Abstract
This study evaluated whether herbivorous insects can be expected to have particular adaptations to withstand the harsh dry season in tropical dry forests (TDFs). We specifically investigated a possible escape in space, with herbivorous insects moving to the few evergreen trees that occur in this ecosystem; and escape in time, with herbivores presenting an increased nocturnal rather than diurnal activity during the dry season. We determined the variation in the free-feeding herbivorous insects (sap-sucking and leaf chewing) between seasons (beginning and middle of both rainy and dry seasons), plant phenological groups (deciduous and evergreen trees) and diel period (diurnal and nocturnal) in a Brazilian TDF. We sampled a total of 5827 insect herbivores in 72 flight-interception traps. Contrary to our expectations, we found a greater herbivore diversity during the dry season, with low species overlap among seasons. In the dry season, evergreen trees supported greater richness and abundance of herbivores as compared to deciduous trees. Insects were also more active at night during the dry season, but no diel differences in insect abundance were detected during the rainy season. These results indicate that the strategies used by insect herbivores to withstand the severe climatic conditions of TDFs during the dry season include both small-scale escape in space and time, with evergreen trees playing a key role in maintaining resident insect herbivore populations in TDFs. Relatively more nocturnal activity during the dry season may be related to the avoidance of harsh climatic conditions during the day. We suggest that the few evergreen tree species occurring in the TDF landscape should be especially targeted for protection in this threatened ecosystem, given their importance for insect conservation.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Insects represent the most diverse and well-distributed group of organisms, comprising different trophic guilds, such as herbivores, detritivores, and predators. In tropical forests, the insects achieve their highest diversity (Basset et al. 2012), being an important part of living biomass from the soil to the emerging canopy trees (Basset et al. 2003, 2012). Due to their complex life forms, diverse feeding habits, dispersal capacity, and high diversity and biomass, insects participate in several processes (e.g. soil fertilization and aeration, seed dispersal, pollination and biological control) that are key for the functioning and maintenance of ecosystems (Losey and Vaughan 2006; Nichols et al. 2008). Specifically, herbivorous insects compose the most diverse group of insects in tropical forests (Price 2002; Basset et al. 2012), with a wide array of feeding guilds that respond differently to seasonal changes (Didham and Springate 2003; Novotny et al. 2003; Leal et al. 2015). However, few studies have investigated particular adaptations about how insect herbivores cope with the harsh dry periods in seasonal ecosystems.
Tropical dry forests (TDFs) are characterized by a marked dry season (i.e. at least 3 months with less than 100 mm/month of rainfall) when deciduous plants drop more than 50% of their leaves (Murphy and Lugo 1986; Sánchez-Azofeifa et al. 2005). During this period, environmental conditions become harsh, with increasing insolation and wind intensity and decreasing humidity (Murphy and Lugo 1986; Lebrija-Trejos et al. 2011). In the case of free-feeding leaf insects (e.g. chewers and suckers), resource availability sharply decreases because deciduous plants dominate the landscape (Villalobos et al. 2013; Pezzini et al. 2014). These organisms exhibit two strategies to survive the prolonged dry season: (1) temporal adjustment via diapause or synchronization of their life cycle with the rainy season; or (2) spatial adjustment, moving to habitats with non-deciduous trees (Janzen 1973; Dirzo and Domínguez 1995; Silva and Neves 2014).
In spite of such strategies, there is a general reduction in insect herbivore diversity and activity during the dry season (Janzen 1973), suggesting that the temporal adjustment may prevail. In fact, finding leaves in TDFs during the dry season is not trivial. It involves long-distance migration to other vegetation types, such as semi-deciduous riparian forests, savannahs (the Cerrado in Brazil) or altitudinal fields (Leal et al. 2015); or searching for isolated evergreen or brevi-deciduous plants that compose a small proportion of TDF species (Silva and Neves 2014). In this way, these plant phenological groups may play a key role in the maintenance of at least some insect guilds during the dry season (Janzen 1973; Silva and Neves 2014). According to Villalobos et al. (2013), only a small fraction of the TDFs flora is evergreen, ranging between 1.1 and 9.7%. Despite the few species, this phenological group could contribute to diversity and ecosystem process maintenance in TDFs, and areas with a high abundance of these species could be prioritized for conservation. Changes in plant diversity and abundance in these threatened forests could underlie changes in herbivore abundance (Leal et al. 2016; Silva et al. 2016). Nevertheless, information on life history or movement patterns for insect species in the tropics are scant, and the relevance of non-deciduous plants to insect population dynamics in TDFs is still unclear. To our knowledge no detailed study has investigated the role of evergreen trees within TDFs in maintaining insect diversity during the severe dry season.
In addition to seasonal variations, insect activity shows small-scale temporal patterns (i.e. diel cycles) that are frequently overlooked (Janzen 1973; Springate and Basset 1996; Basset et al. 2001). Several studies performed in tropical forests have revealed higher diel activity of arboreal insects, mostly herbivores, during the night (Basset and Springate 1992; Novotny et al. 1999; Wardhaugh 2014). Janzen (1983) proposed two non-exclusive hypotheses for a greater nocturnal activity of herbivores: (1) the existence of a “temporal window” of enemy-free space; and (2) the availability of higher leaf concentration of photoassimilates which have not yet been translocated or used in respiration. Moreover, specifically for TDFs, the intensity of diel variation in abiotic conditions, such as temperature and humidity, shows strong seasonal variations (Lebrija-Trejos et al. 2011). Hence, due to more adverse hygrothermal conditions (i.e. high temperatures and low relative humidity) and wider climatic diel range in the dry season, we expect that insect nocturnal activity would be more intense compared to the diurnal activity during this season.
