using ropes to shake the canopy (Marques et al. 2006; Barringer et al. 2019). After 2 h of the first collection, a second collection was performed to collect further arthropods. Arthropods were stored in 95% ethanol and transported to the Agroecology Laboratory of Universidad Michoacana de San Nicolás de Hidalgo (UMSNH) for taxonomic identification. Arthropods were identified to family level with the aid of one of the coauthors (ELB) a specialist taxonomist and using specialized texts. Additionally, each arthropod individual was assigned to a trophic guild (phytophages, predators, detritivores-saprophages, parasitoids, hematophages, mycophages) (Triplehorn and Johnson 2005; Ubick et al. 2017). Arthropod abundance and species richness were estimated using morphospecies criteria, which are recognized as taxonomic units (Majer et al. 2000; Stiegel and Mantilla-Contreras 2018).
Statistical analysis
Oak community composition
To determine the differences in the abundance of oak species and abundance per oak taxonomic section (Lobatae vs Quercus) between study sites along the diversity gradient, we performed generalized linear models (GLM) analyses respectively, using a Poisson error distribution and a log link function. Study sites were used as the independent variable and the total abundance of oaks and oak abundance per taxonomic section were considered as the response variables (SAS, Stokes et al. 2000).
Canopy arthropod composition
Canopy arthropod abundance and species richness were compared between study sites using a GLM analysis with a Poisson error distribution and a log link function. The study sites were used as the independent variable and arthropod abundance and species richness as the response variables. Furthermore, we performed a logistic regression analysis with the CATMOD procedure (SAS 2000) that is a general procedure for modelling categorical data, to evaluate the differences in the frequency of arthropod orders between study sites. The frequency of arthropod orders was considered as the response variable and study sites as independent variable. Because the distribution of the dependent variable did not follow a normal distribution, we used a Poisson distribution with a logarithmic link function (Stokes et al. 2000).
The Pielou-evenness index was used at the order level to calculated arthropod diversity in each study site. This measure of equitability compares the observed Shannon–Wiener index against the distribution of individuals between the observed species which would maximize diversity (Magurran 1988; Wan et al. 2014).
The richness of the canopy arthropod families of each study site was obtained by rarefaction curves using the program EstimateS 9.1.0 (Colwell 2013). The scale of the independent variable (X) was represented by the number of estimated individuals of arthropods in the canopy. For the application of the rarefaction method, a data set was standardized and compared using the number of individuals as the sampling effort (Gotelli and Colwell 2001). A 95% confidence intervals (CI) were used in each rarefaction curve to determine if the differences in richness between localities was a result of true richness or due to abundance.
To determine the influence of the sites and the parameters of the oak community on the canopy arthropod diversity, as well on the diversity of each guild, we perform multiple GLMs analyses. In the first two GLMs analyses, the response variables were the abundance and richness of canopy arthropods, while the explanatory variables were the study sites and the following parameters of the oak community: (i) oak species richness (OSR), total density of all oak species (TDOS), density of oaks Lobatae section (DOLS) and density of oaks Quercus section (DOQS). For the GLMs analyses of the diversity of arthropod guilds, the abundance and richness of each guild (i.e. phytophagous, predators, detritivorous-saprophagous and parasitoids) were considered as the response variables. The study sites and the same parameters of the oak community used for the first GLMs were considered as the explanatory variables. The mycophagous and hematophagous guilds were excluded from the analyses because they presented a very low abundance and richness in each study site. In addition, Shannon's diversity index also was excluded from the models and did not show significant effects on any of the GLMs performed. For all GLMs a Poisson error distribution and a logarithmic link function were used (Stokes et al. 2000).
A principal component analysis on correlations was carried out on the parameters of oak community and the abundance and richness of canopy arthropod at the five study sites along the gradient. The purpose of this analysis was to create a multidimensional representation of the parameters of the oak community of the study sites and their probably correlation with the abundance and richness of arthropods along the gradient.
Multivariate analyses were used to evaluate differences in arthropod composition between the five study sites. Thus, the five communities of arthropods were ordered by non-metric multidimensional scaling (NMDS) using an abundance similitude matrix and the Bray–Curtis index as a distance metric. Afterwards, a non-parametric permutation procedure (ANOSIM) was used to check whether arthropod species composition differed among the five study sites. Again, the ANOSIM was based on a binary matrix arthropod species occurrence in each study site, using the Bray–Curtis index as a distance metric and 5000 permutations (Hammer et al. 2001). Pairwise ANOSIMs were performed between all pairs of sites as a post-hoc test. These analyses were developed using PAST software (Hammer et al. 2001).
Relationships between local climate variables, oak community and canopy arthropod diversity
We also assessed the effects of climate variables on the composition of the oak community and the canopy arthropod community of Q. laurina between all study sites. Climatic data were obtained for each of the 19 bioclimatic variables available in WorldClim (http://worldclim.org/version2) for each study site, and then, we eliminated redundant variables on the basis of the results of a paired correlation analysis (the criterion was to eliminate one variable from each pair with R > 0.80 retaining the more general variable). The variables selected were mean annual temperature (MAT), mean annual precipitation (MAP), mean temperature of the warmest quarter (TWQ) and mean temperature of the coldest quarter (TCQ). Hence, we performed a canonical correspondence analysis (CCA) to determine whether there is any correlation between climate variables and the oak community composition and the canopy arthropod community in the study sites. CCA has proved a valuable tool for exploring species-environment correlations (Jin et al. 2016). For this analysis, we used the statistical package PAST 3.23 (Hammer et al. 2001).
