Abstract
As pre-hibernating larvae of the marsh fritillary (Euphydryas aurinia) have limited mobility essential resources need to be available at a very local scale. We surveyed larval webs (2011–2013), the host plant devil’s bit scabious (Succisa pratensis) (2012), and derived variables from digital orthophotos and digital elevation models (Normalized Differenced Vegetation Index, accumulated sun hours, slope, aspect) to explain the presence–absence and abundance of larval webs at three different spatial grain sizes (5 × 2.5 m, 10 × 10 m, 25 × 25 m) across seven study sites in northern Jutland, Denmark. Two-component hurdle models indicated that host plant abundance was the only important predictor of presence–absence and abundance of larval webs across the seven sites. The strength of the host plant effect on larval web prevalence increased when enlarging spatial grain size. For presence–absence (and less for abundance), the effect of host plants on larval webs varied across study sites. Using mixed effects models, we additionally analysed presence–absence of larval webs (in 1 × 1 m plots) in relation to detailed host plant measurements (abundance and size), vegetation height, and environmental variables (soil temperature, air temperature and soil moisture) across four of the sites. This showed that larval webs were located in the densest parts of the host plant patches. Given the low mobility of pre-hibernating larvae (<0.5 m), our results suggest that females select dense parts within large patches of host plants as oviposition sites. Future management should concentrate on establishing large patches of the larval host plant.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Insects have evolved complex strategies to access essential resources. Migratory species like the monarch butterfly (Danaus plexippus) travel remarkable distances to reach places where the necessary resources are available to sustain their lives and to reproduce (Milner-Gulland et al. 2011). Other species are less mobile and thus more dependent on local resources. For instance, larvae of the Glanville fritillary (Melitaea cinxia) move a maximum of 1–2 m before winter diapause (Kuussaari et al. 2004) and the mobility of adults of the silver-spotted skipper (Hesperia comma) and the silver studded blue (Plebejus argus) can be extremely low (Hill et al. 1996; Thomas 1985). Even in species with better adult dispersal capacity like the Marsh fritillary (Euphydryas aurinia), >10 km dispersal based on mark recapture (Zimmermann et al. 2011), the low mobility of pre-hibernating larvae (Liu et al. 2006) suggests that oviposition site selection is highly important. Thus, to sustain viable populations of species with low mobility life stages their habitat must be composed of all the resources required at a local spatial scale (Dennis 2010; Hill et al. 1996; Thomas 1985).
The viability of butterfly populations is not only affected by habitat size and landscape characteristics (Ellis et al. 2011) but also by habitat quality (Anthes et al. 2003; Betzholtz et al. 2007; Fowles and Smith 2006), which has often been assumed to correlate with the extent of suitable habitat (Fowles and Smith 2006). Conservation guidelines therefore often focus on the ability of populations to maintain a metapopulation structure (Wahlberg et al. 2002) whereby connectivity between subpopulations is seen as a critical factor for the persistence of butterfly species. However, site-specific carrying capacities are determined by the quality and quantity of habitat patches both of which can strongly affect the viability of butterfly metapopulations (Schtickzelle et al. 2005). Thus, enhancing the quality of existing habitat patches is now increasingly recognised as a major component of endangered butterfly conservation strategies (Gutierrez et al. 1999; Konvicka et al. 2003). There is also evidence that resource requirements for specialist butterflies need to be met at the local scale (e.g. as seen in the Spanish fritillary Euphydryas desfontainii) (Pennekamp et al. 2013). As the proximity of different resources are likely to play a major role for larval development and survival (Pennekamp et al. 2013), the spatial scale at which patch selection occurs (e.g. for oviposition) should be considered when identifying the suitability of habitat patches. For instance, large and south-facing patches might constitute a more favourable habitat than smaller patches with otherwise similar topographic characteristics, and larger patches of host plants might be more attractive than smaller patches. Hence, the relative strength of resource selection at different local spatial grain sizes might indicate at which scale adult butterflies select oviposition sites and thus where larvae locate and exploit their resources.
Recent advances in remote sensing techniques have extended the use of high-resolution surface-information into a wide range of environmental applications (Forsberg 2007). For instance, airborne scanning laser altimetry (LIDAR) data can be used to derive detailed topographic information (slope, aspect) as well as information related to physical and climatic conditions, i.e. water accumulation and accumulated sun hours, even at fine spatial resolutions (<1 m). In addition, digital orthophotos can be used to derive variables related to habitat quality or primary production such as the Normalized Differenced Vegetation Index (NDVI) (Wiegand et al. 2008). Such data have been suggested to be useful for research related to butterfly conservation, e.g. for the investigation of species-habitat relationships (Hess et al. 2013) or predictive distribution modelling (Mason et al. 2003). However, it remains to be investigated at which spatial grain size these variables are most relevant, e.g. for the selection of oviposition sites of female butterflies at local spatial scales.
Here, we use field measurements of habitat quality (host plant abundance, host plant size, and vegetation height), environmental variables (soil temperature, air temperature and soil moisture), and habitat characteristics derived from digital orthophotos and digital elevation models (NDVI, accumulated sun hours, slope and aspect) to explain the presence–absence and abundance of larval webs across a subset of the remaining stronghold sites of the univoltine (i.e. with one generation per year) and critically endangered butterfly E. aurinia in Denmark. To study oviposition site selection, we specifically addressed the effect of host plant and environmental predictor variables on the presence–absence and abundance of larval webs at different local spatial grain sizes as well as the spatiotemporal variation between sites and years at a subset of Danish stronghold sites. We hypothesized that E. aurinia females select large patches with high abundance of host plants for egg-laying to ensure the availability of key resources throughout the lifecycle of the immature life stages. We further expected that females would select large-sized host plant individuals (Anthes et al. 2003) and speculated that certain microclimatic conditions (Pennekamp et al. 2013) or specific aspects of vegetation structure (Botham et al. 2011; Konvicka et al. 2003) might be particularly attractive for the Danish population of E. aurinia and that these preferences might vary across sites as an adaptation to local environmental and climatic conditions.
