Abstract
Population dynamics studies in insects mostly focus on a specific life stage of a species and seldom consider different stages. Here, we studied the population demography of a protected Maculinea alcon ‘cruciata’ population and the factors that could influence the distribution of eggs. The results of the mark-recapture survey showed a relatively short flight period between mid-June and mid-July with a clearly marked early peak period. Unlike in many other butterflies, protandry was not strong. The total population of M. alcon ‘cruciata’ was estimated at 699 individuals. The survival rate, and consequently the average life span, was relatively low. Eggs showed a highly aggregated pattern, and egg numbers were positively related to general shoot size, while the number of flower buds and the features of the surrounding vegetation did not display any effect on egg laying. Based on our findings, the studied population appears viable, but specific management techniques could ensure optimal conditions for egg laying in this protected butterfly.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Dynamics of insect populations, mostly in the case of pests, and more recently also in protected species, has been the subject of wide range of studies (Way and Heong 1994; Hunter 2001; Yamamura et al. 2006; Thomas et al. 2009). Most of these concentrate on a single life stage of an insect (e.g., adults, larvae), while usually neglecting parallel investigations into other developmental stages, or the connection between them (Elkinton and Liebhold 1990; Yamamura et al. 2006; Ordano et al. 2015). Admittedly, it is much easier, and therefore much more practical, to determine the viability of any population based solely on the abundance of adults, consequently many pest control and species conservation actions primarily rely on such information (Steytler and Samways 1995; Sunderland and Samu 2000; Thomas et al. 2009). In highly mobile insects, such as butterflies, the information on the existence of eggs in a given area connected to the presence of adults is of major relevance. Therefore, linking the dynamics of adults with, e.g., egg laying patterns can offer a more precise picture of the sustainability of populations in a given area, since the viability of a population is primarily determined by the number of offspring produced in the study area, i.e., in the case of insects by the number of eggs and/or larvae (Begon et al. 1996).
The population dynamics of adult butterflies is frequently connected to weather conditions and environmental stochasticity (Melbourne and Hastings 2008; Nowicki et al. 2009; Cormont et al. 2013), while adult egg laying decisions, and thus the fate of their offspring, primarily relies on the condition of host plants, the strength of intraspecific competition, predatory pressure or other habitat parameters (Stamp 1980 for a review, Bergman 2001; Czekes et al. 2014; Patricelli et al. 2015). Ovipositing females have to choose the optimal site for their offspring, as well as the best available host plant within the site. Additionally, the survival of the offspring can also be affected by the host plants’ direct or indirect responses to the presence of eggs and/or larvae as well (see Hilker and Fatouros 2015 for a review).
Large Blue butterflies of the genus Maculinea Van Eecke, 1915 (synonymised lately with Phengaris Doherty, 1891) are one of the most intensively studied butterfly groups in Europe, being considered flagship and umbrella species in nature conservation. They are highly sensitive to habitat changes, and the conservation of their habitats is beneficial to many other threatened species (Thomas and Settele 2004; Settele et al. 2005). In the past decades severe declines were recorded in most of their Western European populations due to habitat fragmentation and intensification of agriculture (Van Swaay and Warren 1999; Van Swaay et al. 2010). They also raise specific scientific interest due to the intriguing obligate myrmecophylic lifestyle of their larvae (see Witek et al. 2014). Most Maculinea populations are small and isolated (Thomas et al. 1998), characterized by density dependent regulation due to intra-specific competition between larvae on host plants and/or in host ant colonies (Hochberg et al. 1992; Nowicki et al. 2009). Long-term surveys have already shown the importance of weather patterns (Roy et al. 2001; Cormont et al. 2013), but Maculinea populations are also affected by general habitat characteristics (Nowicki et al. 2007), and human activities (e.g., changes in agricultural practices) (Schmitt and Rákosy 2007).
In the case of Maculinea alcon there are two major ecotypes differentiated based on their host plants: the hygrophilous form feeding on Gentiana pneumonanthe (previously treated as M. alcon), and the xerophilous form feeding on G. cruciata (previously treated as M. rebeli, hereafter referred to as M. alcon ‘cruciata’). Recent molecular studies showed that the two forms cannot be regarded as different species (Bereczki et al. 2005; Steiner et al. 2006; Pecsenye et al. 2007). Nevertheless, in addition to habitat and host plant segregation, they typically use different host ant species, and they also have different flight periods (Bálint 1994; Sielezniew et al. 2012), therefore they ought to be treated as separate Evolutionary Significant Units worthy of specific conservation status (Independent Conservation Units) (see Casacci et al. 2014 for a review). Moreover, the North-East European populations of the ‘cruciata’ ecotype are endangered (see Casacci et al. 2014), therefore comprehensive data is of major relevance for conservation management plans. The Romanian populations are outstanding in addition, since it is here that the two ecotypes co-occur syntopically (see Czekes et al. 2014).
Although there are a relatively large number of both field and modelling studies on the ecology of M. alcon ‘cruciata’ (e.g., Hochberg et al. 1992; Czekes et al. 2014) in which data concerning population parameters and data concerning egg laying patterns were combined (see Meyer-Hozak 2000; Kőrösi et al. 2008), there are no studies available investigating adult population size and egg laying patterns the data for which originate in the same time period. In addition, there is a need for complex information on populations of protected butterflies, but such studies are generally rare in the case of other butterfly species as well (see Bergman 2001). Consequently, the aims of our research were to (a) study the within-season dynamics of an adult M. alcon ‘cruciata’ population, while also (b) examining the temporal changes in the deposition of eggs, and (c) identifying the factors influencing the distribution of eggs.