Results
Composition of the oak community
In total, we found five oak species in the study sites, three grouped in the Lobatae section (Q. laurina, Q. crassifolia and Q. calophylla) and two in the Quercus section (Q. obtusata and Q. rugosa). Quercus laurina was the most abundant species at all sites, representing from 50% of the individuals in the Tequila Volcano and to 95% in Cerro Burro. The second most abundant species was Q. rugosa which was present in four sites, while Q. calophylla was found in three sites and finally Q. crassifolia in two sites (Fig. 2). The Tequila Volcano presented the highest oak species abundance and richness, followed by Los Azufres, Indaparapeo, Carindapaz and Cerro Burro (Table 1). The oak community with the highest density (930 trees/ha considering all species) was also Tequila Volcano, and then Los Azufres, Cerro Burro, Indaparapeo and Carindapaz (Table 2). In all sites, species belonging to the Lobatae section were more abundant, ranging from 96% of the individuals (Tequila Volcano) to 75% (Los Azufres) (Table 2).
We found significant differences in the abundance of oaks species between the study sites (χ2 = 409.9, df = 4, P = 0.0001), as well as between the oak taxonomic sections (χ2 = 358.3, df = 1, P = 0.0001) (Fig. 2).
Arthropod community along the oak diversity gradient
We collected a total of 7479 arthropods associated to Q. laurina in all study sites, distributed as follows: 3408 in Tequila Volcano, 1393 in Los Azufres, 1120 in Indaparapeo, 747 in Carindapaz and 811 in Cerro Burro. These arthropods were separated into a total of 1154 morphospecies (Online Appendix 1) which grouped in 15 orders (Fig. 3). In general, the frequency of arthropod orders was different between study sites (χ2 = 35.1; d.f. = 4; P < 0.0001) (Fig. 3). Per site, the number of arthropod orders found varied between 12 (Tequila Volcano) and 9 (Carindapaz) (Fig. 3). The most abundant order (in terms of number of individuals) at all study sites was Coleoptera, followed by Psocoptera and Hemiptera. Particularly, Tequila Volcano, which was the site with higher oak species richness had a higher frequency of the Coleoptera (26.1%), Hymenoptera (14.7%) and Araneae (12.5%), while the site with lowest richness of oaks (i.e. Cerro Burro) had 30.6% of Coleoptera, 19.4% of Psocoptera and (12.1%) of Hemiptera (Fig. 3).
Shannon’s diversity index values per site at the order level were 2.14, 2.13, 2.11, 1.78 and 1.93 for Tequila Volcano, Los Azufres, Indaparapeo, Carindapaz and Cerro Burro, respectively. All study sites had low values of evenness in the abundance of orders according to Pielou-evenness index, which ranged from J = 0.77 in Carindapaz to J = 0.88 in Tequila Volcano. The rarefaction analysis showed that the richness of arthropod families was significantly higher in the Volcano Tequila, followed of Indaparapeo, Los Azufres, Cerro Burro and Carindapaz (Fig. 4).
Arthropod abundance (measured as the mean number of individual arthropods per tree) associated to the canopy of Q. laurina was significantly different along the oak diversity gradient (χ2 = 274.1; d.f. = 4, P < 0.0001) (Fig. 5a). Trees of Q. laurina in Tequila Volcano harbored higher arthropod abundance (681.6 ± 13.6) in comparison with trees of Los Azufres (348 ± 5.24), Indaparapeo (224 ± 18.5), Carindapaz (149.4 ± 6.72) and Cerro Burro (162.2 ± 19.32) (Fig. 4a). Similarly, the mean richness of arthropod morphospecies per tree was greater in the Tequila Volcano (65.6 ± 7.62) than in Los Azufres (53 ± 5.1) and Indaparapeo (51.2 ± 9.67 SE) and lower in Carindapaz (28.2 ± 3.8) and Cerro Burro (34.4 ± 5.3) (χ2 = 72.9; d.f. = 4, P < 0.0001) (Fig. 5b).
In total, we recorded six different guilds distributed as follows, in terms of the total number of individual arthropods in each guild: phytophages (55.5%), detritivores-saprophages (16.5%), predators (14.5%), parasitoids (12.6%), hematophages (0.5%), and microphages (0.5%). The relative abundance of these arthropod guilds also differed significantly among the study sites. Phytophages represented a higher proportion of the arthropod community at Tequila Volcano (51.7%) and a lower proportion at Los Azufres (37.3%), while the other sites showed intermediate values (χ2 = 269.31; d.f. = 4, P < 0.0001) (Fig. 6a). In contrast, predators were more abundant in Indaparapeo (26.7%) and least abundant in the Tequila Volcano (11.1%), with intermediate values in the other three sites (χ2 = 5.51; d.f. = 4, P < 0.018). In turn, parasitoids showed a higher relative abundance in Los Azufres and Cerro Burro (around 19%), while they were least abundant in Indaparapeo (6.9%). The frequency of detritivores varied less across sites, being between 18.7 and 21.6%.