This study evaluated temporal variations in the abundance and richness of herbivorous insects in the TDF canopy across different seasons (beginning and middle of rainy and dry seasons), plant phenological groups (deciduous and evergreen) and diel periods (day- and night-time). We addressed the following hypotheses: (1) there are changes in herbivorous species composition between seasons, with reduced abundance and richness of insects during the dry season; (2) the richness and abundance of herbivorous insects are greater on evergreen compared to deciduous tree species in the dry season; and (3) herbivorous insects have greater nocturnal activity compared to the diurnal activity in the dry season, whereas the inverse pattern is expected during the rainy season.
Methods
Study area and system
This study was conducted at the Mata Seca State Park (MSSP), created in 2000, and managed by the Instituto Estadual de Florestas (IEF—Forestry State Institute). The MSSP has an area of 15,466.44 ha and is located in the valley of the São Francisco River, Minas Gerais state, Brazil, between 14°48′36″–14°56′59″S and 43°55′12″–44°04′12″W, at an altitude between 400 and 500 m above sea level. The climate of the region is considered as tropical semi-arid (Aw in Köppen’s classification), with an average temperature of 24 °C, and average annual precipitation of 871 mm (Antunes 1994). The dry season extends from May to October, and rains are unevenly distributed along the rainy season from November to April (Antunes 1994). The precipitation in the rainy season is 744 mm in average, with only 60 mm during the marked dry season (data available from 1936 to 2012 at the Mocambinho Station, situated at 15 km from the MSSP).
The MSSP is covered with a mosaic of TDFs in different successional stages, and this study was conducted in an old-growth forest. According to the former farm manager, forest structure has been the same since the beginning of the 1970s, although occasional selective logging and sparse free-ranging cattle occurred in the area until the park creation. This forest is characterized by two vertical strata: the first is composed of deciduous trees that reach more than 16 m in height and form a closed canopy. The second stratum is composed of a dense understory, with lianas, shade-tolerant adult trees, and juvenile canopy trees. The average basal area is 22.0 ± 2.3 m2/ha and tree density is 98.8 ± 6.1 individuals/0.1 ha (Madeira et al. 2009). The level and duration of deciduousness vary among tree species, but evergreen trees can hold their green leaves during the dry season (Pezzini et al. 2014). Previous floristic and phenological studies conducted at the MSSP registered the presence of four leaf-exchanger evergreen species (see Pezzini 2008): Aspidosperma polyneuron Müll. Arg. (Apocynaceae), Goniorrhachis marginata Taub. (Fabaceae-Ceasalpinioideae), Machaerium scleroxylon Tul. (Fabaceae-Faboideae) and Ziziphus joazeiro Mart. (Rhamnaceae). Individuals of these species retain their leaves for approximately 11.5 months of the year, and replace them within a few days at the end of the dry season (September) (Pezzini et al. 2014).
The beginning of the rainy season is characterized by rapid leaf production, with canopy formation (22.32 ± 1.41% of canopy openness) within 2 weeks after the occurrence of the first rains in October/November (Fig S1). In this period, there is a high abundance of leaf buds and expanding leaves with high nutritional quality for herbivores (Silva et al. 2012). In the peak of the rainy season (January) there is high foliage availability (16.47 ± 1.68% of canopy openness) with fully expanded green leaves. The onset of the dry season (May) is marked by rapid leaf senescence (40.54 ± 3.18% of canopy openness), with leaf nutrients being translocated to other plant organs (Silva et al. 2012). In the peak of the dry season (August), there is no precipitation and plants drop up to 95–100% of their leaves (70.18 ± 1.80% of canopy openness) (see Fig. S1).
Sampling design
Samples were taken between 2011 and 2012 in the following periods: (a) middle of the dry season (August-2011), (b) beginning of the rainy season (October-2011); (c) middle of the rainy season (January-2012) and (d) beginning of the dry season (May-2012). We determined the month corresponding to each season through a cluster analysis (using complete linkage method for amalgamation and Euclidean distance as the coefficient of association; see Madeira and Fernandes 1999), using the monthly rainfall recorded between 1976 and 2012 from Mocambinho Station (see Fig. S2).
Insects were collected with flight-interception traps, modified from Malaise and Window traps, set up in tree crowns (see Springate and Basset 1996; Novais et al. 2016; Fig S3). The main body of the trap consisted of a rectangular cross-panel of black netting (mesh width of 0.5 ram, collecting surface of 4.5 m2). A funnel was attached at both ends of the main body of the trap (upper and lower diameter of funnel of 90 and 75 cm, respectively) and connected to a collecting recipient (4 cm in diameter). The length of the trap, from the top of the upper collector to the base of the lower recipient, was 2.65 m. Collecting recipients were filled with ethanol 70% and glycerin 5%. This trap is very effective in capturing winged insects, but it is not appropriate to collect wingless adult insects or crawling larvae (see Grimbacher and Stork 2009).