Materials and methods
Study species and site
Maculinea alcon ‘cruciata’ prefers semi-natural calcareous grasslands (Rákosy and Vodă 2008), and it uses quite a wide range of host ant species from the genus Myrmica Latreille, 1804, which adopt them due to their efficient chemical and acoustical mimicry (see Fiedler 2006 and Witek et al. 2014 for a review). Their development continues inside the ant nest, where they are fed by the ant workers (Elmes et al. 1991). The flight period of adult butterflies is from mid-June to mid-July (Kőrösi et al. 2008). The conservation status of M. alcon is Least Concern according to the IUCN Red List in Europe and Near Threatened in the European Union (Van Swaay et al. 2010).
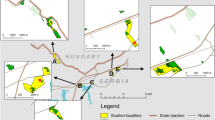
The field study was performed on a 9,252 m2 semi-natural calcareous dry grassland of southeastern exposure in the surroundings of Rimetea village (N46°27′51.45″, E23°33′46.26″, ca. 620 m a.s.l., Romania). The grassland is a plant species-rich meadow dominated by Brachypodium pinnatum, Carex humilis and Festuca rupicola with other characteristic species including Dorycnium pentaphyllum, Cytisus albus, Hieracium bauhinii, Teucrium montanum and Thymus serpyllum, and it is intensively grazed by goats and sheep. The meadow is partially surrounded by a mixed forest and shrubs of Crataegus monogyna, Prunus spinosa, Pyrus pyraster and Rosa canina. The site is part of the ROSCI0253 ‘Trascău’ Natura 2000 protected site.
Data collection
Population dynamics survey
A mark-recapture study of adult M. alcon ‘cruciata’ butterflies was conducted between 15 June and 16 July 2012 covering the entire flight period. The survey plan followed the requirements of the Pollock’s Robust Design approach (Pollock 1982; Pollock et al. 1990), i.e. relatively infrequent but highly intensive capture days were established, which constituted primary sampling periods. The sampling took place on every fourth day, with a single exceptional case in which the interval between consecutive capture days was reduced to 3 days due to the forecast of unfavourable weather conditions on the following days. Butterflies were surveyed between 10 am and 5 pm during five one-hour capture sessions, separated by 30 min breaks to allow free mixing of butterflies between the sessions. These sessions were regarded as independent capture occasions, constituting secondary sampling periods of the Robust Design. In other words, recaptures occurring on the same day but within different sessions were treated as independent events and they were used for estimating the number of butterflies present on that particular day.
Captured individuals were marked on the underside of their hind-wing with unique identity numbers using a fine-tipped waterproof pen (© Schneider GmbH), and then immediately released at the place of capture. For each capture we recorded the date, the exact time and the position of each capture (GPS coordinates), as well as the identity number and the sex of the adult.
Distribution of butterfly eggs
Prior to the adult butterfly survey, we randomly placed out 22 sampling plots within the study site. The plots were circles of 2 m radius, as generally applied in the case of Maculinea species based on the average foraging radius of the host ant Myrmica (see Elmes et al. 1998), with a focal G. cruciata plant in the middle. Within the plots we recorded the number of all G. cruciata host plants, and the number of their shoots. Shoots were considered to belong to the same plant when they were obviously connected above the soil surface. In order to minimise disturbance, we recorded the number of eggs on the shoots of the focal host plant within each plot (n = 22) only at the end of each mark-recapture sampling day. At the end of the whole study period we counted all eggs found on all host plant shoots within the sampling plots in addition to the characteristics of the host plants and general vegetation features. The following parameters were recorded: (a) the total number of butterfly eggs laid on the host plant shoots, and separately on different verticils, (b) shoot height as the length of the shoot (cm), (c) number of shoot leaves, and (d) number of flowers (only flower buds with coloured sepals were taken into account since small green flower buds are impossible to count sometimes) on separate verticils of shoots, (e) the number of host plants in each plot, (f) the maximum height of the surrounding vegetation (cm), and (g) the proportion of vegetation cover visually estimated to the nearest 5 %.
Data analysis
Mark-recapture data was analysed with the use of Mark 7.0 program (White and Burnham 1999) applying the Robust Design (RD) model (Pollock 1982; Kendall et al. 1995). The RD model allows relatively high precision of population estimates, and it has proved its applicability in butterfly population studies (Nowicki et al. 2008). The analyses were conducted separately for males and females, because sex-specific population parameters were of interest for our study. The data from capture sessions (i.e. secondary periods of the RD model) within sampling days were used to estimate daily population sizes for these days (N i ). In the estimation we accounted for individual heterogeneity in capture probabilities, since its existence was revealed by the tests for violations to the equal catchability assumption (Otis et al. 1978; Chao 1988). In turn, the data pooled together within capture days (i.e. primary periods of the RD model) were used for assessing survival rate between these days (φ i ). The model variant assuming no time variation in survival rate performed the best as indicated by its lowest value of the Akaike Information Criterion corrected for small samples (AICc; Hurvich and Tsai 1989), which implies that adult survivorship was fairly constant throughout the flight period. Subsequently, we calculated the average adult lifespan as e = (1 − φ)−1 − 0.5 (Nowicki et al. 2005a).