In terms of the number of morphospecies, the higher proportion corresponded to the phytophagous guild (37.6% of the total), while 26.5, 17.9, 17.12, 1 and 0.4% of the morphospecies corresponded to detritivorous-saprophagous, predators, parasitoids, hematophagous and mycophagous, respectively. The proportion of morphospecies belonging to each guild also varied among sites. Phytophagous morphospecies represented 44.9% of the total in Tequila Volcano, while they represented 30.7% in Los Azufres, with intermediate values in the other three sites (χ2 = 435.69; d.f. = 4, P < 0.0001). Significant differences were also observed for predators (χ2 = 32.25; d.f. = 4, P < 0.018), parasitoids (χ2 = 63.65; d.f. = 4, P < 0.0001) and detritivorous-saprophagous (χ2 = 63.65; d.f. = 4, P < 0.0001) (Fig. 6b).
The GLM models indicated that parameters of the oak community and the study sites had significant effects on the canopy arthropod abundance and richness, as well on the diversity of arthropod guilds associated to the canopy of Q. laurina. The parameters of oak community OSR (F = 2197; d.f. = 22; P < 0.0001), TDOS (F = 304.78; d.f. = 21; P < 0.0001), DOLS (F = 180.75; d.f. = 20; P < 0.0001), DOQS (F = 33.02; d.f. = 19; P < 0.0001) had significant effects on the arthropod abundance and richness; as well the study sites (F = 31.05; d.f. = 18; P < 0.0001) (Table 3). For the case of arthropod guilds, the phytophagous abundance was affected only by three parameters of the oak community: OSR (F = 182.43; d.f. = 22; P < 0.0001), TDOS (F = 734.16; d.f. = 21; P < 0.0001) and DOLS (F = 227.53; d.f. = 20; P < 0.0001). The phytophagous richness was influenced for OSR (F = 12.14; d.f. = 22; P < 0.0001), TDOS (F = 26.91; d.f. = 21; P < 0.0001) and DOQS (F = 6.61; d.f. = 19; P < 0.0001) (Table 3). The predator abundance was significant influenced for two parameters of the oak community: OSR (F = 26.76; d.f. = 22; P < 0.0001) and DOLS (F = 43.37; d.f. = 20; P < 0.0001). We did not find significant effects on the richness of predators (Table 3). We found a similar pattern for the abundance of detritivores–saprophagous and parasitoids. The abundance of the detritivores–saprophagous was significant affected by OSR (F = 64.45; d.f. = 22; P < 0.0001) and TDOS (F = 118.75; d.f. = 20; P < 0.0001). The parasitoids abundance was influenced by OSR (F = 349.66; d.f. = 22; P < 0.0001), TDOS (F = 256.44; d.f. = 21; P < 0.0001) and DOLS (F = 19.45; d.f. = 20; P < 0.0001) (Table 3).
In turn, the principal components analysis revealed that the PC1 was positively correlated with parameters of the oak community as OSR, TDOI, DILS and arthropods abundance. The sites of Tequila Volcano and Indaparapeo were high on PC1. PC2 was positively correlated with DIQS, H' and the richness of arthropods. The site of Los Azufres was high on PC2; and for the other sites we did not find a clear correlation between the oak community and the abundance and richness of arthropods (Fig. 7).
Principal component analysis of the parameters of the oak community and the abundance (AA) and richness (AR) of arthropods in the canopies of Q. laurina at the five sites presents along the oak’s diversity gradient. Vectors show the strength and direction of the relationship between the parameters of the oak community and axes. The parameters of the oak community are: Oak species richness (OSR), Shannon diversity index (H′), total density of all oak individuals (TDOI), density of individuals in the Lobatae section (DILS), density of individuals in the Quercus section (DIQS)
The results of the similarity of arthropod community showed that NMDS ordination explained 62% of the variance between sampling points (axis 1 = 52% and axis 2 = 10%), where each point is a two-dimensional representation of the composition of arthropod species in a single tree based on global NMDS. The composition of arthropod species associated to Q. laurina varied between the study sites (ANOSIM r = 0.52, n = 25, P < 0.001) (Fig. 8). The results of post-hoc pairwise ANOSIM tests showed different composition of arthropod species between all study sites. Particularly, Tequila Volcano and Cerro Burro had totally different assemblages in comparison with the other three study sites (P < 0.05). Moreover, the arthropod species composition of Carindapaz also differed from Los Azufres (P < 0.05).
Non-metric multidimensional scaling (NMDS) ordinations illustrating similarity of arthropod taxonomic composition between the study sites along the oak diversity gradient. Each point is a two-dimensional (axis 1 and axis 2) representation of arthropod species composition on an individual tree based on global, non-metric multidimensional scaling (NMDS)
Local climate variables, oak community and canopy arthropod diversity
Canonical correspondence analyses (CCA) between climate variables and the oak community composition along the oak diversity gradient showed significant correlations in the Permutation test of all canonical axes (Trace = 0.22, P = 0.004). The first two axes explained 85.15% (axis 1 = 65.41% and axis 2 = 19.74%) of the spatial variation of the oak community relative to climate variables, where MAP, TCQ and TWQ had a strong correlation with the oak community composition. Particularly, Tequila Volcano, Los Azufres and Indaparapeo showed a greater correlation between climatic variables and the composition of the oak community.
The results of the canonical correspondence analysis (CCA) between the climate variables and arthropod community did not show significant correlations in the Permutation test of all canonical axes (Trace = 0.1294, P = 0.34). This indicates that the composition of the canopy arthropod community associated to Q. laurina was not influenced by local environmental variables of each study site.
