Abstract
The genus Anthrax Scopoli is a well-known genus of bee flies, with 16 species recorded from Egypt, out of about 250 species known worldwide. Six of these species seem to be endangered or threatened (at least in a part of their distribution range) as they disappeared from most of their Egyptian localities which faced severe changes in the floral and faunal composition during the last 50 years. These changes may be attributed to urbanization or modern agricultural activities that caused severe degradation of natural habitats of these species. Maximum entropy (Maxent) modeling was used to predict the potential climatic niches of these six Anthrax species, namely: Anthrax aethiops, Anthrax chionanthrax, Anthrax dentate, Anthrax melanista, Anthrax lucidus, and Anthrax trifasciatus. The Maxent model performed better than random for the six species, with an average test AUC value of 0.95 for A. aethiops bezzii, 0.83 for A. chionanthrax, and 0.98 for A. dentate, 0.86 for A. melanista, 0.96 for A. lucidus and 0.99 for A. trifasciatus. A set of four variables were selected for each species from 19 bioclimatic and 3 topographic variables, based on correlation analyses. The study showed that the current distributions of A. dentata, A. lucidus and A. trifasciatus were largely explained by the altitude, but A. chionanthrax and A. melanista were so far dominated by temperature, whereas on A. aethiops bezzii was strongly related to precipitation variables. St. Catherine protected area showed high habitat suitability for all species except A. lucidus. The suitable habitat for A. trifasciatus was restricted to this protected area, however, future scenarios of climatic changes through the area predict a great degradation in habitat suitability for this species. These results can be used for making powerful conservation management decisions in St. Catherine Protectorate to incorporate these Anthrax spp. in any present or future conservation programs.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Over the last few years, the studies of species niche modeling have substantially increased (Boucher et al. 2012; Schnitzler et al. 2012; Nyári and Reddy 2013; Sallam et al. 2013; Rato et al. 2015; Alahmed et al. 2015; Algar and Mahler 2015; Naeem et al. 2016; Mulieri and Patitucci 2019). The huge accumulative data of species records, availability of geographical, ecological and climatological information, in addition to the development of several statistical modeling tools helped in such revolution for studying biological units (Elith et al. 2011; Salariato and Zuloaga 2016). This is synchronizing with the raise of the new term ‘biodiversity informatics’. The term which apply to the science that benefit from our biological heritage records by manipulating these data with information techniques and geographical information systems (GIS) for improving our understanding of species spatial distributions (Maldonado et al. 2015; Asase and Peterson 2016).
The study of insects’ niche modeling received little attention than that of other creatures such as vertebrates. Only the highly medical important insect groups were studied using these techniques (Sallam et al. 2013; Alahmed et al. 2015; Naeem et al. 2016), neglecting the majority of insects’ fauna which represents 80% of earth’s biodiversity (Hochkirch 2016). One of these neglected groups is the family Bombyliidae, or bee flies as they are commonly called. Bee flies are one of the largest families in the order Diptera, with about 4800 species known worldwide and virtually cosmopolitan in distribution (Evenhuis and Greathead 2015). They are found primarily in warm arid and semi-arid habitats (Greathead and Evenhuis 1997). In Egypt, as predominantly having arid and semi-arid habitats, bee flies are very well represented, and the diversity of their appearance can scarcely fail to excite interest (El-Moursy et al. 1999).
El-Hawagry and Gilbert (2014) analyzed the distribution of 229 bee fly species based on their occurrence in the different Egyptian ecological zones showing a high species richness in Eastern Desert, Sinai and Coastal Strip. Species richness in Lower Nile Valley & Delta and Gebel Elba was moderate, while species richness in the Upper Nile Valley, Fayoum Basin and Western Desert were the lowest. The bee fly species richness, according to the previous study and El-Hawagry’s (2015) study, was positively correlated with plant diversity, and probably reflecting varying climatic factors and vegetation patterns in different Egyptian ecological zones.