We sampled three individuals of each one of the following evergreen tree species: A. polyneuron, G. marginata and M. scleroxylon. Ziziphus joazeiro was not sampled in this study due to its low abundance and absence among emerging and canopy trees (see Pezzini 2008; Madeira et al. 2009). Sampled individuals were at least 30 m apart from any other evergreen tree. We also sampled nine deciduous trees (regardless of species identity and paired to evergreen trees) at least 15 m distance from each trapped evergreen tree. We sampled 18 trees in each period (18 traps × 4 periods = 72 total traps), with one trap winched into the centre of the crown (see Fig. S3). Traps were raised and lowered using ropes passed over supporting branches. All trees were between 15 and 18 m tall and were trapped during five consecutive days. During each period, the traps were surveyed twice a day in the same sequence, with the surveys commencing at 6.00 a.m. and at 6.00 p.m. Thus, samples were separated into diurnal (from 6 a.m. to 6 p.m) and nocturnal (from 6 p.m. to 6 a.m.) catches. During this study, daily mean sunrise and sunset were respectively at 06.11 a.m. and at 6.25 p.m. (i.e. considering the coordinated universal time—in summer: −2.00 and winter: −3.00). On average, the inspection of the 18 traps in each daily survey was completed in 55 min.
All samples were taken to the laboratory and free-feeding herbivorous insects (chewing and sap-sucking) were identified to the family level and sorted into morphospecies (Rafael et al. 2012). We consider as herbivores all the insects from families in which the herbivorous habit predominates (see Moran and Southwood 1982; Neves et al. 2014).
Statistical analyses
The family composition of free-feeding herbivorous insects (chewing and sap-sucking) was determined per trap and compared among different periods using nonmetric multidimensional scaling (NMDS). Since we used abundance data of different taxa simultaneously, the NMDS is indicated to calculate a matrix of dissimilarity for community composition data (Clarke 1993). This analysis to determine data dispersion is based on changes in the mean–variance relationship (Warton et al. 2012). A few morphospecies were captured per trap regardless of the season period, thus most morphospecies was scored as “zero” in the matrix. This could cause only small changes in the mean–variance between groups and generate misleading results when comparing seasonal groups. To solve this problem, traps from different seasons were ordered using the density of families of herbivorous insects calculated through the Bray–Curtis index. To test whether the community structure of different herbivore guilds differed among periods, we performed an analysis of similarity (ANOSIM; Clarke 1993). In this analysis, a nonparametric permutation procedure was applied to rank similarity matrices underlying sample ordinations (Clarke 1993). The calculated R-value (Clarke 1993) was obtained and used to determine similarity patterns between the insect communities sampled at each period. These analyses were conducted using the PAST software (Hammer et al. 2001).
The effects of seasonality (beginning and middle of both rainy and dry seasons) and phenological group (deciduous vs. evergreen trees) on the abundance and richness of free-feeding herbivores (chewing and sap-sucking) were evaluated using generalized linear models (GLMs). In these models, the abundance and richness of the insects were used as response variables, whereas the period, phenological group and their interaction were used as explanatory variables. Initially, all models were adjusted for Poisson error distribution (link-log function), followed by an adjustment when the data were overdispersed. Afterward, the junction of non-significant categorical treatments (amalgamation) was performed using a post hoc contrast analyses (Crawley 2007).
To verify the diel activity (diurnal vs. nocturnal) of the insect fauna, we constructed generalized linear mixed models (GLMMs). We used GLMMs due to the existence of a nested structure (diel shift/host plant) that was included in the model as the random effect (Crawley 2007). In these models, the abundance and richness of free-feeding herbivores (chewing and sap-sucking) were used as response variables and the error distribution was adjusted for the negative binomial family (count data), since our data showed overdispersion (GLMMADMB package). The diel period in each season was used as an explanatory variable. All models were developed in R software (R Development Core Team 2014), followed by residual analyses and evaluation of error distribution adequacy (Crawley 2007).
Results
A total of 5827 free-feeding herbivorous insects were sampled during the study (3463 sucking and 2364 chewing insects), belonging to 396 morphospecies in 36 families (see Tables S1 and S2). The Cicadellidae family was the most abundant (3,405 individuals) and richest (53 morphospecies) among the sap-sucking herbivores (Table S1). Among the chewing insects, the Chrysomelidae family presented the highest abundance (411 individuals) and richness (78 morphospecies; see Table S2).
For sap-sucking insects, the community composition at the family level clearly differed between rainy and dry periods (RANOSIM = 0.16, p < 0.001; Fig. 1a). The community of chewing insects showed differences in composition at the family level among the four periods (RANOSIM = 0.29, p < 0.001; Fig. 1b).
Ordination of 72 traps sampled in four periods (beginning and middle of both rainy and dry seasons) through a nonmetric multidimensional scaling (NMDS), using the family abundance from a chewing and b sap-sucking herbivorous insects (p < 0.05). BRS beginning of rainy season, MRS middle of rainy season, BDS beginning of dry season, and MDS middle of dry season
We recorded a greater abundance of sap-sucking insects in the middle of the dry season, whereas their richness showed two peaks: in the middle of both rainy and dry seasons. A morphospecies belonging to the Neozygina genus (Cicadellidae: Typhlocybinae) dominated the samples with 2941 individuals (up to 50.5% of all the insects sampled in this study and 85% of all sap-sucking insects). This morphospecies occurred exclusively on Goniorrhachis marginata evergreen trees and was responsible for the huge discrepancy in the mean abundance and standard error observed in the middle of the dry season (Table 1; Fig. 2). We recorded the lowest abundance and richness of chewing herbivores at the beginning of the dry season (Table 1; Fig. 3).