Discussion
Oaks represent one of the most diverse groups of woody plants in the Northern Hemisphere, with Mexico being a center of oak diversity and endemism (Cavender-Bares 2016). Our results agree with this idea, since at the local scale, we detected a diversity gradient of four species, ranging from localities with only two species to localities with oak species coexisting. In terms of the number of species of the Lobatae section, we observed the coexistence of Q. laurina with up to two other species of red oaks, such as Q. crassifolia and Q. calophylla. In general, we found a greater abundance of oaks from the Lobatae section, with Q. laurina being the dominant species with an average 88% of the individuals present in the study sites. In addition, we found correlations between climatic variables (MAP, TCQ and TWQ) and oak community composition in study sites with greater richness and abundance of oaks. These results can be explained by the high climatic variation present along the Sierra Madre del Sur and the Trans-Mexican Volcanic Belt (Currie and Francis 2004). Some environmental variables such as temperature, precipitation, habitat heterogeneity, and past climate change have been proposed as the drivers of contemporary species richness patterns of oaks (Hipp et al. 2019). Since Mexico is a diversity center for the genus Quercus, the coexistence among different species is expected, and particularly, the co-occurrence of the two main sections seem to be the norm rather than the exception (Rodríguez-Correa et al. 2015; Cavender-Bares et al. 2018; McCauley et al. 2019).
In total, we detected 1154 morphospecies of arthropods grouped in 15 orders associated to the canopy of Q. laurina. The abundance and richness of the arthropod community associated to Q. laurina was different along the gradient of diversity of oak species. Trees of Q. laurina that occurred in the site with highest oak species diversity (i.e. Tequila volcano) had the highest richness and abundance of arthropods. Additionally, the NMDS showed differences in the composition of the arthropod community along the oak diversity gradient, agreeing with the fact that Tequila Volcano had a significantly different composition of the arthropod community compared with the other study sites. Two hypotheses that are not mutually exclusive can explain our results. “The plant species richness hypothesis” (Fernandes and Price 1988) that proposes that communities with greater plant diversity represent more potential sites to be colonized by a greater number of insect species (“bottom-up effects”) (Hertzog 2017). Some studies have shown that plant diversity is a factor that contributes to the maintenance of herbivorous insect diversity (Haddad et al. 2011). A meta-analysis, involving 52 independent comparisons, confirmed that diversity of herbivorous insects increases with a greater diversity of plants (i.e. 18 correlations derived from galling herbivores and 34 from non-galling herbivores) (Araújo 2013). In our case, oak communities represent to arthropods a higher resource and niches availability to be colonized by a greater number of species (bottom-up forces: plants-herbivores), where species richness of arthropods in the canopy of Q. laurina increases as more oak species are available. Additionally, it has been proposed that plant diversity is a key driver for increasing herbivory by insects because heterospecific neighbors can decrease a plant resistance (i.e. associative resistance) or increase herbivory susceptibility (i.e. associative susceptibility), increasing the likelihood of focal plants being attacked by herbivorous insects. (Root 1973; Tahvanainen and Root 1972; White and Whitham 2000). This fact has been confirmed by meta-analyses suggesting that associative resistance is the most common pattern in forest ecosystems communities (Jactel & Brockerhoff 2007; Castagneyrol et al. 2014, 2017). In either of the two ideas raised, our results support the “plant species richness hypothesis” (Fernandes and Price 1988) where an increase in the richness of oak species generates a greater diversity of arthropods in the canopy of Q. laurina.
A second hypothesis “The natural enemy hypothesis” (Root 1973) suggests that the diversity of natural enemies (i.e. predators and parasitoids) increases in communities with a great plant diversity because of the presence of more potential prey and availability of microenvironments (“top-down effects”) (Wilby and Thomas 2002; Sobek et al. 2009). This hypothesis implies an indirect mechanism between plants and herbivorous insects, where the presence of natural enemies exerts a top-down control on herbivore abundance (Pearce et al. 2003). Therefore, a greater diversity of resources provided by a high herbivore diversity present in highly diverse oak community can increase the diversity of predators (bottom-up forces: arthropod-arthropod) affecting the community structure of arthropods (Randlkofer et al. 2010; Haddad et al. 2011; Moreira et al. 2016; Hertzog 2017).
Another important result is the fact that trees of Q. laurina growing in Tequila Volcano harbored higher abundance and species richness of phytophages, predators and parasitoids. Similar results have been shown in other ecological studies supporting the idea that communities with high plant diversity can represent more availability of resources, niches and microenvironments to be colonized by more arthropod guilds (Sobek et al. 2009; Hertzog 2017). However, the great diversity of predators and parasitoids registered in the most diverse oak community also can suggests that natural enemies can be regulating arthropod diversity (top-down forces). Some studies suggest that top-down forces in communities increase with resource availability to primary producers (Root 1973; Boyer et al. 2003). Therefore, we propose that our results can be explained by a combination of both factors, where bottom-up forces will set the stage for top-down forces to act. For example, plant diversity can increase the abundance of herbivores through greater availability and/or quality of food, niches, microenvironments and shelter, which in turn will determine the abundance and diversity of predators, affecting the predation pressure (Forkner and Hunter 2000). The role of top-down and bottom-up forces in terrestrial communities has been a subject of debate in the ecological literature (Schmitz et al. 2000; Halaj and Wise 2001). Particularly, in oak communities, some studies have suggested bottom-up forces as the main regulatory mechanism of the distribution and abundance of herbivorous insects through leaf nutritional quality and phenology (Feeny 1970; Schultz and Baldwin 1982; West 1985; Faeth and Bultman 1986; Rossiter et al. 1988). However, more recent studies suggest that bottom-up and top-down forces interact together by molding and structuring arthropod communities (Forkner and Hunter 2000; Castagneyrol et al. 2014, 2017). For example, a meta-analysis including 172 studies showed that top-down forces were stronger than bottom-up forces to control herbivorous insects, where, chewing, sucking and gall-making herbivores were more affected by top-down than bottom-up forces, and parasitoids and predators had equally strong top-down effects on insect herbivores (Vidal and Murphy 2018).