El-Hawagry (2015) merely listed the localities from which Egyptian bee fly species have been recorded based on all previous studies and on the preserved material in the main Egyptian insect collections. These records provide information on only a subset of localities occupied by a species and do not provide information on localities that have not been surveyed, or that may be colonized in the future following climate changes (Rondinini et al. 2006; Hoegh-Guldberg et al. 2008). However, this information is very important as it can be employed for making powerful conservation management decisions and can be exploited for the predictions of species occurrences derived from environmental suitability models that combine biological records with spatial environmental data (Guisan et al. 2013).
The genus Anthrax Scopoli (family: Bombyliidae, subfamily: Anthracinae, tribe: Anthracini) is a well-known genus of bee flies, with 16 species recorded from Egypt, out of about 250 species known worldwide. It is characterized by the following combination of characters: small to large species, up to 15 mm; very short proboscis; pedicel globular or disc-shaped, not moulded to flagellum; flagellum onion-shaped; wings usually with a more or less extensive brown or black infuscation, seldom almost completely hyaline, never completely infuscated, second longitudinal vein in the wing is rather long; body with dense scales, lying flat, predominantly black with patches of white or silver scales on abdomen. Larvae are usually parasites of wild bees (Coquillett 1887; Steyskal and El-Bialy 1967; El-Hawagry and Evenhuis 2008).
Six of these species seem to be endangered or threatened (at least in parts of their distribution range) as they disappeared from most of their Egyptian localities which faced severe changes in the floral and faunal composition during the last 50 years. These changes may be attributed to urbanization or modern agricultural activities that caused severe degradation of natural habitats of these species. These endangered or threatened species are: Anthrax aethiopsbezzii (Paramonov 1957), Anthrax chionanthrax (Bezzi 1926), Anthrax dentata (Becker 1906), Anthrax lucidus (Becker 1902), Anthrax melanista (Bezzi 1925) and Anthrax trifasciatus Meigen 1804. The present study aims to propose environmental niche models, and to locate the sites of the highest habitat suitability for the studied species, based on climatological and topographical data. Also, to evaluate their present status in St. Catherine protected area and predict the climate change effects on the habitat suitability of Anthrax trifasciatus under four different climate change scenarios on 2070.
Materials and methods
This study was conducted in Egypt, the country that covers approximately 1001,449 square kilometers of land area. Egypt is divided into eight ecological zones: Coastal Strip, Lower Nile Valley & Delta, Upper Nile Valley, Fayoum Basin, Eastern Desert, Western Desert, Sinai and Gebel Elba (Larsen 1971, 1990; Boulos 1999–2005; El-Hawagry 2002, 2011, 2017; El-Hawagry and Gilbert 2014). It is characterized by a warm and almost rainless climate, and it is one of the driest countries in the world with a long-term average precipitation of 51 mm per year (Hoath 1992; FAO 2012).
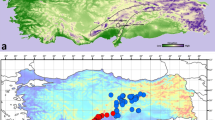
A total of 80 records for the six Anthrax spp. (Fig. 1) were gathered from literature (Efflatoun 1945, 2002, 2015), museum collections, in addition to material collected by the second author (M. El-Hawagry) during a large series of collecting trips to various localities in the eight Egyptian ecological zones from 1991 to 2015.