Based on the estimates of daily population sizes and survival rates, we also estimated the recruitment (B i ), i.e. the numbers of individuals eclosing from pupae and entering the adult population during the intervals between consecutive capture days. As the adult life span was relatively short when compared with the length of these intervals (d i ), we used the formula of Nowicki et al. (2005a; see this reference for the rationale), which accounts for the individuals eclosing and dying within the same intervals: B i ′ = d × (N i+1 − N i ·φ d) × (φ − 1)/(φ d − 1). The sum of recruitment for the entire flight period makes-up the seasonal population size (N total ). In a similar way, by summing female recruitment prior to each capture day, we derived the total number of females present.
Poulin’s discrepancy index (Poulin 1993) was used to characterize the distribution of eggs on host plant shoots. The index is generally used to describe the tendency of a parasite to aggregate, or overdisperse within the host population. We applied it to characterize the distribution of eggs among host plant shoots. Biases in the distribution of eggs among different host plant verticils were checked using a Generalized Linear Mixed Model approach (GLMM, Poisson error, maximum likelihood approximation; n = 133). The number of eggs laid on different verticils of egg-bearing plant shoots was introduced as dependent variable, and the ID of verticil as independent factor. Sampling plot and plant IDs were introduced as nested random factors to handle dependency of data. Only egg data from the top four verticils were taken into account since no eggs were recorded on lower verticils.
We tested the relationship between the estimated number of females present before each sampling day, and the total number of eggs laid in the same period (n = 8) in order to reveal whether the number of eggs laid was related to the number of female butterflies. Spearman rank correlation analysis was applied due to the lack of normality of both variables. In addition, the effect of the abundance of eggs already present on oviposition was checked by testing the relationship between the number of eggs present and the number of newly-laid eggs in the following period for seven consecutive periods between the eight sampling days. Again, Spearman rank correlation analysis was applied in this case.
The effects of host plant and vegetation characteristics on egg distribution were analyzed using GLMM (Poisson error, maximum likelihood approximation; n = 410). Correlation between host plant characteristics were checked using Spearman rank correlation analysis due to non-normality of datasets. A principal component analysis (PCA) was applied to obtained uncorrelated derived variables for plant characteristics, and the principal components were used as independent variables in the GLMM analysis. The number of eggs laid on each focal host plant shoot was introduced as a dependent variable, while independent variables were the host plant shoot morphological characteristics [PC1 (correlated shoot height and number of leaves) and PC2 (correlated number of flower buds)], the number of host plants in sample plots, the maximum height of the surrounding vegetation, and vegetation cover. Sampling plot and host plant IDs were introduced as random factors to handle dependencies. Automated model selection procedure was carried out, and the effects of different explanatory variables were averaged across the supported models with delta AICc <4, i.e. those with the AICc differing by <4 from the best model (see Grueber et al. 2011).
All statistical analyses were carried out using the R 3.1.1 Statistical Environment (R Development Core Team 2014) and Quantitative Parasitology 3.0 (Rózsa et al. 2000). Normality of datasets was regularly checked with the Shapiro–Wilk test. The Relevel function was used in order to carry out post hoc sequential comparisons among factor levels when performing GLMM. GLMMs were carried out with the use of the glmer function in the package lme4 (Bates et al. 2015), and the dredge function in the MuMIn package (Barton 2015) was applied for automated model selection. Table-wide Bonferroni-Holm correction was applied in the case of sequential comparisons, such as Spearman rank correlations and comparison of factor levels in the GLMM analysis concerning the location of eggs on different verticils.
Results
Demography and dynamics of adult butterflies
During the entire study we captured and marked 152 (67.5 %) males and 73 (32.5 %) females, out of which 85 males and 14 females, respectively, were recaptured at least once. The total adult population was assessed at 699 individuals, with a relatively balanced sex ratio (55 % males vs. 45 % females) (Table 1). The estimated survival was fairly low, which translates to a rather short adult lifespan of ca. 2 days with no major inter-sexual difference (Table 1).
The butterfly had a relatively short flight period between mid-June and mid-July, with a clearly pronounced peak occurrence in the early part of the period (Fig. 1). More than 50 % of individuals emerged within the first week, and more than 80 % within the first two weeks (Fig. 1). Besides, in comparison to many other butterfly species, we found rather weak protandry (cf. Pfeifer et al. 2000; Nowicki et al. 2005a): the number of females peaked only three days after the peak of males. Most of the butterflies clearly preferred the close proximity of shrubs (Fig. 2).
Egg laying dynamics and preferences
At the end of the study altogether 94 eggs were recorded on a total of 410 G. cruciata shoots of 201 plants within the 22 study plots. More than 90 % of the shoots lacked eggs, and the maximum number of eggs on a single shoot was 23. The overall mean egg density was 0.47 eggs/plant, and 0.23 eggs/shoot (4.48 eggs/plant and 4.09 eggs/shoot for plants with eggs); while the mean host plant density was 0.72 plants/m2 (9.13 plants/plot), and 1.48 shoots/m2 (18.6 shoots/plot). The distribution of eggs on plants showed a highly aggregated pattern (Fig. 3) as indicated by Poulin’s discrepancy index (D = 0.97). Eggs were laid only on the top four verticils of the plants. Most eggs were laid on the 2nd verticil (33.93 % of total), but no significant differences were revealed between the number of eggs on the different verticils (GLMM, z ≤ 1.487, p = NS, n = 133).