Additionally, the presence of geographic barriers such as mountainous terrains that are characteristic of the Trans-Mexican Volcanic Belt (TMBV), also could be affecting the canopy arthropod community, limiting their distribution and reducing the arthropod migration between localities (Novotny and Weiblen 2005; Arriaga-Jiménez et al. 2018). Furthermore, given that some arthropod groups exhibit high levels of intraspecific aggregation as a result of oviposition behavior, it is possible to expect a limited spatial dispersion between communities (Caballero et al. 2011), mainly in the Tequila volcano, which was the locality most separated from the other study sites and with highest arthropod diversity.
Our results show significant differences in the composition of the arthropod community associated with the canopy of Q. laurina along the gradient of diversity of oak species. The sites with more diversity of oak species presented higher levels of arthropod abundance and richness, thus a greater presence of arthropod guilds (phytophages, predators, parasitoids) and a greater presence of adult arthropods and nymphs. These results are consistent with the premise that for habitats in temperate forests, tree communities are a key element for shaping the environmental physical structure; reason that has been suggested that tree species diversity could influence an increase in richness and abundance of canopy arthropod (Lassau et al. 2005; Affeld 2008; Moreira et al. 2016). This fact is consistent with the absence of significant relationships between environmental variables (i.e. temperature, precipitation) and the canopy arthropod community in all study sites, suggesting that the differences in the oak community structure between localities are the main factor that is modulating the community of arthropods associated to the canopy of Q. laurina along the oak diversity gradient.
Finally, one little discussed factor in the ecological literature that potentially affect the insect abundance on a tree is the problem associated with the assignment of arthropod species to guilds (Stork 1987). It has been shown that the incorrect assignment of the food guilds of only a few insect species can result in important differences in the composition and diversity of the arthropod communities of the canopy (Stork 2018). For example, for many groups of canopy insects, the larval and adult stages have the same feeding habits. However, in some groups of hymenopterans, the larvae act as parasites and the adults may feed on honey, nectar, or not feed, which affects the estimates of insect diversity patterns (Stork 1987).
In conclusion, our study showed changes in arthropod diversity of the canopy of Q. laurina along an oak species diversity gradient. We detected differences in arthropod diversity, abundance and guild composition between oak communities. The general pattern showed higher arthropod diversity in Tequila volcano, which represented the site with the greatest diversity of oaks. Our findings confirm that at the local scale, plant species richness is the main factor that determines the arthropod diversity associated to canopy of Q. laurina, affecting trophic levels and the structure and composition of arthropod guilds. Mexico is the main center of richness, diversification and endemism of oaks in the northern hemisphere. Thus, we highlight the importance of conserving oak species because they represent key elements of temperate forest that harbor high diversity of animals.
References
Affeld K (2008) Spatial complexity and microclimatic responses of epiphyte communities and their invertebrate fauna in the canopy of northern rata (Metrosideros robusta A. Cunn.: Myrtaceae) on the West Coast of the South Island, New Zealand. Dissertation, Lincoln University
Arriaga-Jiménez A, Rös M, Halffter G (2018) High variability of dung beetle diversity patterns at four mountains of the Trans-Mexican Volcanic Belt. PeerJ 6:e4468. https://doi.org/10.7717/peerj.4468
Barringer LE, Bartlett CR, Erwin TL (2019) Canopy assemblages and species richness of planthoppers (Hemiptera: Fulgoroidea) in the Ecuadorian Amazon. Insecta Mundi 0726:1–16
Basset Y, Hammond PM, Barrios H, Holloway JD, Miller SE (2003) Vertical stratification of arthropod assemblages. In: Basset Y, Novotny V, Miller SE, Kitching RL (eds) Arthropods of tropical forests: spatio-temporal dynamics and resource use in the canopy. Cambridge University Press, Cambridge, pp 17–27
Boyer AG, Swearingen RE, Blaha MA, Fortson CT, Gremillion SK, Osborn KA, Moran MD (2003) Seasonal variation in top-down and bottom-up processes in a grassland arthropod community. Oecologia 136(2):309–316. https://doi.org/10.1007/s00442-003-1272-x
Caballero M, Lozano-García S, Vázquez-Selem L, Ortega B (2011) Evidencias de cambio climático y ambiental en registros glaciales y en cuencas lacustres del centro de México durante el último máximo glacial. Boletín De La Sociedad Geológica Mexicana 62(3):359–377