Materials and methods
Conservation status of study species
Within the European continent, E. aurinia is mainly distributed in the South and Central parts and reaches Scandinavia up to 62° N in Sweden and Finland (Kudrna et al. 2011). Although the species is widespread in Europe, it has a patchy distribution restricted to areas with a sufficiently good quality of habitat, and in Denmark it now only persists in a few fragmented subpopulations (Sigaard et al. 2008). The Danish population of E. aurinia has suffered a severe population decline throughout the last century (Stoltze 1996) and the species is currently listed as critically endangered in the Danish red list (Wind 2010). The same trend is evident from other parts of Europe (Warren 1994) and the species has become listed at the Annex II of the EU Habitats Directive (Council Directive 92/43/EEC).
Mapping of larval webs
A number of populations of E. aurinia were monitored at irregular intervals during the first decade of the twentieth century, but the entire Danish distribution was poorly known at the time. Based on a comprehensive national survey of larval webs in 2011, a total of seven sites (mean area ± SD 1.77 ± 0.87 ha) were selected for this study. We selected this particular subset in order to cover a range of environmental gradients (e.g. wetness, turf height, landscape topography and grazing regimes) characterizing the remaining stronghold sites of E. aurinia in Denmark (Fig. 1). A systematic survey for larval webs was performed from early to late August in 2011, 2012 and 2013 at all seven sites covering the entire area of each site. Although pre-hibernating larvae of E. aurinia are known to have a restricted mobility, the spatial location of oviposition sites could differ from those of larval webs if larvae move considerable distances before hibernation. To assess the assumption that the location of the larval web in August can be used as proxy for the location of the oviposition site, we measured the distance (in cm) between the natal host plant (identified by having withered and consumed leaves) and the position of the larval webs (n = 24) at four of the sites.
Mapping of host plants
Although historic observations in Denmark have reported the use of other host plants (e.g. from genera Veronica and Plantago), the present populations of E. aurinia in Denmark seem to be monophagous, i.e. exclusively feeding on S. pratensis. A survey of host plant abundance covering the entire area of each site was conducted in August 2012 along transect lines 5 meters apart at all sites. For the mapping of host plant abundance, a 5 × 2.5 m plot in front of the observer was surveyed every 2.5 m by estimating the number of rosettes of the host plant (S. pratensis) in one of five categories (0, 1–10, 11–50, 51–100, and >100 rosettes). Each survey plot was georeferenced using a handheld GPS (Trimble GeoExplorer 6000). Based on the obtained field data, distribution maps of the host plants were later derived in ArcGIS 10.1 (ESRI 2013) based on inverse-distance-weighted interpolation (with raster cell sizes 0.32–0.58 m). Each host plant abundance range estimate was converted to a single number (0, 5, 30, 75, 150) to allow for the calculation of mean abundances across multiple plots. The host plant S. pratensis exhibits slow demographic responses to competition and management changes (Herben et al. 2006). Hence, we assumed a relatively stable host plant distribution across neighbouring years and used the mapped abundances of host plants in 2012 as a predictor of larval webs across all years (2011–2013).
Additionally to the surveys across all seven sites, twenty plots of 1 × 1 m size were established in a subset of four sites (Lundby Hede, Overklitten Sø, Sandmosen and Tryn Mose; Fig. 1) and the location was registered using a handheld GPS (Trimble, Juno SB). The installation of these plots was done to map more detailed habitat characteristics such as small-scale variation in host plant abundance, host plant size, vegetation height and locally sampled environmental data (i.e. soil moisture, air and soil temperature). Logistically it was not possible to conduct these detailed surveys at all seven sites because the advancement of autumn (harsher weather conditions and leaf withering) would have biased sampling results. Based on larval web surveys and the mapping of host plant distribution at the sites mentioned above, twenty plots were randomly selected (Appendix 1) at each of the four sites to cover areas with and without larval webs (ten “Web” plots and ten “No web” plots). The ten “No Web” plots were characterized by >10 S. pratensis rosettes but no larval webs (in either 2011 or 2012) whereas the ten “Web” plots were selected randomly among the plots with larval webs recorded in 2012. The minimum number of ten rosettes in the “No Web” plots was chosen to ensure that both “Web” and “No web” plots had at least a certain amount of host plants available (a basic requirement for larval habitat suitability).
For each of the twenty plots, three plant-related measurements (vegetation height, host plant abundance and host plant cover) were taken. Vegetation height was measured at each side of selected 1 × 1 m plots according to the methods described by Fredshavn et al. (2009), where vegetation height is measured using an upright plate with horizontal lines 5 cm apart. Vegetation height (range 5–40 cm) was then estimated as the height of the horizontal line for which 50 % of the plate was still visible. In cases when ten or less host plant rosettes were found within a 1 × 1 m plot of the “No web” category, the frame was moved east, west, north, or south of the centre until the minimum number of ten rosettes was available. Plots in the “Web” category were placed with the larval web in the centre of the frame with the sides of the frame facing north–south. In most cases, the larval web was successfully relocated in the field, but when relocation was not possible, the frame was placed according to the GPS-position of the larval web. The percentage host plant cover (%) was estimated visually within sixteen sub-squares (each 25 × 25 cm) within the 1 × 1 m frame. For recording host plant size, the number of S. pratensis rosettes was counted in every second sub-square (starting from the top left corner) and average host plant size was then calculated as the area covered by S. pratensis divided by the number of rosettes. The mean across all eight sub-squares was used as a measure of host plant size for each plot.
Finally, three environmental parameters (soil temperature, air temperature and soil moisture) were also measured for each of the twenty plots. We used a Hobo microstation datalogger (manufacturer: onset) and recorded the following parameters: air temperature (°C), soil (10 cm below the ground) temperature (°C), and soil moisture (water content, m3 of water/m3 of soil). Synchronous recording of one plot in each of the two categories was performed for 1 h with a 1 min logging interval. Hobo loggers were not installed at all sites simultaneously and the time since the first measurement (time lag) was used as a linear correction factor for seasonal change in soil and air temperature and soil moisture. All environmental parameters were collected between 10:00 am and 7:00 pm during the period 15th September–12th October 2012.