Eggs were recorded even during the first part of the study period on the focal host plants of the sampling plots (n = 22), even if less <10 % of the focal plants bore eggs on the 2nd sampling day (22.06). By the 6th sampling day (08.07) 63 % of the plants had eggs, after this the percentage of egg bearing plants decreased (Fig. 4). The number of eggs laid before each capture period did not correlate with the number of females recruited in the same period (Spearman r = 0.53, p = 0.13, n = 8). In turn, the number of newly-laid eggs correlated negatively with the number of eggs already present on host plants, the negative correlations between the two variables reached statistical significance during all but two sampling periods (Table 2).
Host plant morphological characteristics were mostly correlated according to the results of the Spearman rank-correlation analysis (n = 410): shoot height versus number of leaves r = 0.5, p < 0.001; number of leaves versus number of flower buds r = 0.25, p < 0.001; shoot height versus number of flower buds r = 0.10, p < 0.05. The PCA yielded 1st (PC1) and 2nd (PC2) principal components that explained 52 and 31 % of the variance, respectively. PC1 represented shoot height and number of leaves with loadings of 0.66 and 0.68, respectively, as a measure of general shoot size, while PC2 reflected the number of flower buds with a loading of 0.94. All input variables were retained in the best average GLMM model for egg laying preferences (see Tables 3, 4 for details on the best average model), but only the general shoot size (PC1) had a significant positive effect on the number of eggs laid (Table 4). Thus, there were more eggs on taller shoots with more leaves (Fig. 5). None of the other input variables displayed any significant effects (Table 4).
Discussion
The results of the present study show that the butterfly population studied appears fairly viable based on the comparison with other studies concerning the size of M. alcon ‘cruciata’ populations. During a 3 year long MRR study Árnyas et al. (2005) found that on a 0.75 ha site the studied M. alcon ‘cruciata’ population was stable with nearly 1,000 individuals, while Timuş et al. (2013) estimated the size of another population in Romania to 1,073 individuals for a 40 ha site. In comparison, the size of our studied population (699 individuals on ~1 ha) suggests that the population is relatively big. Generally, Maculinea alcon populations show very small fluctuations (Hochberg et al. 1994; Elmes et al. 1996), thus there is a considerable chance that our studied population is stable as well.
Similarly to other European populations (Meyer-Hozak 2000; Árnyas et al. 2005), we found that the butterflies fly from mid-June to mid-July. In some cases the flight period takes <1 month (Timuş et al. 2013), which can reflect differences in habitat or/and meteorological conditions of different populations. Although we found a relatively weak indication of protandry, the peak emergence of males still preceded that of females. This phenomenon is in fact common for all Maculinea species and for butterflies in general. According to Elmes and Thomas (1987) the males pupate a few days before females, and thus during the initial part of the flight period the population is dominated by males. During the entire study we caught roughly twice as many male individuals as female, but the estimated sex ratio was relatively balanced, which corresponds with results of other studies (Árnyas et al. 2005; Timuş et al. 2013). Considerably higher capture and recapture rates of males may be attributed also to the fact that they fly more often and higher searching for the less mobile females. The latter tend to fly lower because they are searching for food plants in the undergrowth (Árnyas et al. 2005).
Phytophagous butterfly species mostly lay their eggs separately one by one or in clusters (Thompson and Pellmyr 1991). Both strategies can positively influence the survival of eggs and larvae. Females can lower the chances of predation and competition for their offspring by depositing their eggs individually. In these cases eggs are usually cryptic (light yellow or green) and are laid on protected parts of the host plants (see Stamp 1980). Laying eggs in clusters can be advantageous when other factors can affect negatively the reproduction, like the patchy distribution of host plants, the scarcity of resources for larvae and adults, low population density or unfavorable weather conditions (Stamp 1980; Karlsson and Johansson 2008). Besides, as clusters of eggs and larvae are more protected from desiccation when clumped together, clusters can ensure higher survivability through lower sensitivity to ambient conditions (Stamp 1980; Clark and Faeth 1998). During our study we found a low mean egg density per host plant (0.47 eggs/plant) compared to that recorded by Czekes et al. (2014) in another population (8.89 eggs/plant). In addition, the distribution of eggs among host plants showed a clearly aggregated pattern, thus most of the eggs were concentrated only on a few host plants. This could suggest the patchy distribution of host ant colonies, since some studies indicate that ovipositing females in some Maculinea species could detect the presence of host ants indirectly (Van Dyck et al. 2000; Patricelli et al. 2015; Wynhoff et al. 2015), although there is no evidence that this holds true for all Maculinea (Thomas and Elmes 2001; Nowicki et al. 2005b; Fürst and Nash 2010). The recent comprehensive study of Patricelli et al. (2015) showed that egg laying M. arion butterflies may detect the host ant colonies by sensing the defensive volatile compounds released by those host plants which have host ant colonies residing in their roots. A similar mechanism might help M. a. ‘cruciata’ to detect its host ants, but further inquiries would be required to confirm it.