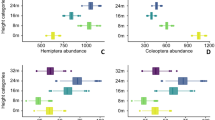
Average a abundance and b richness of sap-sucking insect herbivores per trap on deciduous and evergreen trees sampled in four periods (beginning and middle of both the rainy and dry seasons) in a tropical dry forest (n = 72). The use of different letters upon bars indicates differences among periods, whereas the use of asterisk indicates differences between phenological groups (deciduous vs. evergreen) within the same period (p < 0.05). Error bars indicate one standard error. BRS beginning of rainy season, MRS middle of rainy season, BDS beginning of dry season, and MDS middle of dry season
Average a abundance and b richness of chewing insect herbivores per trap on deciduous and evergreen trees sampled in four periods (beginning and middle of both the rainy and dry seasons) in a tropical dry forest (n = 72). The use of different letters upon bars indicates differences among periods, whereas the use of asterisk indicates differences between phenological groups (deciduous vs. evergreen) within the same period (p < 0.05). Error bars indicate one standard error. BRS beginning of rainy season, MRS middle of rainy season, BDS beginning of dry season, and MDS middle of dry season
The abundance of sap-sucking insects was higher in deciduous than evergreen trees during the rainy season, a pattern that was inverted during the dry season (Table 1; Fig. 2). In general, the insect abundance was higher in the middle of the dry season (Fig. 2a) because of the occurrence of two Cicadellidae morphospecies on G. marginata (see Tables S1, S2). On the other hand, the richness of sap-sucking insects on deciduous trees peaked in the middle of the rainy season, whereas the same occurred on evergreen trees in the middle of the dry season (Fig. 2b). The abundance and richness of chewing insects was significantly lower for evergreen and deciduous trees at the beginning of the dry season (Table 1; Fig. 3a). However, the abundance of this guild did not differ between phenological groups. The richness of chewing insects was significantly higher on deciduous trees during the rainy season, and the opposite occurred during the dry season (Table 1; Fig. 3b).
Finally, diel activity for insect herbivores showed significant diurnal/nocturnal changes during the dry season, during which the abundance of both sap-sucking and chewing insects was higher during the night (Table 2). No diel differences were detected during the rainy season.
Discussion
We detected an unexpected higher diversity of herbivorous insects during the dry season in the studied TDF, mainly due to an increase in insect abundance and richness on evergreen tree species. To our knowledge this is the first study to show the role of evergreen trees within TDFs in maintaining insect diversity during the severe dry season. Also, the observed variations in the abundance and richness of two leaf-chewing and sap-sucking insects indicate small-scale spatial and temporal adaptations (i.e. moving to evergreen trees and shifting to nocturnal periods) to survive the harsh dry season of TDFs. Given its scarce occurrence in the TDF landscape and its importance to sustain insect herbivores (and maybe species from higher trophic levels) during the dry season, evergreen trees should receive special attention in management and conservation strategies for TDFs.
Seasonality
We recorded pronounced seasonal changes in community composition, abundance, and richness of herbivorous insects on TDF trees. The sap-sucking insect fauna was equally distributed among families during the rainy season, but was dominated by Cicadellidae (Typhlocybinae) both at the beginning and in the middle of the dry season. The Cicadellidae family is characterized by a morpho-physiological specialization of the Malpighian tubules, with the secretion of the protein-lipid named brochosomes (Rakitov 2000). Brochosomes form a thin hydrophobic layer on the insect integument (on all ontogenetic stages), providing protection from contamination with sticky honeydew, and creating a barrier against attachment and penetration of spores of entomopathogenic fungi (Rakitov 2002). Specifically, in arid environments, the main advantages of the brochosomes could be the protection against heat by reflecting the ultraviolet rays (Rakitov 2002). Most Typhlocybinae feed on sap in the leaf mesophyll (Novotny and Wilson 1997; Novotny et al. 2003), and this would explain the high abundance of the mesophyll cell feeders Alconeura sp. (Typhlocybinae: Dikraneurini) and Neozygina sp. (Typhlocybinae: Erythroneurini) on evergreen trees in the dry season.
Changes in sap-sucking composition were observed between rainy and dry seasons, but not between periods within each season. This pattern may be possibly related to a relatively low variability in sap quality within a season (Santos et al. 2014). On the other hand, chewing herbivores were more sensitive to seasonal variations in abiotic and biotic conditions, given that changes in chewing composition were observed among the four periods. The community of chewing herbivores was characterized by prevalence, in the rainy season, of families that feed on young and highly nutritional leaves, such as Chrysomelidae and Curculionidae. The abundance of these groups decreased at the beginning and in the middle of the dry season, being replaced by large generalist herbivores with robust mouthparts and well-developed digestive systems (Orthopteroids: Orthoptera and Phasmida) that are able to cut relatively tough, mature leaves with low nutritional quality (Didham and Springate 2003; Novotny et al. 2003; Wardhaugh 2014). Moreover, there is an increase in the amount of recently dead wood suspended in the canopy (e.g. fallen branches) during the dry season, favoring the occurrence of generalist wood-eating or mixed-diet beetles, such as Cerambycidae, Elateridae and Dermestidae (Novotny et al. 2003; Novotny and Basset 2005; Grimbacher and Stork 2009). The presence of chewing herbivores in leafless trees during the dry season is intriguing, but it is likely that these insects are searching for food or suitable microhabitats and mates, avoiding predation and eventually dispersing or migrating.