Extraction of remote sensing data at all seven study sites
To link the field surveys from the seven study sites to remote sensing data, the GPS recorded coordinates from the host plant survey were aligned to the closest transect line in ArcGIS. In addition, each coordinate was moved 1.25 m along the transect line to mark the centroid rather than the edge of the plot from which the waypoint was recorded and the plot surveyed. The nearest plant score (Euclidean distance) was assigned to some of the 5 × 2.5 m plots (n = 250) for which larval webs (2011, 2012 or 2013) but no host plant data were available.
To investigate the effect of local spatial scale (i.e. grain size), three grids with cell sizes of 5 × 2.5, 10 × 10 and 25 × 25 m were constructed in ArcGIS. These were aligned and overlaid on each of the seven study sites. The three grids were used to extract four remotely-sensed environmental parameters: slope (in degrees), aspect (i.e. the compass direction that a slope faces, in degrees), the NDVI, and accumulated sun hours (Watt-hours/m2). Slope and aspect were derived from a high-resolution Digital Elevation Model (DEM, raster cell size of 0.16 m × 0.16 m, TIFF-format) (Rosenkranz and Frederiksen 2011), depicting the elevation in meters above sea level for each raster cell. The NDVI was derived from two spectral bands of the Danish Digital Orthophotos (Cowi A/S 2010) and calculated as follows:
where NIR and RED are the measured surface reflectance in the near-infrared and red regions of the electromagnetic spectrum. The digital orthophotos used to calculate NDVI were taken between 15th of May and 15th of July 2010. Accumulated sun hours from 1st August to 31st August (main period of pre-hibernating larval activity) was calculated based on the Danish Surface Model (DSM, raster cell size 1.6 m × 1.6 m, TIFF-format) (Rosenkranz and Frederiksen 2011) using the ArcGIS spatial analyst tool ‘Solar radiation’. The DSM, in contrast to the DEM, incorporates information about canopy height as well as elevation in open areas. For three of the four remotely-sensed variables (slope, NDVI, and accumulated sun hours), mean values were extracted for each of the three spatial grain sizes at each site using ‘Zonal statistics’ in ArcGIS. The calculation of aspect, being a circular measure, required the following vector addition: \(C = \sum\nolimits_{i = 1}^{n} {\cos \theta_{i} } ,S = \sum\nolimits_{i = 1}^{n} {\sin \theta_{i} } ,R^{2} = C^{2} + S^{2} \left( {R \ge \, 0} \right)\) (Fisher 1995), where C is the sum of cosine to all vector angles, S is the sum of sine to all vector angles, and R is the direction vector. The direction θ of the resultant vector is then given by Cos θ = C/R. We then calculated in ArcGIS an index of aspect (Cos(aspect)) for each grid cell at the three spatial grain sizes which transformed the circular measure (0–360°) into a non-circular measure, with values ranging from +1 (north) to −1 (south). Below we refer to this index as ‘aspect’.
Statistical modelling
To test which factors determine the local occurrence of larval webs, two separate analyses were performed. First, variation in presence–absence and abundance of larval webs across all seven study sites were investigated to test for the effects of host plant abundance, NDVI, accumulated sun hours, slope, aspect, and the interaction term slope × aspect. These analyses were performed separately at all three spatial grain sizes (i.e. grids with different cell sizes). Second, presence–absence of larval webs at the subset of four sites was investigated to test for the effects of vegetation height, host plant abundance, host plant size, soil temperature, air temperature and soil moisture.
Across all seven study sites, presence–absence and abundance of larval webs was analysed in 5 × 2.5 m plots, 10 × 10 m plots and 25 × 25 m plots. The sum of larval webs per grid cell for the larger grain sizes was derived by spatial joining in ArcGIS. We then used hurdle models as a modelling framework (Potts and Elith 2006) because strong overdispersion was present in our response variable, especially at the 5 × 2.5 m scale. This excess of zeroes in the data (i.e. grids with host plants but without larval webs) could be better accommodated with the hurdle model than with a simple Poisson model. Moreover, we were interested to differentiate the effects of predictor variables on both the presence and the abundance of larval webs, which can be achieved by hurdle models (Potts and Elith 2006). The hurdle models consist of a binary (presence/absence) model (i.e. a logistic regression) and a count model based on a Poisson distribution truncated at 0 (i.e. no stochastic absence on presence sites). We only included grid cells from the seven sites with host plant presence because this resource is a necessity for the presence of larval webs. Predictor variables (for both the binary and count-component of the hurdle model) were host plant abundance, NDVI, slope, aspect, slope × aspect and site as a fixed effect. We did not include the predictor variable ‘accumulated sun hours’ in the modelling because it was correlated with aspect (Spearman rank, r s = −0.41). Prior to modelling, predictor variables were standardized to mean = 0 and standard deviation SD = 1 to facilitate the interpretation of coefficients (Schielzeth 2010). The maximum number of larval webs over the three years (2011, 2012, 2013) was used as the response variable. Before deciding on a global model (with all predictor variables), we tested for potential non-linear effects of predictor variables, but none of the polynomial terms improved the Akaike Information Criterion (AIC) of the models by more than 5 % and hence they were consequently not included in the final models.
To specifically test for the effect of site and year on resource selection by E. aurinia, we ran additional hurdle models which included host plant abundance (number of S. pratensis rosettes), site (seven study sites, see Fig. 1), and year (2011, 2012, 2013), and the interaction terms host plant abundance × site and host plant abundance × year as predictor variables. Here, the number of webs per grid cell for each year individually (2011, 2012, and 2013) was used as the response variable. We examined the interaction term host plant abundance × site (in our results, expressed as a relative difference with the site Jerup Railway for ease of interpretation and parameter identifiability) to test for site-specific resource selection of E. aurinia, and the interaction term host plant abundance × year (relative to 2011) to test for temporal variation in resource selection. One site (Tryn Mose) had to be omitted from these analyses because only one larval web was registered at that site in 2013.