Castagneyrol B, Régolini M, Jactel H (2014) Tree species composition rather than diversity triggers associational resistance to the pine processionary moth. Basic Appl Ecol 15(6):516–523. https://doi.org/10.1016/j.baae.2014.06.008
Castagneyrol B, Bonal D, Damien M, Jactel H, Meredieu C, Muiruri EW, Barbaro L (2017) Bottom-up and top-down effects of tree species diversity on leaf insect herbivory. Ecol Evol 7(10):3520–3531. https://doi.org/10.1002/ece3.2950
Cavender-Bares J (2016) Diversity, distribution and ecosystem services of the North American oaks. Int Oaks 27:37–49
Cavender-Bares J (2019) Diversification, adaptation, and community assembly of the American oaks (Quercus), a model clade for integrating ecology and evolution. New Phytol 221(2):669–692. https://doi.org/10.1111/nph.15450
Cavender-Bares J, Kothari S, Meireles JE, Kaproth MA, Manos PS, Hipp AL (2018) The role of diversification in community assembly of the oaks (Quercus L.) across the continental US. Am J Bot 105(3):565–586. https://doi.org/10.1002/ajb2.1049
Colwell RK (2013) Estimates: statistical estimation of species richness and shared species from samples. Version 9. User's Guide and application. http://purl.oclc.org/estimates
Cook-Patton SC, McArt SH, Parachnowitsch AL, Thaler JS, Agrawal AA (2011) A direct comparison of the consequences of plant genotypic and species diversity on communities and ecosystem function. Ecology 92:915–923. https://doi.org/10.1890/10-0999.1
Cuevas-Reyes P, Quesada M, Hanson P, Dirzo R, Oyama K (2004) Diversity of gall-inducing insects in a Mexican tropical dry forest: the importance of plant species richness, life-forms, host plant age and plant density. J Ecol. https://doi.org/10.1111/j.0022-0477.2004.00896.x
Currie DJ, Francis AP (2004) Taxon richness and climate in angiosperms: is there a globally consistent relationship that precludes region effects? Am Nat 163:780–785
de Araújo WS (2013) Different relationships between galling and non-galling herbivore richness and plant species richness: a meta-analysis. Arthropod Plant Interact 7(4):373–377. https://doi.org/10.1007/s11829-013-9259-y
Erwin TL, Geraci CJ (2009) Chapter 4. Amazonian rainforests and their richness of Coleoptera, a dominant life form in the critical zone of the Neotropics. In: Foottit RG, Adler PH (eds) Insect biodiversity: science and society. Wiley-Blackwell, West Sussex
Faeth SH, Bultman TL (1986) Interacting effects of increased tannin levels on leaf-mining insects. Entomology 40(3):297–301. https://doi.org/10.1111/j.1570-7458.1986.tb00515.x
Feeny P (1970) Seasonal changes in oak leaf tannins and nutrients as a cause of spring feeding by winter moth caterpillars. Ecology 51(4):565–581. https://doi.org/10.2307/1934037
Fernandes GW, Price PW (1988) Biogeographical gradients in galling species richness. Oecologia 76(2):161–167. https://doi.org/10.1007/BF00379948
Forkner RE, Hunter MD (2000) What goes up must come down? Nutrient addition and predation pressure on oak herbivores. Ecology 81(6):1588–1600. https://doi.org/10.1890/0012-9658(2000)081
García-Guzmán OM, Garibay-Orijel R, Hernández E, Arellano-Torres E, Oyama K (2017) Word-wide meta-analysis of Quercus forests ectomycorrhizal fungal diversity reveals southwestern Mexico as a hotspot. Mycorrhiza 27(8):811–822. https://doi.org/10.1007/s00572-017-0793-9
Gotelli N, Colwell RK (2001) Quantifying biodiversity: procedures and pitfalls in the measurement and comparison of species richness. Ecol Lett 4:379–391. https://doi.org/10.1046/j.1461-0248.2001.00230.x
Haddad NM, Crutsinger GM, Gross K, Haarstad J, Tilman D (2011) Plant diversity and the stability of foodwebs. Ecol Lett 14:42–46. https://doi.org/10.1111/j.1461-0248.2010.01548.x
Halaj J, Wise DH (2001) Terrestrial trophic cascades: how much do they trickle? Am Nat 157:262–281. https://doi.org/10.1086/319190
Hamilton AJ, Novotny VWEK, Bassset Y, Benke KK, Grimbacher PS, Samuelson GA, Weiblen GD, Yen JDL, Stork NE, Weiblen GD, Yen JDL (2013) Estimating global arthropod species richness: refining probabilistic models using probability bounds analysis. Oecologia 171:357–365. https://doi.org/10.1007/s00442-012-2434-5
Hammer Ø, Harper DA, Ryan PD (2001) PAST: Paleontological statistics software package for education and data analysis. Palaeontol Electron 4(1):9
Hertzog L (2017) Plant diversity impacts on arthropod communities and arthropod-mediated processes. Dissertation, Technische Universität München
Hipp Andrew L, Manos PS et al (2019) Genomic landscape of the global oak phylogeny. New Phytol 226:1198–1212. https://doi.org/10.1111/nph.16162
Hipp Andrew L et al (2020) Sympatric parallel diversification of major oak clades in the Americas and the origins of Mexican species diversity. New Phytol 217:439–452
Jactel H, Brockerhoff EG (2007) Tree diversity reduces herbivory by forest insects. Ecol Letters 10(9):835–848. https://doi.org/10.1111/j.1461-0248.2007.01073.x
Jin J, Wang Y, Jiang H, Kong Y, Zhang X (2016) Improvement of ecological geographic regionalization based on remote sensing and canonical correspondence analysis: A case study in China. Sci China Earth Sci 59(9):1745–1753