A reduction in insect diversity during the dry season seems to be common in tropical habitats (Novotny and Basset 1998; Pinheiro et al. 2002; Freire-Jr et al. 2014; Kishimoto-Yamada and Itioka 2015), and this pattern would be even more drastic for herbivorous insects in TDFs with intense drought-deciduousness (Janzen 1973, 1981; Vasconcellos et al. 2010). Nonetheless, we recorded a higher abundance and richness of sap-sucking herbivorous insects during the dry season, an unusual pattern already detected for insects in general in other TDFs (Kishimoto-Yamada and Itioka 2015). However, our result must be interpreted in the context of the sampling design used in this study. When the two most abundant Cicadellidae morphospecies were removed from the analyses, insect abundance peaked during the rainy season. Furthermore, we sampled the same number of evergreen and deciduous trees, but this proportion is quite different in TDFs. In general, only up to 1.7–9.7% of tree species in TDFs are evergreen (Lott and Atkinson 2002; Ragusa-Netto and Silva 2007). Thus, it is very likely that the overall insect diversity (i.e. at the landscape level) is highest during the rainy season when all deciduous species have leaves. In spite of that, it is possible that the high diversity of herbivorous insects in the dry season is related to an increased foraging activity to compensate for the resource scarcity (see Grimbacher and Stork 2009), or to an increased searching for humid refuges provided by evergreen trees (Silva and Neves 2014). Temporal and spatial replicates (i.e. more than 1 year and one site) are necessary to corroborate the unexpected higher diversity of herbivorous insects in the dry season and elucidate the mechanisms underlying such a pattern.
Phenological groups
The insect diversity on plants from different phenological groups varied seasonally. In the dry season, a higher herbivore richness (for both sap-sucking and chewing insects) and abundance (only for sap-sucking) was recorded on evergreen trees. This result suggests that these different guilds of insect herbivores show a small-scale spatial adjustment to withstand the harsh dry season by moving to trees with leaves. The greater abundance of sap-sucking herbivores (e.g. Neozygina sp.; Cicadellidae: Typhlocybinae) on evergreen trees in the dry season may be associated with increased availability of amino acids, nitrogen and soluble carbohydrates in the sap (Walter et al. 2012; Gonda-King et al. 2014), and reduced water potential of evergreen plants (Hasselquist et al. 2010). In addition, the phloem does not contain large concentrations of defenses during the dry season (Walter et al. 2012), which can increase the performance of sap-sucking herbivores in this period. Moreover, as mentioned above, Typhlocybinae species feed on sap in the mesophyll of leaves (Novotny and Wilson 1997), which are only available on evergreen trees during the dry season.
For chewing herbivores, the availability of leaves on evergreen trees in the dry season does not necessarily constitute a food resource, since in this period the leaves are tougher and better defended (Silva and Neves 2014; Silva et al. 2015). Although leaf-chewing insects and sap-sucking mesophyll-feeders exploit the same resource, the latter guild can avoid certain leaf parts (e.g. channels of resin, latex, and trichomes), and feed on leaves that possess defensive barriers (Novotny and Wilson 1997; Novotny et al. 2003). Such differences in feeding mechanisms and limitations would explain the higher sap-sucking abundance on evergreen trees in the dry season. Alternatively, a large proportion of the chewing insect herbivore community spends the dry season as non-reproductive adults with reduced leaf consumption rates (Dirzo and Domínguez 1995). Further research is needed to understand what these insects are doing on these trees and how they complete their life cycle in the studied TDF.
Although the three evergreen species sampled in this study are leaf-exchangers, during our sampling dates in the middle of the dry season, only A. polyneuron and G. marginata had young leaves, whereas M. scleroxylon supported mature/old leaves. This is a possible explanation for the higher similarity in their associated insect fauna when compared to M. scleroxylon. At the beginning of the rainy season, the young leaves from both deciduous and evergreen tree species, were still expanding and the humid conditions probably favored the absence of differences in insect diversity between evergreen and deciduous trees. However, in the middle of the rainy season, deciduous trees with a shorter lifespan and faster leaf expansion may have accumulated fewer carbon-based defenses, being preferred by herbivores (see Silva et al. 2015). Thus, small interspecific differences in the timing of leaf exchange (i.e. like leaf flushing by evergreen species in the dry season) may affect herbivore colonization.