Across the four study sites with 1 × 1 m plots (Lundby Hede, Overklitten Sø, Sandmosen and Tryn Mose), presence–absence of larval webs in 2012 was used as the response variable and analysed using mixed effects models for binary data (Zuur et al. 2009). Predictor variables were standardized prior to modelling (as above). All possible models nested within the global model were fitted. The global model included the log-transformed number of S. pratensis rosettes as a measure of host plant abundance, mean S. pratensis size (host plant size), mean vegetation height, soil moisture, soil temperature, air temperature, the time lag since the first environmental sampling event, and site (categorical variable). The number of S. pratensis rosettes was log-transformed due to a high variance: mean ratio. The site effect was included here as a random effect to account for variation among sites.
All statistical analyses were performed in R version 2.15.0 (R Development Core Team 2012) using the packages ‘lme4’, ‘MUMIn’, and ‘pscl’. Using the R function ‘dredge’ (package: MUMIn), all possible models were fitted and ranked on the basis of the weights w i of the AIC corrected for small sample sizes (AICc) (Burnham and Anderson 2002). The parameters of the 95 % confidence set of models (cumulative sum of w i ≤0.95) were then averaged and weighted by w i . Confidence intervals not including zero were considered to indicate significant effects on the response variable. The relative importance of each predictor variable (RIV) was further computed as the sum of w i over all selected models in which the variable of interest appeared (Burnham and Anderson 2002).
Spatial autocorrelation
Because spatial autocorrelation can influence inference from statistical models (Bini et al. 2009) we quantified residual spatial autocorrelation in each global model at each spatial grain size to identify spatial structure in the response variables that is not accounted for by the included predictor variables. We also quantified spatial autocorrelation in the raw data, i.e. the response variable itself (number of larval webs). We calculated Moran’s I values based on geographic coordinates (latitude, longitude) of the centroids of the grid cells using the R function ‘moran.mc’ (R package: spdep). A spatial weights matrix was constructed using the R function ‘knearneigh’ (R package: spdep), considering up to ten nearest neighbours. We only quantified residual spatial autocorrelation rather than including it into the statistical models because (1) reliable spatial implementations of hurdle or mixed effects models are currently not available, and (2) the spatial scale and magnitude of this effect was minimal (short distance and low Moran’s I values, see “Results”).
Results
A total of 628 larval webs were located across the three year study period. Across 24 larval webs, travel distances of pre-hibernating larvae never exceeded half a meter and the mean (± SD) travel distance was 23 ± 11.5 cm (range 6–50 cm). At the 5 × 2.5 m resolution, a total of 1,762 grid cells with host plants only and 555 grid cells with host plants and larval webs (in one of the years 2011–2013) were included in the analyses. The seven study sites differed in terms of host plant distribution, with some sites having large areas covered by S. pratensis and others having a more fragmented distribution of host plants (Fig. 2). Average host plant abundance also varied across sites (range 1.85–9.25 plants per 5 × 2.5 m grid cell) with the highest abundance at Overklitten Sø and the lowest at Tryn Mose (compare Fig. 2c, e). Within host plant patches, the highest average abundance of S. pratensis was also recorded at Overklitten Sø (28.91 plants per 5 × 2.5 m grid cell) and the lowest at Sandmosen (14.40 plants 5 × 2.5 m per grid cell) (compare Fig. 2c, d).
Spatial distribution of host plant abundance at seven study sites (a Lundby Hede, b Tranum Military Area, c Overklitten Sø, d Sandmosen, e Tryn Mose, f Napstjert, g Jerup Railway) modelled by Inverse-Distance-Weighted Interpolation based on a host plant survey conducted in August 2012. Larval webs (triangles) registered in 2011, 2012 and 2013 are shown in each panel
Larval web presence–absence and abundance at all seven study sites
Consistently across the three spatial grain sizes (5 × 2.5 m, 10 × 10 m and 25 × 25 m) at the seven study sites, larval webs were most likely to be present where the local abundance of host plants was high (Fig. 3a; Table 1). A similar result was obtained for larval web abundance (i.e. maximum number of larval webs across the years 2011, 2012, and 2013) suggesting that the density of larval webs is higher in dense patches of host plants (Fig. 3b). Interestingly, the strength of the effect of S. pratensis abundance increased with increasing spatial grain size (Fig. 3a, b), suggesting that larger patches with a high host plant abundance were more favorable for the presence and abundance of larval webs than were smaller patches. In contrast to host plant abundance, NDVI and topographic variables (i.e. slope, aspect, and their interaction slope × aspect) did not show a statistically significant effect on the presence–absence of larval webs across the three spatial scales (Fig. 3a). Similar results were obtained for larval web abundance (Fig. 3b) except for topographic slope which showed a negative effect at the 25 × 25 m grain size (Fig. 3b). Finally, comparing the relative importance of predictor variables (based on AICc weights) across scales (Table 1) showed that NDVI and topographic variables were more important for larval web presence at the 5 × 2.5 m and 10 × 10 m scale than at the 25 × 25 m scale.
Average coefficients ±95 % confidence intervals (CI) at three spatial grain sizes (5 × 2.5 m, 10 × 10 m, 25 × 25 m) as derived from two-component hurdle models relating a presence and b abundance of larval webs to five environmental predictor variables. Host plant abundance: Abundance of Succisa pratensis rosettes, NDVI: Normalized Difference Vegetation Index, Slope: topographic slope, Aspect: compass direction of the slope (non-circular index), Slope × aspect: interaction between slope and aspect. All possible models were ranked on the basis of the Akaike Information Criterion corrected for small sample sizes (AICc). Parameters were then averaged for the 95 % confidence set of models (cumulative sum of AICc weights ≤0.95)
Resource selection by E. aurinia (i.e. larval web presence–absence and abundance relative to host plant abundance) varied across sites (Fig. 4a, b). Examining the interaction term host plant abundance × site showed that the presence–absence of larval webs in relation to host plant abundance differed at three sites (relative to the site Jerup Railway) (Fig. 4a) whereas the abundance of larval webs differed at one site (Fig. 4b). These results indicated a higher presence of larval webs on high density host plant patches at Napstjert, Sandmosen and Tranum Military Area, and a lower larval web abundance relative to host plant availability at Overklitten Sø. In contrast to the site effect, there was no evidence of differences in host plant selection among years (Fig. 4c, d).