The number of females did not explain the number of newly-laid eggs, which can also be attributed to the emigration of females. During the study we observed a few females flying out of the study site. It is possible that some of these females also laid their eggs in the surrounding land fragments, as we noticed eggs laid outside the investigated area. Another cause of this result could be the loss of a large number of eggs during the egg laying season due to predation (Bergman 2001), but also to meteorological factors and the grazing of host plants (authors’ unpubl. data; M. Dolek pers. comm.). There was a negative relationship between the number of eggs already present on the plants and the quantity of newly-laid eggs. This result could indicate that females would prefer ovipositing on empty plants or at least with a small number of eggs present only. However, this evidence is very circumstantial and would require a specifically-designed study for confirmation (see e.g. Kőrösi et al. 2008).
Earlier studies about egg laying preferences showed that the most important factors influencing oviposition are the morphological characteristics of host plants, such as the height of the plant, the number, the size and the phenology of buds, and the number of leaves (e.g. Czekes et al. 2014; Wynhoff et al. 2015). In concordance with the aforementioned studies, our research suggests that females preferred the taller shoots with many leaves for oviposition. A visually conspicuous host plant (i.e. tall ones with many leaves) may be more attractive or more perceptible for females than smaller ones (Czekes et al. 2014; Wynhoff et al. 2015). The large number of eggs on tall plants with a large number of leaves can be beneficial for the butterflies due to an increased egg laying surface, decreased larval competition, or even better climatic conditions. Wynhoff et al. (2015) suggested that larger host plants might provide high quantities of food for the caterpillars as they would subsequently develop more fully-developed flower buds than smaller plants. Maculinea alcon ‘cruciata’ females laid their eggs exclusively on the four top verticils of their host plants, which could be attractive sites for oviposition presumably also because of the lower predation risk for adult females (Van Dyck and Regniers 2010) and the better microclimate for larval development (Alonso 2003). In addition, ovipositing mostly on the 2nd verticil from the top, as suggested by our data, could ensure better climatic conditions to eggs through reduced exposure to sun and wind.
Our findings also highlight the vulnerability of the studied M. alcon ‘cruciata’ population. The discrepancy between the number of females and the number of eggs, and the reduced lifespan of adults compared to other European populations suggests that the long-term stability of our population might be threatened. Linking population demography data to oviposition preferences could help the protection of the focal butterfly species not only by offering data to nature conservationists, but also by revealing that specific management techniques could ensure better conditions for egg laying. Specifically, sustaining a low grazing pressure could have a positive effect on the butterfly population (WallisDeVries and Raemakers 2001), and it would also keep shrubs from invading the grassland. Unfortunately, the solely species-based approach of current European legislation (see Casacci et al. 2014 for discussion therein) hinders the elaboration and application of specific management plans for particular ecotypes as e.g. M. alcon ‘cruciata’. However, the survival of traditional land use strategies in the study region, and more widely in Transylvania, Romania, may offer a good chance for the survival of this very specific ecotype of Maculinea alcon.
References
Alonso C (2003) Choosing a place to grow. Importance of within plant abiotic microenvironment for Yponomeuta mahalebella. Entomol Exp Appl 83:171–180. doi:10.1046/j.1570-7458.1997.00169.x
Árnyas E, Bereczki J, Tóth A, Varga Z (2005) Results of the mark-release-recapture studies of a Maculinea rebeli population in the Aggtelek karst (N Hungary) between 2002–2004. In: Settele J, Kühn E, Thomas J (eds) Studies on the ecology and conservation of butterflies in Europe. Vol. 2. Species ecology along a European Gradient: Maculinea butterflies as a model. Pensoft Publishers, Sofia-Moscow, pp 111–114
Bálint ZS (1994) Magyarország nappali lepkéi a természetvédelem tükrében (Lepidoptera, Rophalocera). Somogyi Múzeumok Közleményei 10:183–206
Barton K (2015) MuMIn: Multi-model inference. R package version 1.10.5. http://CRAN.R-project.org/package=MuMIn
Bates D, Mächler M, Bolker BM, Walker SC (2015) Fitting linear mixed-effects models using lme4. J Stat Softw 67(1):1–48. doi:10.18637/jss.v067.i01