Climatic data were downloaded from the WorldClim database with spatial resolution 30 arc-s (~ 1 km) (http://www.worldclim.org/). In this study, a combination of 19 climatic and three topographical variables was initially used to assess current climatic conditions (Table 1). We remove bioclimatic variables 8–9 and 18–19 from the analysis, due to spatial artifacts in those four variables (Samy et al. 2016). We first used the jackknifing function in Maxent to estimate the most important set of variables, also this analysis produce jackknife that obtain the variables that had no effect (Zero effect) for species distribution. The variables that showed any contribution for each species were undergoing the test in SDMTools in ArcGIS 10.3 (Universal tool; Remove highly correlated variables) to remove variables with high correlations and which consequentially have no biological effect on the species (Alkishe et al. 2017). Accordingly, we used a set of four most important variables that contribute to habitat suitability for each species (Table 2). All these bioclimatic layers were clipped to match the dimensions of Egypt and saved in ASCII grid format for using in Maxent, for clipping these layers ArcGIS software V. 10.3 was used (Alahmed et al. 2015). These variables are used in species distribution modeling (Kumar et al. 2009; Sanchez et al. 2011). The data were extracted for each species by using the “extract by mask” function in Arc Map to focus the results on the St. Catherine protected area. Climatic data projected to the year 2070 from the global climate models of the Meteorological Research Institute (MRI-CGCM3), Beijing Climate Center (BCC-CSM 1_1), National Center for Atmospheric Research (CCSM4) and Norwegian Climate Centre (NorESM 1-M). We used these four climate models to assess the effects of climate change on the species A. trifasciatus for RCP2.6 and RCP8.5. These data are among the recent GCM climate projections that are used in the fifth Assessment IPCC Report.
Maximum entropy model (MaxEnt version 3.3.3k http://www.cs.princeton.edu/wschapire/maxent/) were used in this study because Maxent perform better with small sample sizes compare to other modeling methods (Pearson et al. 2007). The program estimate the suitable habitat for species that varies from 0 (lowest suitability) to 1 (highest suitability) and generate response curve for each bioclimatic variable to estimate the relationships between the habitat suitability for a species and bioclimatic variables (Khanum et al. 2013). Maxent automatically removes the duplicate records in the same cell. In our models, we selected 75% of the occurrence records for training and 25% of the records for testing the model (Phillips 2008). To improve the model performance, tenfold cross-validation was used in which the model was run ten replicates for each species and the results were averaged. The area under curve (AUC) of the receiver operating characteristics (ROC) was used to estimate the model performance and its value varies from 0 (random discrimination) to 1 (perfect discrimination) (Peterson et al. 2008; Swets 1988).
Results
Models for six Anthrax spp. performed better than random, with averages test AUC values ranging from 0.83 to 0.99. The current distributions of the species as modeled are as follows: The Maxent model for A. aethiops bezzii performed very well with an average AUC value of 0.95. Current suitable habitat predicted that zones with high habitat suitability were North Sinai, some areas in South Sinai and Coastal Strip but the habitat suitability was low in the rest of the country (Fig. 2a). St. Catherine protected area showed high habitat suitability in some areas (Fig. 3a). Precipitation of wettest quarter (Bio 16) showed higher effects on the distribution of A. aethiops bezzii relative to other bioclimatic variables (Fig. 4). This variable was the top predictor in the Maxent model with contributions of 92.1% (Table 2). The probability of presence of A. aethiops bezzii increased with an increase in precipitation of wettest quarter (Bio 16) (Fig. 5).
The model performance for A. chionanthrax was better than random with an AUC value of 0.83. The predicted distribution in Egypt showed areas with high suitability for this species in North Sinai, Eastern Desert especially in the Red Sea coast, and Coastal Strip. The habitat suitability was medium in the rest of Sinai, some areas in Lower Nile Valley & Delta and Western Desert. However, the habitat suitability was low in the rest of the country (Fig. 2b). The habitat suitability was high in almost all areas in St. Catherine protected area, but some parts showed very low habitat suitability (Fig. 3b). Temperature seasonality (Bio 4) provided the most useful and unique information for A. chionanthrax, that was not present in other variables (Fig. 4). Temperature seasonality (Bio 4) had the top contribution of 60.1% (Table 2). The suitability of habitat for A. chionanthrax decreased sharply with the increasing of temperature seasonality (Bio 4) (Fig. 5).
The Maxent model for A. dentata gave satisfactory results with an AUC value of 0.98. Current suitable habitat predicted that zones with high habitat suitability were Lower Nile Valley & Delta, some areas in Eastern Desert, Western Dessert, and South Sinai. The rest of the country showed low suitable habitat for this species (Fig. 2c). Some areas in St. Catherine protected area showed high habitat suitability to the species (Fig. 3c). Altitude was the most important predictor of A. dentata’s habitat distribution (Fig. 4). This variable presented the higher contribution of 62.8% compared to other predictors (Table 2). The suitability of habitat for A. dentata increased with increasing altitude also the species preferred the low altitude (Fig. 5).