JMP®, Version .2008, SAS Institute Inc., Cary, NC, USA ISBN 978-1-59994-924-6
Lassau SA, Hochuli DF, Cassis G, Reid CA (2005) Effects of habitat complexity on forest beetle diversity: do functional groups respond consistently. Divers 1:73–82. https://doi.org/10.1111/j.1366-9516.2005.00124.x
Leroux SJ, Loreau M (2015) Theoretical perspectives on bottom-up and top-down interactions across ecosystems. Trophic Ecol 3:28. https://doi.org/10.1017/CBO9781139924856.002
Maclean JE, Mitchell RJ, Burslem DF, Genney D, Hall J, Pakeman J (2017) The epiphytic bryophyte community of Atlantic oak woodlands shows clear signs of recovery following the removal of invasive Rhododendron ponticum. Biol Conserv 212:96–104. https://doi.org/10.1016/j.biocon.2017.06.003
Magurran AE (1988) Ecological diversity and its measurement. Princeton University Press, Princeton
Majer JD, Recher HF, Ganesh S (2000) Diversity patterns of eucalypt canopy arthropods in eastern and western of Australian. Ecol Entomol 25:295–306. https://doi.org/10.1046/j.1365-2311.2000.00259.x
Maldonado-López Y, Vaca-Sánchez MS, González-Rodríguez A, Oyama K, López-Barbosa, Fagundes M, Cuevas-Reyes P (2018) Hybridization increases canopy arthropod diversity in the Quercus affinis× Quercus laurina complex. J Insect Conserv 22(5–6):781–793. https://doi.org/10.1007/s10841-018-0103-7
Marques MI, Adis J, dos Santos GB, Battirola LD (2006) Terrestrial arthropods from tree canopies in the Pantanal of Mato Grosso, Brazil. Rev Bras Entomol 50:257–267. https://doi.org/10.1590/S0085-56262006000200007
McCauley RA, Cortés-Palomec AC, Oyama K (2019) Species diversification in a lineage of Mexican red oak (Quercus section Lobatae subsection Racemiflorae) the interplay between distance, habitat, and hybridization. Tree Genet 15:27. https://doi.org/10.1007/s11295-019-1333-x
Moreira X, Abdala-Roberts L, Rasmann S, Castagneyrol B, Mooney KA (2016) Plant diversity effects on insect herbivores and their natural enemies: current thinking, recent findings, and future directions. Curr Opin Insect 14:1–7. https://doi.org/10.1016/j.cois.2015.10.003
Novotny V, Weiblen GD (2005) From communities to continents: beta diversity of herbivorous insects. In: Annales Zoologici Fennici. Finnish Zoological and Botanical Publishing Board. pp 463–475
Novotny V, Basset Y, Miller SE, Weiblen GD, Bremer B, Cizek L, Drozd P (2002) Low host specificity of herbivorous insects in a tropical forest. Nature 416(6883):841–844
Pearce JL, Venier LA, McKee J, Pedlar J, McKenney D (2003) Influence of habitat and microhabitat on carabid (Coleoptera: Carabidae) assemblages in four stand types Influence of habitat and microhabitat on carabid (Coleoptera: Carabidae) assemblages in four stand types. Can Entomol 135(3):337–357. https://doi.org/10.4039/n02-031
Randlkofer B, Obermaier E, Hilker M, Meiners T (2010) Vegetation complexity: the influence of plant species diversity and plant structures on plant chemical complexity and arthropods. Basic Appl Ecol 11:383–395. https://doi.org/10.1016/j.baae.2010.03.003
Rodríguez-Correa H, Oyama K, MacGregor-Fors I, González-Rodríguez A (2015) How are oaks distributed in the neotropics? A perspective from species turnover, areas of endemism, and climatic niches. Int J Plant Sci 176:222–231. https://doi.org/10.1086/679904
Root RB (1973) Organization of a plant–arthropod association in simple and diverse habitats: the fauna of collards (Brassica oleracea). Ecol Monogr 43:95–124. https://doi.org/10.2307/1942161
Rossiter M, Schultz JC, Baldwin IT (1988) Relationships among defoliation, red oak phenolics, and gypsy moth growth and reproduction. Ecology 69(1):267–277. https://doi.org/10.2307/1943182
SAS (2000) Categorical data analysis using the SAS system. SAS Institute, Cary
Schmidl J, Bussler H (2008) Xylobiontic beetle guild composition and diversity driven by forest canopy structure and management. In: Floren A, Schmidl J (eds) Canopy arthropod research in Europe. Bioform Entomology, Nuremberg, pp 299–323
Schmitz OJ, Hamback PA, Beckerman AP (2000) Trophic cascades in terrestrial systems: a review of the effects on carnivore removals on plants. Am Nat 155:141–153. https://doi.org/10.1086/303311
Schultz JC, Baldwin IT (1982) Oak leaf quality declines in response to defoliation by gypsy moth larvae. Science 217(4555):149–151. https://doi.org/10.1126/science.217.4555.149
Sobek S, Scherber C, Steffan-Dewenter I, Tscharntke T (2009) Sapling herbivory, invertebrate herbivores and predators across a natural tree diversity gradient in Germany’s largest connected deciduous forest. Oecologia 160:279–288. https://doi.org/10.1007/s00442-009-1304-2
Stiegel S, Mantilla-Contreras J (2018) Environment vs. plant ontogeny: arthropod herbivory patterns on European beech leaves along the vertical gradient of temperate forests in Central Germany. Insects 9:9–28. https://doi.org/10.3390/insects9010009
Stokes ME, Davis CS, Koch GG (2000) Categorical data analysis using the SAS system. Wiley, Hoboken, p 626