Diel activity
In the dry season, the abundance of both herbivore guilds was higher during the night (see Table 2), a pattern probably driven by the interaction of abiotic and biotic factors such as suitable microclimate and reduced competition and predation (Janzen 1973; Basset and Springate 1992; Novotny et al. 1999). According to Lebrija-Trejos et al. (2011), in the dry season, the canopy is more exposed to intense solar radiation, wind speed and extreme temperatures, and is therefore much less humid than in the rainy season. However, such harsh conditions are attenuated during the night (see Janzen 1973; Wardhaugh 2014). In a study conducted during the dry season in a tropical rainforest in New Guinea with Ficus wassa (Moraceae), Novotny et al. (1999) found higher insect diversity at night and related this result to a lower predation risk for herbivores (i.e. mostly Chrysomelidae, Curculionidae and Cicadellidae). The community of herbivore insects differed between seasons, but our data cannot be directly used to determine changes in species behavior between seasons (i.e. same species flying during the day in rainy season and at night during the dry season) because most morphospecies were captured only in one season. Our results suggest that insect morphospecies become more nocturnal during the dry season and not that nocturnal morphospecies were relatively more common during the dry season. Thus, we indicate the existence of seasonally-conditioned variations in diel activity of herbivorous insects in TDFs, a pattern which may be typical of these ecosystems.
Conclusions
Our study contributes to filling the gaps in knowledge about insect seasonality patterns at the community level in TDFs. Unexpectedly, our results indicate a higher diversity of herbivorous insects during the dry season. Their activity during the dry season in TDFs is probably of importance in the maintenance of higher trophic levels and interaction networks, and certainly deserves further attention. Our results indicate that the strategies used by insect herbivores to withstand the severe climatic conditions of TDFs during the dry season include both small-scale escape in space and time, with evergreen trees playing a key role in maintaining resident insect herbivore populations in TDFs.
Based on the fact that TDFs represent 42% of the tropical forests (Miles et al. 2006) and have been considered the most threatened tropical ecosystem (Quesada et al. 2009; Portillo-Quintero and Sánchez-Azofeifa 2010), conservation efforts must be urgently directed to the maintenance of their biodiversity and ecosystem process (Quesada et al. 2009). Evergreen species serve as dry season refuges for insect herbivores (and probably their predators) in TDFs and, due to their scarcity in the TDF landscape, these trees should be especially targeted for protection in this threatened ecosystem as key micro-habitats for insect conservation.
References
Antunes FZ (1994) Caracterização Climática—Caatinga do Estado de Minas Gerais. Info Agro 17:15–19
Basset Y, Springate ND (1992) Diel activity of arboreal artbropods associated with a rainforest tree. J Nat Hist 26:947–952
Basset Y, Aberlenc HR, Barrios H, Curletti G, Bérenger JM, Vesco JP, Causse P, Haug A, Hennion AS, Lesobre L, Marquès F, O’meara R (2001) Stratification and diel activity of arthropods in a lowland rainforest in Gabon. Biol J Linn Soc 72:585–607
Basset Y, Novotny V, Barrios H, Holloway JD, Miller E (2003) Vertical stratification of arthropod assemblages. In: Basset Y, Novotny V, Miller SE, Kitching RL (eds) Arthropods of tropical forests: spatio-temporal dynamics and resource use in the canopy. Cambridge University Press, Cambridge, pp 17–27
Basset Y, Cizek L, Cuénoud P, Didham RK, Guilhaumon F, Missa O, Novotny V, Ødegaard F, Roslin T, Schmidl J, Tishechkin AK, Winchester NN, Roubik DW, Aberlenc HP, Bail J, Barrios H, Bridle JR, Castaño-Meneses G, Corbara B, Curletti G, Rocha WD, Bakker D, Delabie JHC, Dejean A, Fagan LL, Floren A, Kitching RL, Medianero E, Miller SE, Oliveira EG, Orivel J, Pollet M, Rapp M, Ribeiro SP, Roisin Y, Schmidt JB, Sørensen L, Leponce M (2012) Arthropod diversity in a Tropical Forest. Science 338:1481–1484
Clarke KR (1993) Nonparametric analyses of changes in community structure. Aust J Ecol 18:117–143
Crawley MJ (2007) Statistical computing—an introduction to data analysis using S-Plus. Wiley, London
Didham RK, Springate ND (2003) Determinants of temporal variation in community structure. In: Basset Y, Novotny V, Miller SE, Kitching RL (eds) Arthropods of tropical forests: spatio-temporal dynamics and resource use in the canopy. Cambridge University Press, Cambridge, pp 28–39
Dirzo R, Domínguez CA (1995) Plant-herbivore interactions in Mesoamerican tropical dry forest. In: Bullock SH, Mooney A, Medina E (eds) Seasonally dry tropical forests. Cambridge University Press, Cambridge, pp 304–325
Freire-Jr G, Nascimento AR, Konstantinov IM, Diniz IR (2014) Temporal occurrence of two Morpho butterflies (Lepidoptera: Nymphalidae): influence of weather and food resources. Environ Entomol 43:274–282