Begon M, Mortimer M, Thompson DJ (1996) Population ecology: a unified study of animals and plants. Blackwell Science, Hoboken. doi:10.1002/9781444313765.fmatter
Bereczki J, Pecsenye K, Peregovits L, Varga Z (2005) Pattern of genetic differentiation in the Maculinea alcon species group (Lepidoptera, Lycaenidae) in Central Europe. JZS 43:157–165. doi:10.1111/j.1439-0469.2005.00305.157-165
Bergman K-O (2001) Population dynamics and the importance of habitat management for conservation of the butterfly Lopinga achine. J Appl Ecol 38:1303–1313. doi:10.1046/j.0021-8901.2001.00672.x
Casacci LP, Barbero F, Balletto E (2014) The “Evolutionary Significant Unit” concept and its applicability in biological conservation. Ital J Zool 81:182–193. doi:10.1080/11250003.2013.870240
Chao A (1988) Estimating animal abundance with capture frequency data. J Wildl Manag 52:295–300. doi:10.2307/3801237
Clark BR, Faeth SH (1998) The evolution of egg clustering in butterflies: a test of the egg desiccation hypothesis. Evol Ecol 12:543–552. doi:10.1023/A:1006504725592
Cormont A, Wieger Wamelinka GW, Jochema R, WallisDeVries MF, Wegmana RMA (2013) Host plant-mediated effects of climate change on the occurrence of the Alcon blue butterfly (Phengaris alcon). Ecol Model 250:329–337. doi:10.1016/j.ecolmodel.2012.11.022
Czekes ZS, Markó B, Nash DR, Ferencz M, Lázár B, Rákosy L (2014) Differences in oviposition strategies between two ecotypes of the endangered myrmecophilous butterfly Maculinea alcon (Lepidoptera: Lycaenidae) under unique syntopic conditions. Insect Conserv Divers 7:122–131. doi:10.1111/icad.12041
Elkinton JS, Liebhold AM (1990) Population dynamics of gypsy moth in North America. Annu Rev Entomol 35:571–596. doi:10.1146/annurev.en.35.010190.003035
Elmes GW, Thomas JA (1987) Die Gattung Maculinea. In: Geiger W (ed) Tagfalter und ihre Lebensraeume: Arten, Gefaehrdung, Schutz. Schweizerische Bund für Naturschutz, Basel, pp 354–368
Elmes GW, Wardlaw JC, Thomas JA (1991) Larvae of Maculinea rebeli, a large blue butterfly, and their Myrmica host ants: patterns of caterpillar growth and survival. J Zool 224:79–92. doi:10.1111/j.1469-7998.1991.tb04789.x
Elmes GW, Clarke RT, Thomas JA, Hochberg ME (1996) Empirical tests of specific predictions made from a spatial model of the population dynamics of Maculinea rebeli, a parasitic butterfly of red ant colonies. Acta Oecol 17:61–80
Elmes GW, Thomas JA, Wardlaw JC, Hochberg ME, Clarkel RT, Simcox DJ (1998) The ecology of Myrmica ants in relation to the conservation of Maculinea butterflies. J Insect Conserv 2:67–78. doi:10.1023/A:1009696823965
Fiedler K (2006) Ant-associates of Palaearctic lycaenid butterfly larvae (Hymenoptera: Formicidae; Lepidoptera: Lycaenidae)—a review. Myrmecol Nachr 9:77–87
Fürst MA, Nash DR (2010) Host ant independent oviposition in the parasitic butterfly Maculinea alcon. Biol Lett 6:174–176. doi:10.1098/rsbl.2009.0730
Grueber CE, Nakagawa S, Laws RJ, Jamieson IG (2011) Multimodel inference in ecology and evolution: challenges and solutions. J Evol Biol 24:699–711
Hilker M, Fatouros NE (2015) Plant responses to insect egg deposition. Annu Rev Entomol 60:493–515. doi:10.1146/annurev-ento-010814-020620
Hochberg ME, Thomas JA, Elmes GW (1992) A modelling study of the population dynamics of a large blue butterfly, Maculinea rebeli, a parasite of red ant nests. J Anim Ecol 61:397–409. doi:10.2307/5331
Hochberg ME, Clarke RT, Elmes GW, Thomas JA (1994) Population dynamic consequences of direct and indirect interactions involving a large blue butterfly and its plant and red ant hosts. J Anim Ecol 63:375–391. doi:10.2307/5555
Hunter MD (2001) Insect population dynamics meets ecosystem ecology: effects of herbivory on soil nutrient dynamics. Agric For Entomol 3:77–84. doi:10.1046/j.1461-9563.2001.00100.x
Hurvich CM, Tsai C (1989) Regression and time series model selection in small samples. Biometrika 76:297–307. doi:10.1093/biomet/76.2.297
Karlsson B, Johansson A (2008) Seasonal polyphenism and developmental trade-offs between flight ability and egg laying in a pierid butterfly. Proc R Soc B 275:2131–2136. doi:10.1098/rspb.2008.0404
Kendall WL, Pollock KH, Brownie C (1995) A likelihood-based approach to capture-recapture estimation of demographic parameter under the robust design. Biometrics 51:293–308
Kőrösi Á, Örvössy N, Batáry P, Kövér S, Peregovits L (2008) Restricted within habitat movement and time-constrained egg laying of female Maculinea rebeli butterflies. Oecologia 156:455–464. doi:10.1007/s00442-008-0986-1
Melbourne BA, Hastings A (2008) Extinction risk depends strongly on factors contributing to stochasticity. Nature 454:100–103. doi:10.1038/nature06922
Meyer-Hozak C (2000) Population biology of Maculinea rebeli (Lepidoptera: Lycaenidae) on the chalk grasslands of Eastern Westphalia (Germany) and implications for conservation. J Insect Conserv 4:63–72. doi:10.1023/A:1009695031802