Stork NS (1987) Guild structure of arthropods from Bornean rain forest trees. Ecol Entomol 12:69–80. https://doi.org/10.1111/j.1365-2311.1987.tb00986.x
Stork NS (2018) How many species of insects and other terrestrial arthropods are there on Earth? Annu Rev Entomol 63:31–45. https://doi.org/10.1146/annurev-ento-020117-043348
Stork NS, Grimbacher PS, Storey R, Oberprieler RG, Reid C, Slipinski C (2008) What determines whether a species of insect is described? Evidence from a study of tropical forest beetles. Insect Conserv Divers 1:114–119. https://doi.org/10.1111/j.1752-4598.2008.00016.x
Tahvanainen JO, Root RB (1972) The influence of vegetational diversity on the population ecology of a specialized herbivore, Phyllotreta cruciferae (Coleoptera: Chrysomelidae). Oecologia 10(4):321–346. https://doi.org/10.1007/bf00345736
Tal O, Freiberg M, Morawetz W (2008) Micro-climatic variability in the canopy of a temperate forest. In: Floren A, Schmidl J (eds) Canopy arthropod research in Europe. Bioform Entomology, Nuremberg, pp 49–59
Torres-Miranda A, Luna-Vega I, Oyama K (2013) New approaches to the biogeography and areas of endemism of red oaks (Quercus L., Section Lobatae). Syst Biol 62(4):555–573. https://doi.org/10.1093/sysbio/syt021
Tovar-Sánchez E, Oyama K (2006a) Community structure of canopy arthropods associated to Quercus crassifolia x Quercus crassipes complex. Oikos 112:370–381. https://doi.org/10.1111/j.0030-1299.2006.14029.x
Tovar-Sánchez E, Oyama K (2006b) Effect of hybridization of the Quercus crassifolia x Quercus crassipes complex on the community structure on endophagous insects. Oecologia 147:702–713. https://doi.org/10.1007/s00442-005-0328-5
Triplehorn CA, Johnson NF, Borror DJ (2005) An introduction to the study of insects. Thompson Brooks/Cole, Belmont
Ubick, D, P Paquin, P, Cushing, Roth VD (2017)) Spiders of North America. An Identification Manual. Second Edition. American Arachnological Society. Keene, New Hampshire USA 377pp.
Ulyshen MD (2011) Arthropod vertical stratification in temperate deciduous forests: implications for conservation-oriented management. For Ecol Manag 261:1479–1489. https://doi.org/10.1016/j.foreco.2011.01.033
Valencia S (1994) Contribución a la delimitación taxonómica de tres especies del género Quercus subgénero Erythrobalanus. Tesis de Maestría, Facultad de Ciencias, Universidad Nacional Autónoma de México, Mexico
Valencia S (2004) Diversidad del género Quercus (Fagaceae) en México. Bol Soc Bot México 75:33–53. https://doi.org/10.17129/botsci.1692
Valencia-Cuevas L, Mussali-Galante P, Piñero D, Castillo-Mendoza E, Rangel-Altamirano G, Tovar-Sánchez E (2015) Hybridization of Quercus castanea (Fagaceae) across a red oak species gradient in Mexico. Plant Syst Evol 301:1085–1097. https://doi.org/10.1007/s00606-014-1151-4
Vidal MC, Murphy SM (2018) Bottom-up vs top-down effects on terrestrial insect herbivores: a meta-analysis. Ecol Lett 21(1):138–150. https://doi.org/10.1111/ele.12874
Wan NF, Ji XY, Jiang JX (2014) Testing the enemies hypothesis in peach orchards in two different geographic areas in eastern China: the role of ground cover vegetation. PLoS ONE 9(6):e99850. https://doi.org/10.1371/journal.pone.0099850
West C (1985) Factors underlying the late seasonal appearance of the lepidopterous leaf-mining guild on oak. Ecol Entomol 10(1):111–120. https://doi.org/10.1111/j.1365-2311.1985.tb00540.x
White JA, Whitham TG (2000) Associational susceptibility of cottonwood to a box elder herbivore. Ecology 81(7):1795–1803. https://doi.org/10.1890/0012-9658(2000)081
Wilby A, Thomas MB (2002) Natural enemy diversity and pest control: patters of pest emergence with agricultural intensification. Ecol Letters 5:353–360. https://doi.org/10.1046/j.1461-0248.2002.00331.x
Acknowledgements
Vaca-Sánchez is a PhD student from Programa de Doctorado en Ciencias Biomédicas, Universidad Nacional Autónoma de México and is supported by CONACyT (scholarship no. 305245). The study was funded by Coordination of Scientific Research (UMSNH) project 001. This project was supported by CONACYT/PDC2016 Project 3053.
Author information
Authors and Affiliations
Contributions
All authors contributed to the study conception and design. Material preparation, data collection and analysis were performed by [MSV-S], [YM-L], [MF], [A-PJS] and [PC-R]. The first draft of the manuscript was written by [MSV-S], YM-L], [AG-R], [KO], [GWF], [PC-R] and all authors commented on previous versions of the manuscript. All authors read and approved the final manuscript.
Corresponding author
Ethics declarations
Conflict of interest
All authors declare that they have no conflict of interest.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Vaca-Sánchez, M.S., Maldonado-López, Y., González-Rodríguez, A. et al. Canopy arthropod diversity associated with Quercus laurina: importance of an oak species diversity gradient on abundance, species richness and guild composition. J Insect Conserv 25, 859–874 (2021). https://doi.org/10.1007/s10841-021-00352-5
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10841-021-00352-5