The Maxent model for A. lucidus had an AUC value of 0.96. The predicted distribution in Egypt showed areas with high suitability for this species in Lower Nile Valley & Delta, Fayoum Basin, some areas in Coastal Strip and Western Desert. The habitat suitability was low in the rest of the country (Fig. 2d). Almost all parts of St. Catherine protected area had low habitat suitability for A. lucidus (Fig. 3d). Altitude contributed most to the model (Fig. 4). The contribution of this factor is 69.6% (Table 2). The suitability of habitat for A. lucidus initially decreased sharply with the increasing of altitude (Fig. 5).
The model performance for A. melanista performed very well with an average AUC value of 0.86. The predicted distribution in Egypt showed areas with high suitability for this species as parts in South Sinai, Eastern Desert especially in the Red Sea coast and in the north-eastern part of the country, North Sinai, Gebel Elba and Coastal Strip. The habitat suitability was medium in some areas in Lower Nile Valley & Delta, Western Desert and Eastern Desert. The habitat suitability was low in the rest of the country (Fig. 2e). St. Catherine protected area is considered as a highly suitable habitat for this species in some parts (Fig. 3e). Temperature annual range (Bio 7) was the strongest predictor A. melanista distribution (Fig. 4). This factor gave the highest influence on the model with 47.6% contribution. The probability of presence A. melanista decreased with the increasing of the temperature annual range (Bio 7) (Fig. 5).
The current and future distribution for A. trifasciatus showed that Maxent model provided satisfactory results with an AUC value of 0.99. Current suitable habitat predicted that the only zone with high habitat suitability was South Sinai especially St. Catherine protected area (Fig. 2f, 3f), whereas the rest zones of the country showed a very low habitat suitability. Altitude contained more useful information than the other variables (Fig. 4). Altitude had the top contribution to the model with 98% (Table 2). The suitability of habitat for A. trifasciatus increased sharply with the increasing of altitude (Fig. 5). The habitat suitability in RCP 2.6 for the year 2070 in four selected GCM showed that A. trifasciatus decreased in their suitable habitat and this species suffered from future habitat loss in RCP 8.5 larger than in RCP2.6 (Fig. 6).
Predicted future habitat suitability of A. trifasciatus in St. Catherine protected area for 2070 year under four different climate change scenarios (GCM); a Beijing Climate Center (BCC-CSM 1_1), b National Center for Atmospheric Research (CCSM4), c Meteorological Research Institute (MRI-CGCM3) and d Norwegian Climate Centre (NorESM 1-M) [under RCP 2.6 emission scenario (1), under RCP 8.5 emission scenario (2)]
Discussion
In response to global environmental changes, species ranges are fragmenting, contracting, expanding or shifting (Chen et al. 2011). The development of biodiversity databases in the last decade has provided new opportunities to analyze species occurrence data to support the efforts to conserve these species (Jetz et al. 2012), and many species distribution models (SDMs) or environmental niche models (ENMs) have increasingly proposed to support conservation decision making (Guisan et al. 2013).
About 100 years ago, late Efflatoun Bey and his co-workers started to collect flies from different localities all over Egypt. They recorded many fly species including the species treated herein from localities in the neighborhood of Cairo such as wadies south east of Cairo, Abu-Rawash, Kerdassa, Kafr Hakim, Kafr Eldems (= Mansheyet Radwan), Borgash, Mansouriah, Mazghouna, Sakkara, Helwan, Ezbet El-Nakhl, etc. (Efflatoun 1934, 1937, 1945). They also recorded many species from Mariout District on the Coastal Strip. However, many of these species have disappeared from these localities because of severe changes in the floral and faunal composition during the last 50 years. These changes may be attributed to urbanization or modern agricultural activities that caused severe degradation of natural habitats of these species (El-Hawagry et al. 2000; El-Hawagry 2015). However, the present study showed that the climatic and topographic variables in the Lower Nile Valley & Delta and Coastal Strip including these localities still have high suitability for most studied species.