Gonda-King L, Gómez S, Martin JL, Orians CM, Preisser EL (2014) Tree responses to an invasive sap-feeding insect. Plant Ecol 215:297–304
Grimbacher PS, Stork NE (2009) Seasonality of a diverse beetle assemblage inhabiting lowland tropical rain forest in Australia. Biotropica 41:328–337
Hammer Ø, Harper DAT, Ryan PD (2001) Past: paleontological statistics software package for education and data analysis. Palaeontol Electron 4:1–9
Hasselquist NJ, Allen MF, Santiago LS (2010) Water relations of evergreen and drought-deciduous trees along a seasonally dry tropical forest chronosequence. Oecologia 164:881–890
Janzen DH (1973) Sweep samples of tropical foliage insects: effects of seasons, vegetation types, elevation, time of day, and insularity. Ecology 54:687–708
Janzen DH (1981) Patterns of herbivory in a tropical deciduous forest. Biotropica 13:271–282
Janzen DH (1983) Food webs: who eats what, why, how, and with what effects in a tropical forest. In: Golley FB (ed) Tropical rain forest ecosystems: structure and function. Elsevier, Amsterdam, pp 162–182
Kishimoto-Yamada K, Itioka K (2015) How much have we learned about seasonality in tropical insect abundance since Wolda (1988)? Entomol Sci 18:407–419
Leal CRO, Fagundes M, Neves FS (2015) Change in herbivore insect communities from adjacent habitats in a transitional region. Arthropod-Plant Interact 9:311–320
Leal CRO, Silva JO, Sousa-Souto L, Neves FS (2016) Vegetation structure determines insect herbivore diversity in seasonally dry tropical forests. J Insect Conserv 20:979–988
Lebrija-Trejos E, Pérez García EA, Meave JA, Poorter L, Bongers F (2011) Environmental changes during secondary succession in a tropical dry forest in Mexico. J Trop Ecol 27:1–13
Losey JE, Vaughan M (2006) The economic value of ecological services provided by insects. Bioscience 56:311–323
Lott EJ, Atkinson TH (2002) Biodiversidad y fitogeografica de Chamela-Cuixmala Jalisco. In: Noguera F, Rivera JHV, Aldrete ANG, Quesada M (eds) Historia natural de Chamela. Instituto de Biologia UNAM, Mexico City, pp 83–97
Madeira JÁ, Fernandes GW (1999) Reproductive phenology of sympatric taxa of Chamaecrista (Leguminosae) in Serra do Cipó, Brazil. J Trop Ecol 15:463–479
Madeira BG, Espírito-Santo MM, D’Angelo-Neto S, Nunes YRF, Sánchez-Azofeifa GA, Fernandes GW, Quesada M (2009) Changes in tree and liana communities along a successional gradient in a tropical dry forest in south-eastern Brazil. Plant Ecol 291:291–304
Miles L, Newton AC, Defries RS, Ravilious C, May I, Blyth S, Kapos V, Gordon JE (2006) A global overview of the conservation status of tropical dry forests. J Biogeogr 33:491–505
Moran CV, Southwood TRE (1982) The guild composition of arthropod communities in trees. J Anim Ecol 51:289–306
Murphy PG, Lugo AE (1986) Ecology of tropical dry forest. Annu Rev Ecol Syst 17:67–88
Neves FS, Silva JO, Espírito-Santo MM, Fernades GW (2014) Insect herbivores and leaf damage along successional and vertical gradients in a tropical dry forest. Biotropica 46:14–24
Nichols E, Spector S, Louzada J, Larsen T, Amezquita S, Favila ME, Network TSR (2008) Ecological functions and ecosystem services provided by Scarabaeinae dung beetles. Biol Conserv 141:1461–1474
Novais SMA, Reis LEM, Rocha WD, Neves FS (2016) Effects of habitat management on different feeding guilds of herbivorous insects in cacao agroforestry systems. Rev Biol Trop 64:763–777
Novotny V, Basset Y (1998) Seasonality of sap-sucking insects (Auchenorrhyncha: Hemiptera) feeding on Ficus (Moraceae) in a lowland rain forest in New Guinea. Oecologia 115:514–522
Novotny V, Basset Y (2005) Host specificity of insect herbivores in tropical forests. Proc R Soc Lond 272:1083–1090
Novotny V, Wilson MR (1997) Why are there no small species among xylem-sucking insects? Evol Ecol 11:419–437
Novotny V, Basset Y, Auga J, Boen W, Dal C, Drozd P, Kasbal M, Isua B, Kutil R, Manumbor M, Molem K (1999) Predation risk for herbivorous insects on tropical vegetation: a search for enemy-free space and time. Aust J Ecol 24:477–483
Novotny V, Basset Y, Kitching R (2003) Herbivore assemblages and their food resources. In: Basset Y, Novotny V, Miller S, Kitching R (eds) Arthropods of tropical forests: spatio-temporal dynamics and resource use in the canopy. Cambridge University Press, Cambridge, pp 40–53
Pezzini FF (2008) Fenologia de comunidades arbóreas de mata seca em três estágios sucessionais. Master Dissertation. Universidade Federal de Minas Gerais, Belo Horizonte
Pezzini FF, Ranieri BD, Brandão D, Fernandes GW, Quesada M, Espírito-Santo MM, Jacobi CM (2014) Changes in tree phenology along natural regeneration in a seasonally dry tropical forest. Plant Biosyst 148:956–974
Pinheiro F, Diniz IR, Coelho D, Bandeira MSP (2002) Seasonal pattern of insect abundance in the Brazilian cerrado. Austral Ecol 27:132–136
Portillo-Quintero CA, Sánchez-Azofeifa GA (2010) Extent and conservation of tropical dry forests in the Americas. Biol Conserv 143:144–155