Nowicki P, Witek M, Skórka P, Settele J, Woyciechowski M (2005a) Population ecology of the endangered butterflies Maculinea teleius and M. nausithous, and its implications for conservation. Popul Ecol 47:193–202. doi:10.1007/s10144-005-0222-3
Nowicki P, Witek M, Skórka P, Woyciechowski M (2005b) Oviposition patterns in the myrmecophilous butterfly Maculinea alcon Denis & Schiffermüller (Lepidoptera: Lycaenidae) in relation to characteristics of foodplants and presence of ant hosts. Pol J Ecol 53:409–417
Nowicki P, Pepkowska A, Kudlek J, Skórka P, Witek M, Settele J, Woyciechowski M (2007) From metapopulation theory to conservation recommendations: lessons from spatial occurence and abundence patterns of Maculinea butterflies. Biol Conserv 140:119–129. doi:10.1016/j.biocon.2007.08.001
Nowicki P, Settele J, Henry P-Y, Woyciechowski M (2008) Butterfly monitoring methods: the ideal and the real world. Isr J Ecol Evol 54:69–88. doi:10.1560/IJEE.54.1.69
Nowicki P, Bonelli S, Barbero F, Balletto E (2009) Relative importance of density-dependent regulation and environmental stochasticity for butterfly population dynamics. Oecologia 161:227–239. doi:10.1007/s00442-009-1373-2
Ordano M, Engelhard I, Rempoulakis P, Nemny-Lavy E, Blum M, Yasin S, Lensky IM, Papadopoulos NT (2015) Fruit fly (Bactrocera oleae) population dynamics in the Eastern Mediterranean: influence of exogenous uncertainty on a monophagous frugivorous insect. PLoS ONE 10(5):e0127798. doi:10.1371/journal.pone.0127798
Otis DL, Burnham KP, White DC, Anderson DR (1978) Statistical inference from capture data on closed animal populations. Wildl Monogr 62:1–135
Patricelli D, Barbero F, Occhipinti A, Bertea CM, Bonelli S, Casacci LP, Zebelo SA, Crocoll C, Gershenzon J, Maffei ME, Thomas JA, Balletto E (2015) Plant defences against ants provide a pathway to social parasitism in butterflies. Proc R Soc B 282:20151111. doi:10.1098/rspb.2015.1111
Pecsenye K, Bereczki J, Tihanyi B, Tóth A, Peregovits L, Varga L (2007) Genetic differentiation among the Maculinea species (Lepidoptera: Lycaenidae) in eastern Central Europe. Biol J Linnean Soc 91:11–21. doi:10.1111/j.1095-8312.2007.00781.x
Pfeifer MA, Andrick UR, Frey W, Settele J (2000) On the ecology of a small and isolated population of the Dusky Large Blue Butterfly Glaucopsyche (Maculinea) nausithous (Lycaenidae). Nota Lepid 23:147–172
Pollock KH (1982) A capture-recapture design robust to unequal probability of capture. J Wildl Manage 46:752–757. doi:10.2307/3808568
Pollock KH, Nichols JD, Brownie C, Hines JE (1990) Statistical inference for capture recapture experiments. Wildl Monogr 107:1–97
Poulin R (1993) The disparity between observed and uniform distribution: a new look at parasite aggregation. Int J Parasitol 23(7):937–944. doi:10.1016/0020-7519(93)90060-C
R Core Team (2014) R: a language and environment for statistical computing. R Foundation for Statistical Computing, Vienna, Austria. http://www.Rproject.org/
Rákosy L, Vodă R (2008) Distribution of Maculinea genus in Romania. Entomol Rom 13:9–17
Roy DB, Rothery P, Moss D, Pollard E, Thomas JA (2001) Butterfly numbers and weather: predicting historical trends in abundance and the future effects of climate change. J Anim Ecol 70:201–217. doi:10.1111/j.1365-2656.2001.00480.x
Rózsa L, Reiczigel J, Majoros G (2000) Quantifying parasites in samples of hosts. J Parasitol 86(2):228–232. doi:10.1645/0022-3395(2000)086[0228:QPISOH]2.0.CO;2
Schmitt T, Rákosy L (2007) Changes of traditional agrarian landscapes and their conservation implications: a case study of butterflies in Romania. Divers Distrib 13:855–862. doi:10.1111/j.1472-4642.2007.00347.x
Settele J, Kühn E, Thomas J (eds) (2005) Studies on the ecology and conservation of butterflies in Europe. Vol. 2. Species ecology along a European Gradient: Maculinea butterflies as a model. Pensoft Publishers, Sofia-Moscow
Sielezniew M, Rutkowski R, Ponikwicka-Tyszko D, Ratkiewicz M, Dziekanska I, Svitra G (2012) Differences in genetic variability between two ecotypes of the endangered myrmecophilous butterfly Phengaris (=Maculinea) alcon—the setting of conservation priorities. Insect Conserv Divers 5:223–236. doi:10.1111/j.1752-4598.2011.00163.x
Stamp NE (1980) Egg deposition patterns in butterflies: why do some species cluster their eggs rather than deposit them singly? Am Nat 115:367–380
Steiner FM, Schlick-Steiner BC, Höttinger H, Nikiforov A, Moder K, Christian E (2006) Maculinea alcon and M. rebeli (Insecta: Lepidoptera: Lycaenidae)—one or two Alcon Blues? Larval cuticular compounds and egg morphology of East Austrian populations. Ann Naturhist Mus Wien 107B:165–180
Steytler SN, Samways MJ (1995) Biotope selection by adult male dragonflies (Odonata) at an artificial lake created for insect conservation in South Africa. Biol Conserv 72:381–386. doi:10.1016/0006-3207(94)00052-R