Many of these species seem to be endangered or threatened, and conservation actions should be taken especially in localities or zones that still maintain their natural habitats. Sinai is one of these zones, and it has a merit that it includes seven natural protectorates, namely: Ras Muhammad, Azzaraniq, Ahrash, St. Catherine, Nabaq, Abu Galum and Taba (Leach et al. 2013). Undoubtedly, managing any conservation actions in an area already declared as a natural protectorate will be easier and more applicable than in other areas. The present study aims to contribute to the implementation of desired conservation actions, and to be used as a guide for any management decisions.
The results indicate that St. Catherine area has the highest habitat suitability for the six-studied species except A. lucidus. The species Anthrax trifasciatus, particularly, is recorded only from Sinai and not recorded elsewhere in Egypt. One of most interesting results of the six species through the area is that each species has its own niche (Fig. 3). By analyzing the six maps, it appears clearly that they have different habitat suitability. Such result shows how species of the same genus tend to have different ecological requirements to reduce competition even if they share the same habitat.
Fortunately, the St. Catherine protected area is expected to be maintained and will not face the severe changes in the floral and faunal composition as happened in the Lower Nile Valley & Delta and the Coastal Strip. However, future scenarios of climatic changes in St. Catherine for one of the species, Anthrax trifasciatus predict a great degradation in habitat suitability in the area.
St. Catherine area is about 1650 m above sea level. It also contains the highest mountains in Sinai (Gebel Catherine, 2642 m high, and Gebel Mussa, 2285 m high) (El-Moursy et al. 1999; Zalat et al. 2008). It has unusual climatic conditions as compared to the climate of the rest of Egypt. It is determined primarily by the altitude, the effect of which masks that of latitude. There is a wide difference in temperature between Summer and Winter. August is the hottest month, with a mean temperature of 25.9 °C, and January is the coldest month, with a mean temperature of 7.7 °C. The effect of the high mountains of St. Catherine is to make the climate continental, and to augment the difference between day and night. The maximum mean relative humidity occurs during January (64%), while the lowest mean occurs during May (Semida 1994; El-Rayes 1992).
The vegetation diversity in Sinai Peninsula including St. Catherine is exceptionally large and significant. The vegetation is characterized by the dominance of four large families, namely Asteraceae, Fabaceae, Lamiaceae, and Brassicaceae. One of the most common plants in St. Catherine is Alkanna orientalis (L.) Boiss. (Asclepiadaceae).
El-Moursy et al. (1999) studied the foraging behavior of anthracine bee flies in southern Sinai, especially in Wadi El-Arbaein, St. Catherine area, and they noticed that Anthrax spp. were present abundantly only at certain sites in the wadi, and they were foraging only on Alkanna orientalis in spite of the presence of many other wild plants in the same area. That’s why they thought that there might be a relation between the diversity and abundance of these anthracine flies and the plant cover of Alkanna orientalis (Gilbert et al. 1996). Many dipterans love to visit yellow flowers. The signal effect of the compact inflorescence of Alcanna flowers is striking and attracts insects even from a greater distance-especially those, which look for open flowers. Fabaceae and Lamiaceae have zygomorph flowers, where the nectar sources are hidden. Could it be that the Anthrax flies meet their special hosts on the flowers and follow them into their nest? (Marston 1971). The existence of Alcanna may act as meeting point for the solitaire bees and in the second row to Anthrax.
Recently, some works used environmental niche modeling to analyse the status of some higher Diptera especially of medical and veterinary importance (Mulieri and Patitucci 2019). While the present study represents the first environmental niche modeling study on bee flies, it sheds light on the status of six of the Egyptian species that are thought to be threatened. The results can be used for making powerful conservation management decisions in St. Catherine Protectorate to incorporate these Anthrax spp. in any present or future conservation programs.