The use of host plants by larval webs of the butterfly Euphydryas aurinia varies by site (a, b) and year (c, d). The temporal and spatial variability of host plant use by E. aurinia is shown for larval web presence–absence (a, c) and larval web abundance (b, d). Illustrated are average coefficients ±95 % confidence intervals (CI) derived from the statistical interaction term of host plant abundance and site (abundance × site) and host plant abundance and year (abundance × year) as obtained from a two-component hurdle model which includes host plant abundance (number of Succisa pratensis rosettes), site (six sites) and year (2011, 2012, 2013), and the interaction terms abundance × site and abundance × year as predictor variables. Site-specific parameter estimates for abundance × site are shown for each site (1 Lundby Hede, 2 Napstjert, 3 Overklitten Sø, 4 Sandmosen, 5 Tranum Military Area, see Fig. 1) relative to Jerup Railway, and the estimates for abundance × year are shown for 2012 and 2013 relative to 2011. The response variable is the number of larval webs of E. aurinia in years 2011, 2012, and 2013, with the effects of predictor variables being separated for the binary (presence–absence) part of the model (a, c) and the count (larval web abundance) part of the model (b, d)
Presence–absence of larval webs in 1 × 1 m plots
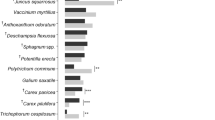
Across the four study sites with data for 1 × 1 m plots, host plant abundance was also the most important variable explaining presence of larval webs (Table 2). A higher host plant abundance (i.e. higher number of S. pratensis rosettes) was positively related to the presence of larval webs (Fig. 5), suggesting that larval webs were most likely present where the within-plot density of host plants was high. Other plant-related variables such as host plant size or vegetation height showed no statistically significant effect, although larval webs tended to be less frequent when vegetation was high (Fig. 5; Table 2). Interestingly, microclimatic variables such as soil moisture, soil temperature, and air temperature did not show any statistically significant effects on the presence of larval webs across the four sites (Fig. 5).
The effect of various predictor variables on presence–absence of larval webs within 1 × 1 m plots at four of the study sites. Shown are estimated average coefficients ±95 % confidence intervals (CI) as derived from model averaging with mixed effects models using seven predictor variables across four study sites (n = 80 grid cells). All possible candidate models were ranked on the basis of the Akaike Information Criterion corrected for small sample sizes (AICc). Parameters were then averaged for the 95 % confidence set of models (cumulative sum of AICc weights ≤0.95). ‘Site’ (categorical variable) was included as a random effect (not shown here). Variable notation: Host plant abundance: Abundance of Succisa pratensis rosettes; Size: host plant size; Vegetation: vegetation height; Soil temp: soil temperature; Air temp: air temperature; Soil moist: soil moisture; Time lag: time lag between sampling events
Spatial autocorrelation
The distribution of larval webs was positively autocorrelated at all three spatial grain sizes (Fig. 6), indicating that the likelihood of larval web presence increased if one or more webs were present in neighboring grid cells. When the environmental and host plant predictor variables were included in the hurdle models, positive spatial autocorrelation in model residuals was removed at all three spatial grain sizes in most cases (Fig. 6). However, a small amount of spatial autocorrelation remained at the finest spatial grain size (5 × 2.5 m) for the smallest spatial distance class (lag of 1), which corresponds to a mean distance of 3.59 ± 1.97 m (Fig. 6). Given the short distance and the low magnitude of this effect we consider it unlikely to have a strong influence on parameter estimates and confidence intervals in our models. However, the remaining small-scale spatial structure is still interesting as it could indicate that other factors not included as predictor variables in this study (e.g. soil conditions or biological causes) might influence the fine-scale spatial structure of larval webs below this neighborhood distance (see “Discussion”).
Correlograms showing Moran’s I values for the raw data (grey circles) and for the residuals of hurdle models (black circles) at spatial grain sizes of a 5 × 2.5 m, b 10 × 10 m, and c 25 × 25 m. Raw data represent the number of larval webs (maximum across the years 2011, 2012, and 2013) and residuals of hurdle models show the remaining residual spatial autocorrelation structure once environmental and host plant predictor variables have been accounted for. Moran’s I values were calculated with up to 10 nearest neighbours (spatial lags). Note that spatial lags >4 could not be calculated for the largest spatial scale (c) because too few neighbours were available in higher lags. The mean distance of lag 1 is 3.59 ± 1.97 m (a), 9.71 ± 1.78 m (b) and 22.01 ± 3.31 m (c)
Discussion
Host plant abundance was the most important predictor for both larval web presence–absence and abundance across seven Danish study sites for the endangered butterfly E. aurinia. The effect of host plant abundance became increasingly evident when considering a larger spatial grain size indicating that larger patches with a high abundance of host plants were preferentially selected by egg-laying females. This supported our initial hypothesis that females select for large patches with high abundance of host plants for egg-laying to ensure the availability of key resources, especially throughout the lifecycle of the early immature life stages. More detailed investigations in 1 × 1 m plots at four of the study sites revealed that the occurrence of larval webs was related to host plant abundance, but not to other local environmental characteristic (e.g. host plant size, vegetation height, soil temperature, or soil moisture), suggesting that larval webs were located in the densest parts of the host plant patches. Resource selection of E. aurinia (i.e. the strength of the effect of host plant abundance on larval web presence–absence and abundance) varied further among sites (but not years), indicating that other site-specific factors could be important for the butterfly species.