Price PW (2002) Resource-driven terrestrial interaction webs. Ecol Res 17:241–247
Quesada M, Sanchez-Azofeifa GA, Alvarez-Añorve M, Stoner KE, Avila-Cabadilla L, Calvo-Alvarado J, Castillo A, Espírito-Santo MM, Fagundes M, Fernandes GW, Gamon J, Lopezaraiza-Mikel M, Lawrence D, Morellato LPC, Powers JS, Neves F de S, Rosas-Guerrero V, Sayago R, Sanchez-Montoya G (2009) Succession and management of tropical dry forests in the Americas: review and new perspectives. For Ecol Manag 258:1014–1024
R Development Core Team (2014) R: a language and environment for statistical computing. R Foundation for Statistical Computing, Vienna. ISBN 3-900051-07-0. http://www.R-project.org
Rafael JA, Melo GAR, Carvalho CJB, Casari AS, Constantino R (2012) Os Insetos do Brasil: diversidade e taxonomia. Holos Editora, Ribeirão Preto
Ragusa-Netto JA, Silva RR (2007) Canopy phenology of a dry forest in western Brazil. Braz J Biol 67:569–575
Rakitov RA (2000) Secretion of brochosomes during the ontogenesis of a leafhopper, Oncometopia orbona (F.) (Insecta:Homoptera:Cicadellidae). Tissue Cell 32:28–39
Rakitov RA (2002) What are brochosomes for? An enigma of leafhoppers (Hemiptera, Cicadellidae). Denisia (Linz) 4:411–432
Sanchez-Azofeifa GA, Quesada M, Rodríguez JP, Nassar JM, Stoner KE, Castillo A, Garvin T, Zent EL, Calvo-Alvarado JC, Kalacska MER, Fajardo L, Gamon JA, Cuevas-Reyes P (2005) Research priorities for Neotropical dry forests. Biotropica 37:477–485
Santos MG, Oliveira MT, Figueiredo KV, Falcão HM, Arruda EP, Almeida-Cortez JS, Sampaio EVSB, Ometto JPHB, Menezes RSC, Oliveira AFM, Pompelli MF, Antonino ACD (2014) Caatinga, the Brazilian dry tropical forest: can it tolerate climate changes? Theor Exp Plant Physiol 26:83–99
Silva JO, Neves FS (2014) Insect herbivores associated with an evergreen tree Goniorrhachis marginata Taub. (Leguminosae: Caesalpinioideae) in a tropical dry forest. Braz J Biol 74:623–631
Silva JO, Espírito-Santo MM, Melo GA (2012) Herbivory on Handroanthus ochraceus (Bignoniaceae) along a successional gradient in a tropical dry forest. Arthropod-Plant Interact 6:45–57
Silva JO, Espírito-Santo MM, Morais HC (2015) Leaf traits and herbivory on deciduous and evergreen trees in a tropical dry forest. Basic Appl Ecol 16:210–219
Silva JO, Espírito-Santo MM, Fernandes GW (2016) Galling insect species richness and leaf herbivory in an abrupt transition between Cerrado and Tropical Dry Forest. Ann Entomol Soc Am 109:705–712
Springate ND, Basset Y (1996) Diel activity of arboreal arthropods associated with Papua New Guinean trees. J Nat Hist 30:101–112
Vasconcellos A, Andreazze R, Almeida AM, Araujo HFP, Oliveira ES, Oliveira U (2010) Seasonality of insects in the semi-arid Caatinga of northeastern Brazil. Rev Bras Entomol 54: 471–476
Villalobos SC, González-Carcacía JA, Rodríguez JP, Nassar J (2013) Interspecific and interannual variation in foliar phenological patterns in a successional mosaic of a dry forest in the central llanos of Venezuela. In: Sánchez-Azofeifa GA, Powers JS, Fernandes GW, Quesada M (eds) Tropical dry forests in the Americas. CRC Press, Boca Raton, pp 301–324
Walter J, Hein H, Auge H, Beierkuhnlein C, Löffler S, Reifenrath K, Schädler M, Weber M, Jentsch A (2012) How do extreme drought and plant community composition affect host plant metabolites and herbivore performance? Arthropod-Plant Interact 6:15–25
Wardhaugh CW (2014) The spatial and temporal distributions of arthropods in forest canopies: uniting disparate patterns with hypotheses for specialization. Biol Rev 89:1021–1041
Warton DI, Wright ST, Wang Y (2012) Distance-based multivariate analyses confound location and dispersion effects. Methods Ecol Evol 3:89–101
Acknowledgements
We are very grateful to A Scariot, J Hay and M Almeida-Neto for their valuable suggestions on early versions of the manuscript, and to TG Silva, JC Santos, A Mendes and SFM Silva for field assistance. We also thank the logistical support provided by the Instituto Estadual de Florestas (IEF), and the financial by Fundação de Amparo à Pesquisa de Minas Gerais-FAPEMIG, Conselho Nacional de Desenvolvimento Científico e Tecnológico (CNPq 563304/2010-3), the Inter-American Institute for Global Change Research (IAI-CRN II-021), and Decanato de Pesquisa e Pós-Graduação of the Universidade de Brasília (UnB). We gratefully acknowledge a scholarship of the Conselho Nacional de Desenvolvimento Científico e Tecnológico (CNPq) to Jhonathan O. Silva and Mário M. Espírito-Santo.
Author information
Authors and Affiliations
Corresponding author
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Silva, J.O., Leal, C.R.O., Espírito-Santo, M.M. et al. Seasonal and diel variations in the activity of canopy insect herbivores differ between deciduous and evergreen plant species in a tropical dry forest. J Insect Conserv 21, 667–676 (2017). https://doi.org/10.1007/s10841-017-0009-9
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10841-017-0009-9