References
Alahmed AM, Naeem M, Kheir SM, Sallam MF (2015) Ecological distribution modeling of two malaria mosquito vectors using geographical information system in Al-Baha Province, Kingdom of Saudi Arabia. Pak J Zool 47(6):1797–1806
Algar AC, Mahler DL (2015) Area, climate heterogeneity, and the response of climate niches to ecological opportunity in island radiations of Anolis lizards. Glob Ecol Biogeogr. https://doi.org/10.1111/geb.12327
Alkishe AA, Peterson AT, Samy AM (2017) Climate change influences on the potential geographic distribution of the disease vector tick Ixodes ricinus. PLoS ONE 12:e0189092
Asase, A., & Peterson, A. T. (2016) Completeness of Digital Accessible Knowledge of the Plants of Ghana. Biodiversity Informatics, 11.
Boucher FC, Thuiller W, Roquet C, Douzet R, Aubert S, Alvarez N, Lavergne S (2012) Reconstructing the origins of high-alpine niches and cushion life form in the genus Androsaces L. (Primulaceae). Evolution 66(4):1255–1268
Boulos L (1999–2005) Flora of Egypt. 4 vols. Al Hadara, Cairo
Chen IC, Hill JK, Ohlemuller R, Roy DB, Thomas CD (2011) Rapid range shifts of species associated with high levels of climate warming. Science 333:1024–1026
Coquillett DW (1887) Monograph of the species belonging to the genus Anthrax from America North of Mexico. Trans Am Entomol Soc Proc Entomol Sect Acad Nat Sci 14:159–182
Efflatoun HC (1934) A monograph of Egyptian Diptera. Part IV, Family Asilidae (Section I). Mem Soc R Entomol Egypt 4(2):1–198
Efflatoun HC (1937) A monograph of Egyptian Diptera. Part IV, Family Asilidae (Section II). Mem Soc R Entomol Egypt 4(3):199–443
Efflatoun HC (1945) A monograph of Egyptian Diptera. Part IV. Family Bombyliidae. Section I: Subfamily Bombyliidae Homeophthalmae. Bull Soc Fouadler Entomol 29:1–483
El-Hawagry MS (2002) Distribution, activity periods, and an annotated list of the bee flies of Egypt. Efflatounia 2:21–40
El-Hawagry MS (2015) Catalogue of superfamily Asiloidea (Diptera: Brachycera) of Egypt. LAP Lambert Academic Publishing, Germany, p 196
El-Hawagry MS (2017) Catalogue of Egyptian Tephritoidea (Diptera: Schizophora: Acalyptratae). Zootaxa 4299(2):151–190. https://doi.org/10.11646/zootaxa.4299.2.1
El-Hawagry MS, Evenhuis NL (2008) An updated key to the genera of Egyptian beeflies (Diptera: Bombyliidae). Egypt J Biol 10:104–121
El-Hawagry MS, Gilbert F (2014) Zoogeographical affinities and faunal relationships of bee flies (Diptera: Bombyliidae) in Egypt. Zool Middle East 60(1):50–56. https://doi.org/10.1080/09397140.2014.892339
El-Hawagry MS, El-Moursy AA, Gilbert F, Zalat S (2000) The tribe Anthracini Latreille (Bombyliidae, Diptera) from Egypt. Egypt J Biol 2:97–117
Elith J, Phillips SJ, Hastie T, Dudík M, Chee YE, Yates CJ (2011) A statistical explanation of MaxEnt for ecologists. Divers Distrib 17(1):43–57
El-Moursy A, Gilbert F, Zalat S, El-Hawagry M (1999) Foraging behavior of anthracine flies (Diptera: Bombyliidae) in southern Sinai, Egypt. Egypt J Biol 1:87–95
El-Rayes AE (1992) Hydrogeological studies of Saint Katherine area, South Sinai, Egypt. M.Sc. Thesis, Faculty of Science, Suez Canal University
Evenhuis NL, Greathead DJ (2015) World catalog of bee flies (Diptera: Bombyliidae). http://hbs.bishopmuseum.org/bombcat. Accessed 1 Oct 2017
FAO (The UN Food & Agriculture Organization) (2012) Aquastat water database. www.fao.org/nr/water/aquastat/data. Accessed 1 Jun 2016