In contrast to our initial hypothesis that large-sized host plant individuals should be preferentially selected (Anthes et al. 2003), we found that host plant size in 1 × 1 m plots at four study sites did not vary between occupied and unoccupied plots. Other studies have found that occupied host plants are larger (or of equal size) than unoccupied plants (Anthes et al. 2003; Porter 1992). However, in some cases host plant size differences may also vary among sites, e.g. as demonstrated in the Hebei province of China where E. aurinia uses Scabiosa tschiliensis Grün (Dipsacaeae) as the only host plant (Liu et al. 2006). Rather than comparing host plant sizes of occupied versus unoccupied host plant individuals, we estimated average host plant sizes at the scale of 1 × 1 m patches, and therefore our results are not directly comparable to previous findings concerning host plant size. Low sample size (10 occupied and 10 unoccupied host plant patches at each site) might also have prevented us from detecting differences in plant architecture among occupied and unoccupied host plant patches. Moreover, the timing of our fieldwork was optimal for locating larval webs, but did not allow us to identify local size differences between occupied and unoccupied host plants at the time of oviposition. It would therefore be interesting to directly observe egg-laying females and compare the development in plant architecture of host plants with and without egg batches. Other studies that have found no effect of host plant size on larval web occurrence suggest that abundant small-sized host plants can equally well form a suitable food source for the larvae of E. aurinia (Stefanescu et al. 2006).
Our results demonstrated that E. aurinia larvae moved less than half a meter before hibernation and thus the location of larval webs was a good approximation of the location of oviposition sites. Host plant exploitation of the larvae in our study area is therefore confined to their natal plant or to a few neighboring plants. Hence, selecting a large patch with a high density of host plants for oviposition (as suggested by our results) might be a way of preventing starvation of the solitary post-hibernating larvae. Starvation is common and important for the population dynamics of several checkerspot butterfly species including the closely related M. cinxia (Kuussaari et al. 2004).
We could hardly detect any statistically significant effect of remotely-sensed variables such as NDVI, slope, aspect or the interaction between slope and aspect on the presence or abundance of larval webs across the seven studies sites (spatial resolutions of 5 × 2.5 m, 10 × 10 m, 25 × 25 m). For instance, NDVI as a measure of vegetation density was the only variable that showed a statistically significant effect on the presence–absence (but not abundance) of larval webs at the two finer spatial grain sizes. Likewise, our measurements of microclimate and vegetation structure around larval webs at four study sites (in 1 × 1 m plots) did not differ statistically from those of random control plots, although the presence of larval webs tended to be lower in higher vegetation. The sample size and the range of vegetation heights might be too small at our four sites to detect a stronger effect (larval webs were usually found in vegetation heights of 10–25 cm). This contrasts with our initial hypothesis and with other studies which have found that microclimatic conditions (Pennekamp et al. 2013) or vegetation structure (Botham et al. 2011; Konvicka et al. 2003) affect the presence of larval webs in checkerspot butterflies. However, additional field observations (R. Tjørnløv personal observation) indicate an association of larval webs with grass tussocks and a tendency for an absence of webs in very low vegetation despite a high abundance of host plants. However, the available data were not sufficient to support this quantitatively.
We found that resource selection differed among sites (i.e. the effect of the interaction term host plant abundance × site on larval web presence and abundance) supporting the idea that patch selection can vary geographically as an adaptation to local environmental and climatic conditions. Specifically, three sites (Napstjert, Sandmosen and Tranum Military Area) had a higher probability of larval webs being present and one site (Overklitten Sø) showed a lower abundance of larval webs, relative to the use of host plant abundance at the reference site (Jerup Railway). Such an effect could imply that resource selection is governed by the local (within site) variation in resource availability or mortality between the time of egg-laying and the time of larval web surveys. The strength of apparent resource selection could be altered if pre-hibernating larvae are more (or less) susceptible to mortality, e.g. due to grazing in parts of a site with high host plant abundance, as has been found elsewhere (Liu et al. 2006). Other causes of mortality could be summer flooding or parasitism by parasitoid wasps (Klapwijk and Lewis 2014), but parasitism has not been reported to occur in the Danish population of E. aurinia despite the fact that surveys have specifically looked for such incidents in recent years. Alternatively, it is conceivable that other factors become important when host plant density is sufficiently high. As indirect support for this idea, it has been shown that the specific habitat requirements for E. aurinia differ depending on the degree of habitat fragmentation in southern UK (Botham et al. 2011). Finally, the strength of resource selection could vary according to the density of the butterfly population at a particular site. Site-specific variation in mortality risk (grazing, flooding or predation) or access to nectar resources could therefore be a driving factor to explain site-specific differences in the relationship between larval webs and host plant abundance (Liu et al. 2006). Extensive flooding has been observed at the site Overklitten Sø and could suggest that the weaker relationship between larval web abundance and host plant abundance at this site relative to the other sites is related to higher overwinter mortality. Our results point towards investigating such site differences in order to increase larval web densities at sites with lower than average densities of webs and weaker relationships to host plant abundance.
The spatial structure of our response variable (i.e. number of larval webs) was mostly caused by the spatial structure of the included environmental and host plant predictor variables. However, a small amount of spatial autocorrelation remained in model residuals at the smallest spatial distance (i.e. <3–5 m distance). From a statistical point of view (Legendre and Legendre 2012), we consider this to be unproblematic because the effect was of low magnitude and only detectable at a very short distance. However, from a biological point of view it is interesting because it could be either caused by one or more important physical predictors that had not been included in the present study (e.g. soil characteristics), or by biological causes (e.g. the egg-laying behaviour of the female butterfly). Females usually lay a large initial egg-batch close to their natal patch and then disperse further away to lay additional smaller batches (Porter 1981). Hence, the location of the first oviposition event is the combined result of the female’s own active choice as well as the choice made by the previous female and subsequent oviposition events may thus to a larger degree reflect independent selection. However, this behaviour might lead to spatially autocorrelated larval webs even at such small spatial distances.