Sunderland K, Samu F (2000) Effects of agricultural diversification on the abundance, distribution, and pest control potential of spiders: a review. Entomol Exp Appl 95:1–13. doi:10.1023/A:1003986225443
Thomas JA, Elmes GW (2001) Food-plant niche selection rather than the presence of ant nests explains oviposition patterns in the myrmecophilous butterfly genus Maculinea. Proc R Soc Lond B 268:471–477. doi:10.1098/rspb.2000.1398
Thomas JA, Settele J (2004) Butterfly mimics of ants. Nature 432:283–284. doi:10.1038/432283a
Thomas JA, Clarke RT, Elmes GW, Hochberg ME (1998) Population dynamics in the genus Maculinea (Lepidoptera: Lycaenidae). In: Dempster JP, McLean IFG (eds) Insect population in theory and in practice. Chapman and Hall, London, pp 261–290. doi:10.1007/978-94-011-4914-3_11
Thomas JA, Simcox DJ, Clarke RT (2009) Successful conservation of a threatened Maculinea butterfly. Science 325:80–83. doi:10.1126/science.1175726
Thompson JN, Pellmyr O (1991) Evolution of oviposition behavior and host preference in Lepidoptera. Annu Rev Entomol 36:65–89. doi:10.1146/annurev.en.36.010191.000433
Timuş N, Craioveanu C, Sitaru C, Rus A, Rákosy L (2013) Differences in adult phenology, demography, mobility and distribution in two syntopic ecotypes of Maculinea alcon (cruciata vs. pneumonanthe) (Lepidoptera: Lycaenidae) from Transilvania (Romania). Entomol Rom 18:21–30
Van Dyck H, Regniers S (2010) Egg spreading in the ant-parasitic butterfly, Maculinea alcon: from individual behavior to egg distribution pattern. Anim Behav 80:621–627. doi:10.1016/j.anbehav.2010.06.021
Van Dyck H, Oostermeijer JGB, Talloen W, Feenstra V, Van der Hidde A, Wynhoff I (2000) Does the presence of ant nests matter for oviposition to a specialized myrmecophilous Maculinea butterfly? Proc R Soc Lond B 267:861–866. doi:10.1098/rspb.2000.1082
Van Swaay C, Warren M (1999) Red data book of European butterflies (Rhopalocera). Nature and Environment 99, Council of Europe Publishing, Strasbourg
Van Swaay C, Cuttelod A, Collins S, Maes D, Munguira ML, Šašić M, Settele J, Verovnik R, Verstrael T, Warren M, Wiemers M, Wynhoff I (2010) European red list of butterflies. Publications Office of the European Union, Luxembourg. doi:10.2779/83897
WallisDeVries MF, Raemakers I (2001) Does extensive grazing benefit butterflies in coastal dunes? Restor Ecol 9:179–188. doi:10.1046/j.1526-100x.2001.009002179.x
Way MJ, Heong KL (1994) The role of biodiversity in the dynamics and management of insect pests of tropical irrigated rice—a review. Bull Entomol Res 84:567–587. doi:10.1017/S000748530003282X
White GC, Burnham KP (1999) Program MARK: survival estimation from populations of marked animals. Bird Study 46:120–138. doi:10.1080/00063659909477239
Witek M, Barbero F, Markó B (2014) Myrmica ants host highly diverse parasitic communities: from social parasites to microbes. Insectes Soc 61:307–323. doi:10.1007/s00040-014-0362-6
Wynhoff I, Bakker RB, Oteman B, Arnaldo PS, Van Langevelde F (2015) Phengaris (Maculinea) alcon butterflies deposit their eggs on tall plants with many large buds in the vicinity of Myrmica ants. Insect Conserv Divers 8:177–188. doi:10.1111/icad.12100
Yamamura K, Yokozawa M, Nishimori M, Ueda Y, Yokosuka T (2006) How to analyze long-term insect population dynamics under climate change: 50-year data of three insect pests in paddy fields. Popul Ecol 48:31–48. doi:10.1007/s10144-005-0239-7
Acknowledgments
We thank Annamária Fenesi and Krisztina Havadtői for the characterization of the study area’s vegetation, Ádám Kőrösi for his helpful comments on the manuscript, and Paul Kirkland for linguistic corrections which contributed to the improvement of the manuscript considerably. M.O.-F.’s work was supported by the Sectoral Operational Programme for Human Resources Development 2007–2013, co-financed by the European Social Fund, under the Project POSDRU/159/1.5/S/133391: “Doctoral and postdoctoral excellence programs for training highly qualified human resources for research in the fields of Life Sciences, Environment and Earth”. Furthermore the study was funded by the Polish National Science Centre Grant DEC-2013/11/B/NZ8/00912. During preparation of the manuscript Zs.C.’s work was supported by a Grant of the Ministry of National Education (Romania), CNCS-UEFISCDI, Project No. PN-II-ID-PCE-2012-4-0595, while B.M.’s work by the Bolyai János Scholarship of the Hungarian Academy of Sciences.
Author information
Authors and Affiliations
Corresponding authors
Rights and permissions
About this article
Cite this article
Osváth-Ferencz, M., Czekes, Z., Molnár, G. et al. Adult population ecology and egg laying strategy in the ‘cruciata’ ecotype of the endangered butterfly Maculinea alcon (Lepidoptera: Lycaenidae). J Insect Conserv 20, 255–264 (2016). https://doi.org/10.1007/s10841-016-9858-x
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10841-016-9858-x