Gilbert F, Willmer P, Semida F, Ghazoul J, Zalat S (1996) Spatial variation in selection in a plant-pollinator system in the wadis of Sinai. Egypt. Oecol 108(3):479–487
Greathead DJ, Evenhuis NL (1997) Family Bombyliidae. In: Papp L, Darvas B (eds) Manual of Palaearctic Diptera, vol 2. Science Herald, Budapest, pp 488–511
Guisan A, Tingley R, Baumgartner JB, Naujokaitis-Lewis I, Sutcliffe PR, Tulloch AIT et al (2013) Predicting species distributions for conservation decisions. Ecol Lett 16:1424–1435
Hoath R (1992) Natural selections: a year of Egypt’s wildlife. American University Press, Cairo, pp 3–6
Hochkirch A (2016) The insect crisis we can’t ignore. Nature 539(7628):141. https://doi.org/10.1038/539141a
Hoegh-Guldberg O, Hughes L, McIntyre S, Lindenmayer DB, Parmesan C, Possingham HP et al (2008) Assisted colonization and rapid climate change. Science 321:345–346
Jetz W, McPherson JM, Guralnick RP (2012) Integrating biodiversity distribution knowledge: toward a global map of life. Trends Ecol Evol 27:151–159
Khanum R, Mumtaz A, Kumar S (2013) Predicting impacts of climate change on medicinal asclepiads of Pakistan using Maxent modeling. Acta Oecol 49:23–31
Kumar S, Spaulding SA, Stohlgren TJ, Hermann KA, Schmidt TS, Bahls LL (2009) Potential habitat distribution for the freshwater diatom Didymosphenia geminata in the continental US. Front Ecol Environ 7:415–420
Larsen TB (1984) Butterflies of Saudi Arabia and its neighbours. Stacey International, London
Larsen TB (1990) Butterflies of Egypt. Apollo Books, Skerninge, Denmark, 112 pp. https://doi.org/10.1002/mmnd.19910380104
Leach K, Zalat S, Gilbert F (2013) Egypt’s protected area network under future climate change. Biol Conserv 159:490–500
Maldonado C, Molina CI, Zizka A, Persson C, Taylor CM, Albán J, Chilquillo E, Rønsted N, Antonelli A (2015) Estimating species diversity and distribution in the era of Big Data: to what extent can we trust public databases? Glob Ecol Biogeogr 24(8):973–984. https://doi.org/10.1111/geb.12326
Marston N (1971) Taxonomic study of the known pupae of the genus Anthrax (Diptera: Bombyliidae) in North and South America. Smithson Contrib Zool. https://doi.org/10.5479/si.00810282.100
Mulieri PR, Patitucci LD (2019) Using ecological niche models to describe the geographical distribution of the myiasis-causing Cochliomyia hominivorax (Diptera: Calliphoridae) in southern South America. Parasitol Res 118(4):1077–1086
Naeem M, Alahmed AM, Kheir SM, Sallam MF (2016) Spatial distribution modeling of Stegomyia aegypti and Culex tritaeniorhynchus (Diptera: Culicidae) in Al-bahah Province, Kingdom of Saudi Arabia. Trop Biomed 33(2):295–310
Nyári ÁS, Reddy S (2013) Comparative phyloclimatic analysis and evolution of ecological niches in the scimitar babblers (Aves: Timaliidae: Pomatorhinus). PLoS ONE 8(2):e55629. https://doi.org/10.1371/journal.pone.0055629
Pearson RG, Raxworthy CJ, Nakamura M, Townsend Peterson A (2007) Predicting species distributions from small numbers of occurrence records: a test case using cryptic geckos in Madagascar. J Biogeogr 34:102–117
Peterson AT, Papeş M, Soberón J (2008) Rethinking receiver operating characteristic analysis applications in ecological niche modeling. Ecol Model 213:63–72
Phillips SJ et al (2008) Transferability, sample selection bias and background data in presence-only modelling: a response to Peterson et al. (2007). Ecography 31:272–278