In conclusion, our results show that the effect of host plant abundance on the presence and abundance of larval webs increases when enlarging the spatial grain size of sampling units at local spatial scales, indicating that large patches (10 × 10 m, 25 × 25 m) with a high abundance of S. pratensis are preferred by E. aurinia. This is most likely caused by the egg-laying behaviour of the adult females because measured movement distances of the pre-hibernating larvae were low (<0.5 m) and therefore the location of larval webs is in close proximity to oviposition sites selected by the female. Other factors related to remotely-sensed variables (NDVI, accumulated sun hours, slope, aspect), additional plant measurements (host plant size and vegetation height), or locally sampled environmental variables (soil temperature, air temperature and soil moisture) played a minor role for the prevalence of larval webs at our study sites. However, we also found substantial variation among sites, suggesting that resource selection varies among particular sites or that other factors (e.g. site-specific variation in mortality risk related to grazing, flooding or predation) could be important. Future nature restoration projects serving to improve the conditions for the endangered E. aurinia in Denmark (and possibly elsewhere) should aim to create and maintain large patches of the host plant S. pratensis. Moreover, the relative importance of host plant abundance (amount) and host plant quality needs further study. The population of E. aurinia in Denmark is small, highly fragmented and several remaining populations are isolated to a degree that dispersal between populations is highly unlikely. The role of isolation of populations and other site-specific factors for the abundance of larval webs need to be further investigated to facilitate future efforts to secure viable populations of this species in Denmark.
References
Anthes N, Fartmann T, Hermann G, Kaule G (2003) Combining larval habitat quality and metapopulation structure—the key for successful management of pre-alpine Euphydryas aurinia colonies. J Insect Conserv 7:175–185. doi:10.1023/a:1027330422958
Betzholtz PE, Ehrig A, Lindeborg M, Dinnétz P (2007) Food plant density, patch isolation and vegetation height determine occurrence in a Swedish metapopulation of the marsh fritillary Euphydryas aurinia (Rottemburg, 1775) (Lepidoptera, Nymphalidae). J Insect Conserv 11:343–350. doi:10.1007/s10841-006-9048-3
Bini LM et al (2009) Coefficient shifts in geographical ecology: an empirical evaluation of spatial and non-spatial regression. Ecography 32:193–204. doi:10.1111/j.1600-0587.2009.05717.x
Botham MS et al (2011) The effects of habitat fragmentation on niche requirements of the marsh fritillary, Euphydryas aurinia, (Rottemburg, 1775) on calcareous grasslands in southern UK. J Insect Conserv 15:269–277. doi:10.1007/s10841-010-9344-9
Burnham KP, Anderson DR (2002) Model selection and multimodel inference: a practical information-theoretic approach, 2nd edn. Springer, New York
COWI A/S (2010) http://www.cowi.dk/menu/service/Geografiskinformationogit/Kortoggeodataprodukter/Ortofotos/TidligereudgaverafDDOland/Pages/tidligereudgaverafddo.aspx. Accessed 15 Nov 2012
Dennis RLH (2010) A resource-based view for habitat conservation. Wiley, Oxford
Directive C (92/43/EEC) Council Directive 92/43/EEC of 21 May 1992 on the conservation of natural habitats and of wild fauna and flora
Ellis S, Wainwright D, Berney F, Bulman C, Bourn N (2011) Landscape-scale conservation in practice: lessons from northern England UK. J Insect Conserv 15:69–81. doi:10.1007/s10841-010-9324-0
ESRI (2013) ArcGIS Desktop: Release 10.1. Redlands, Ca: Environmental Systems Research Institute
Fisher NI (1995) Statistical analysis of circular data. Cambridge University Press, Cambridge
Forsberg R (2007) Luftbåren laserscanning til støtte af klima-og miljøovervågning. Perspektiv 12:31–39
Fowles AP, Smith RG (2006) Mapping the habitat quality of patch networks for the marsh fritillary Euphydryas aurinia (Rottemburg, 1775) (Lepidoptera, Nymphalidae) in Wales. J Insect Conserv 10:161–177. doi:10.1007/s10841-006-6291-6
Fredshavn J, Nielsen KE, Ejrnæs R, Nygaard B, Skov F, Strandberg B, Johannsen VK (2009) Overvågning af terrestriske naturtyper. DCE—Nationalt Center for Miljø og Energi, Aarhus University, Denmark
Gutierrez D, Thomas CD, Leon-Cortes JL (1999) Dispersal, distribution, patch network and metapopulation dynamics of the dingy skipper butterfly (Erynnis tages). Oecologia 121:506–517. doi:10.1007/s004420050957
Herben T, Munzbergova Z, Milden M, Ehrlen J, Cousins SAO, Eriksson O (2006) Long-term spatial dynamics of Succisa pratensis in a changing rural landscape: linking dynamical modelling with historical maps. J Ecol 94:131–143. doi:10.1111/j.1365-2745.2005.01063.x
Hess AN, Falkowski MJ, Webster CR, Storer AJ, Pocewicz A, Martinuzzi S (2013) Employing lidar data to identify butterfly habitat characteristics of four contrasting butterfly species across a diverse landscape. Remote Sens Lett 4:354–363. doi:10.1080/2150704x.2012.734932
Hill JK, Thomas CD, Lewis OT (1996) Effects of habitat patch size and isolation on dispersal by Hesperia comma butterflies: implications for metapopulation structure. J Anim Ecol 65:725–735. doi:10.2307/5671
Klapwijk MJ, Lewis OT (2014) Spatial ecology of host-parasitoid interactions: a threatened butterfly and its specialised parasitoid. J Insect Conserv 18:437–445. doi:10.1007/s10841-014-9653-5
Konvicka M, Hula V, Fric Z (2003) Habitat of pre-hibernating larvae of the endangered butterfly Euphydryas aurinia (Lepidoptera : Nymphalidae): what can be learned from vegetation composition and architecture? Eur J Entomol 100:313–322