Rato C, Harris DJ, Perera A, Carvalho SB, Carretero MA, Rödder D (2015) A Combination of divergence and conservatism in the nicheevolution of the Moorish Gecko, Tarentola mauritanica (Gekkota: Phyllodactylidae). PLoS ONE 10(5):e0127980. https://doi.org/10.1371/journal.pone.0127980
Rondinini C, Wilson KA, Boitani L, Grantham H, Possingham HP (2006) Tradeoffs of different types of species occurrence data for use in systematic conservation planning. Ecol Lett 9:1136–1145
Salariato DL, Zuloaga FO (2016) Climatic niche evolution in the Andean genus Menonvillea (Cremolobeae: Brassicaceae). Org Divers Evol 17:1–18. https://doi.org/10.1007/s13127-016-0291-5
Sallam MF, Al Ahmed AM, Abdel-Dayem MS, Abdullah MAR (2013) Ecological Niche modeling and land cover risk areas for rift valley fever vector, Culex tritaeniorhynchus Giles in Jazan, Saudi Arabia. PLoS ONE 8(6):e65786. https://doi.org/10.1371/journal.pone.0065786
Samy AM, Elaagip AH, Kenawy MA, Ayres CF, Peterson AT, Soliman DE (2016) Climate change influences on the global potential distribution of the mosquito Culex quinquefasciatus, vector of West Nile virus and lymphatic filariasis. PLoS ONE 11:e0163863
Sanchez AC, Osborne PE, Haq N (2011) Climate change and the African baobab (Adansonia digitata L.): the need for better conservation strategies. Afr J Ecol 49:234–245
Schnitzler J, Graham CH, Dormann CF, Schiffers K, Linder PH (2012) Climatic niche evolution and species diversification in the Cape flora, South Africa. J Biogeogr 39(12):2201–2211
Semida FM (1994) Insect–plant pollination relationship in Sinai Desert Ecosystem. PhD Thesis, Faculty of Science, Suez Canal University
Steyskal GC, El-Bialy S (1967) A list of Egyptian Diptera with a bibliography and key to families. Tech Bull U A R Minist Agric 3:12–18
Swets JA (1988) Measuring the accuracy of diagnostic systems. Science 240:1285–1293
Zalat S, Gilbert F, Fadel H, El-Hawagry MS, Saleh M, Kamel S, Gilbert J (2008) Biological explorations of Sinai: flora and fauna of Wadi Isla and Hebran, St Katherine Protectorate, Egypt. Egypt J Nat Hist 5:6–15
Acknowledgements
The authors would like to thank all directors of four main Egyptian insect reference collections (Ain Shams University collection, Cairo University Collection, Collection of Egyptian Society of Entomology, Ministry of Agriculture Collection) from which our flies’ data were collected and for their help. Also, we would appreciate the valuable help provided by the Ph.D. candidate Mr. Saad Sonbati, Collage of Food and Agriculture Science, King Saud University, Saudi Arabia.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Ethical approval
This article does not contain any studies containing direct contact with animal, and all applicable international, national, and/or institutional ethical guidelines were taken in consideration during preparation of the manuscript.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Nasser, M., El-Hawagry, M. & Okely, M. Environmental niche modeling for some species of the genus Anthrax Scopoli (Diptera: Bombyliidae) in Egypt, with special notes on St. Catherine protected area as a suitable habitat. J Insect Conserv 23, 831–841 (2019). https://doi.org/10.1007/s10841-019-00174-6
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10841-019-00174-6