Kudrna O, Harpke A, Lux K, Pennersoft J, Schweiger O, Settele J, Wiemers M (2011) Distribution atlas of butterflies in Europe. Gesellschaft für Schmetterlingsschutz, Halle
Kuussaari M, van Nouhuys S, Hellmann JJ, Singer MC (2004) Larval biology of checkerspots. In: Ehrlich PR, Hanski I (eds) On the wings of checkerspots: a model system for population biology. Oxford University Press, New York, pp 138–160
Legendre P, Legendre L (2012) Spatial structure, spatial dependence, spatial correlation. Numerical ecology, vol 24, 3rd edn. Elsevier, Amsterdam, pp 8–21
Liu W, Wang Y, Xu R (2006) Habitat utilization by ovipositing females and larvae of the Marsh fritillary (Euphydryas aurinia) in a mosaic of meadows and croplands. J Insect Conserv 10:351–360. doi:10.1007/s10841-006-9009-x
Mason DC, Anderson GQA, Bradbury RB, Cobby DM, Davenport IJ, Vandepoll M, Wilson JD (2003) Measurement of habitat predictor variables for organism-habitat models using remote sensing and image segmentation. Int J Remote Sens 24:2515–2532. doi:10.1080/014311602100100848
Milner-Gulland EJ, Fryxell JM, Sinclair ARE (2011) Animal migration: a synthesis. Oxford University Press, Oxford
Pennekamp F, Monteiro E, Schmitt T (2013) The larval ecology of the butterfly Euphydryas desfontainii (Lepidoptera: Nymphalidae) in SW-Portugal: food plant quantity and quality as main predictors of habitat quality. J Insect Conserv 17:195–206. doi:10.1007/s10841-012-9497-9
Porter K (1981) The population dynamics of small colonies of the butterfly Euphydryas aurinia. Oxford University, Oxford
Porter K (1992) Eggs and egg-laying. In: Dennis RLH (ed) The ecology of butterflies in Britain. Oxford University, Oxford, pp 46–72
Potts JM, Elith J (2006) Comparing species abundance models Ecological Modelling 199:153–163. doi:10.1016/j.ecolmodel.2006.05.025
R Development Core Team (2012) R: a language and environment for statistical computing. R foundation for statistical computing. Vienna, Austria
Rosenkranz B, Frederiksen P (2011) National Survey and Cadastre—Denmark, technical report series number 12. http://www.kms.dk
Schielzeth H (2010) Simple means to improve the interpretability of regression coefficients. Methods Ecol Evol 1:103–113. doi:10.1111/j.2041-210X.2010.00012.x
Schtickzelle N, Choutt J, Goffart P, Fichefet V, Baguette M (2005) Metapopulation dynamics and conservation of the marsh fritillary butterfly: population viability analysis and management options for a critically endangered species in Western Europe. Biol Conserv 126:569–581. doi:10.1016/j.biocon.2005.06.030
Sigaard P, Pertoldi C, Madsen AB, Sogaard B, Loeschcke V (2008) Patterns of genetic variation in isolated Danish populations of the endangered butterfly Euphydryas aurinia. Biol J Linn Soc 95:677–687. doi:10.1111/j.1095-8312.2008.01078.x
Stefanescu C, Penuelas J, Sardans J, Filella I (2006) Females of the specialist butterfly Euphydryas aurinia (Lepidoptera : Nymphalinae : Melitaeini) select the greenest leaves of Lonicera implexa (Caprifoliaceae) for oviposition. Eur J Entomol 103:569–574
Stoltze M (1996) Danske dagsommerfugle. Gyldendal, Aalborg
Thomas CD (1985) Specializations and polyphagy of Plebejus argus (Lepidoptera, Lycaenidae) in North-Wales. Ecol Entomol 10:325–340. doi:10.1111/j.1365-2311.1985.tb00729.x
Wahlberg N, Klemetti T, Hanski I (2002) Dynamic populations in a dynamic landscape: the metapopulation structure of the marsh fritillary butterfly. Ecography 25:224–232. doi:10.1034/j.1600-0587.2002.250210.x
Warren MS (1994) The UK status and suspected metapopulation structure of a threatened European butterfly, the marsh fritillary Eurodryas aurinia. Biol Conserv 67:239–249. doi:10.1016/0006-3207(94)90615-7
Wiegand T, Naves J, Garbulsky MF, Fernandez N (2008) Animal habitat quality and ecosystem functioning: exploring seasonal patterns using NDVI. Ecol Monogr 78:87–103. doi:10.1890/06-1870.1
Wind P (2010) Den danske rødliste. Danmarks Miljøundersøgelser. http://redlist.dmu.dk
Zimmermann K, Fric Z, Jiskra P, Kopeckova M, Vlasanek P, Zapletal M, Konvicka M (2011) Mark-recapture on large spatial scale reveals long distance dispersal in the Marsh Fritillary Euphydryas aurinia. Ecol Entomol 36:499–510. doi:10.1111/j.1365-2311.2011.01293.x
Zuur A, Ieno E, Walker N, Saveliev A, Smith G (2009) Mixed effects models and extensions in ecology with R. Springer New York. doi:10.1007/978-0-387-87458-6_1
Acknowledgments
We thank Ib Krag Petersen for GPS and GIS assistance during the early phase of the project, and the field assistants Mette Kjær, Susan Hangstrup, Morten Swayne, Claus Bo Popenda Pedersen and Leif Pedersen for help with data collection. We also thank Flemming Helsing-Nielsen, Einar Flensted-Jensen, Anne Eskildsen, Marie Vissing, Joseph J. Bowden, Emil S. Brandtoft and Oskar Liset Pryds Hansen who all have contributed to the detailed surveys of larval webs. J.Y.B. received partial fundings from the CIRCE project under AU Ideas program.W.D.K. acknowledges support from an University of Amsterdam starting grant and T.T.H. acknowledges funding from 15. Juni Fonden.
Author information
Authors and Affiliations
Corresponding author
Appendix 1
Appendix 1
See Fig. 7.
Grid cells selected for sampling and data analysis at four study sites in Denmark; a Lundby Hede b Overklitten Sø c Sandmosen d Tryn Mose. Two categories with 10 grid cells (5 × 2.5 m) each were randomly selected at each site. Green: grids with host plants but without larval webs of Euphydryas aurinia. Red: grids with host plants and larval webs. (Color figure online)
Rights and permissions
About this article
Cite this article
Tjørnløv, R.S., Kissling, W.D., Barnagaud, JY. et al. Oviposition site selection of an endangered butterfly at local spatial scales. J Insect Conserv 19, 377–391 (2015). https://doi.org/10.1007/s10841-014-9747-0
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10841-014-9747-0