Abstract
The knowledge on species’ habitat preferences at local scales across its range is an essential condition for defining the most appropriate habitat management for the conservation of any species. In this study, we combined field observations from three European countries with breeding experiments under field conditions to identify oviposition and larval preferences of Coenonympha oedippus at the micro-scale level across contrasting habitat types (wet vs. dry). Despite the wide geographical range and the different habitats we found some common features: (1) vegetation structure of the herb layer is an essential factor for oviposition site electivity and successful development of premature stages; (2) high cover of litter and/or dwarf shrubs in the microhabitat (larval 45–70 %, oviposition 40–50 %) creates a herb layer rich in gaps; at their edges eggs are deposited and the caterpillars are adequately sun-exposed; (3) egg-laying females are not selective regarding oviposition substratum; (4) oviposition height is adjusted to positions with direct sunlight or warm substratum; (5) the host-plants coverage in oviposition sites was high: between 45 and 50 % in wet habitats, and between 18 and 41 % in dry habitats (depending on whether only plants observed as hosts in this study are counted, or whether all potential host species are included); (6) the most important host-plant is Carex panicea (wet) and Carex humilis (dry), but Molinia caerulea (wet) and Festuca rupicola (dry) are also used regularly; (7) the availability of winter-green host-plants in the vicinity of hibernated larvae plays a substantial role in their survival. As regular mowing or grazing would remove the litter and destroy the gaps, the management should be restricted to selective reed cutting or manual shrub removal. Only selective mowing during winter (December–February) can be recommended for keeping the habitat open where the reduction of bushes is not sufficient.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
For the successful conservation of any species, knowledge of its habitat preferences at local scales across a species’ range is required (e.g. Anthes et al. 2008), and an understanding of the perceptual world of the target organism and its interactions with the environment from the functional habitat point of view is a benefit (Dennis 2003; Dennis et al. 2006; Van Dyck 2012). The resource-based habitat concept based on species-specific resource distribution and individual movements considers all life stages of an organism and thus offers an advanced approach for successful conservation management of threatened butterfly species.
The False Ringlet, Coenonympha oedippus (Fabricius 1787), is one out of twelve endangered European butterfly species (van Swaay et al. 2010), and listed on the Annexes II and IV of the Habitats Directive. Its distribution occupies only 3.92 % of the area of Europe (Kudrna et al. 2011). The Climatic Risk Atlas of European Butterflies (Settele et al. 2008) placed the species in a category of “climate change risk (R)” due to more than 50 % loss of its current grid cells under at least one of the three simulated scenarios. Previous studies represent the first important part of knowledge for better understanding the species’ habitat use within the functional resource-based habitat approach including both consumables and utilities. They aimed at habitat requirements (Bonelli et al. 2010; Bräu et al. 2010; Čelik and Verovnik 2010; Dušej et al. 2010; Örvössy et al. 2010; Selezniew et al. 2010; Šašić 2010), adult movements and population ecology (Čelik 2003, 2004; Čelik et al. 2009a; Čelik and Verovnik 2010; Örvössy et al. 2010, 2013), together with some initial researches on ecology of early developmental stages (Bonelli et al. 2010; Bräu et al. 2010; Čelik 1997, 2003; Čelik and Verovnik 2010).
Coenonympha oedippus is a satyrine butterfly. Larvae of this subfamily feed on plants from the families Poaceae, Cyperaceae and Juncaceae (Munguira et al. 2009). Such grass-feeding species are generally thought to be less specific in their host-plant and oviposition-site choice (Wiklund 1984; Lindman et al. 2013). The ability of larvae to utilize superabundant (i.e. graminoids) and multiple plant species as host-plants reduces limitations of females in oviposition-site selection (Gripenberg et al. 2010), but can also lead to a loss of benefits gained by female discrimination, such as favourable microhabitats for the pre-mature stages (Zalucki et al. 2002; Eilers et al. 2013; Lawson et al. 2014), higher nutritional quality of hosts (Awmack and Leather 2002), and reduced parasitism or predation or intra- and inter-specific competition (Doak et al. 2006). Nevertheless, it has been discovered recently that grass-feeding species have specific requirements concerning the quality of host plants and microhabitat structures (e.g. Möllenbeck et al. 2009; Beyer and Schultz 2010; Weking et al. 2013) especially in terms of vegetation height and density, amounts of grass-litter and sun exposure.
Across its European range C. oedippus occurs in two contrasting habitat types regarding soil humidity: the majority of populations live in semi-open wet grasslands (ordo Molinietalia, ordo Tofieldietalia) (Čelik 1997, 2003, 2004; Dierks 2006; Bonelli et al. 2010; Bräu et al. 2010; Čelik and Verovnik 2010; Dušej et al. 2010; Örvössy et al. 2010; Selezniew et al. 2010; Šašić 2010), but at the southern range limit also on dry habitats (Ruehl 1895; Hafner 1910; Kolar 1919, 1929; Bischof 1968; Habeler 1972). However, populations occurring on dry abandoned grasslands (class Festuco-Brometea) are presently known only from Slovenia (Čelik 2003; Čelik and Verovnik 2010). Conservation of species using distinct habitat types most likely requires different management strategies (e.g. Kalarus et al. 2013). The habitats of the existent and last strong European populations of the False Ringlet are at least partially abandoned and if they are mown this happens only infrequently/extensively (Bonelli et al. 2010; Bräu et al. 2010; Čelik and Verovnik 2010; Örvössy et al. 2010), while regular mowing each summer within flight period leads to drastic declines and the extinction of populations (Čelik et al. 2009b, own observation).
As already stressed by Thomas (1993), warm early successional habitats are often crucial for oviposition and larval development for Lepidoptera species that reach their northern limit in Central Europe. In contrast, C. oedippus inhabits late successional stages. The species seems to avoid large open ranges and prefers clearing-like habitats with interspersed bushes, enclosed by hedges (own observation, Örvössy et al. 2010). Such species of late successional habitats have been less studied compared to those of early successional stages, resulting in a higher demand of new information.
Previous studies on ecology of C. oedippus contributed to our knowledge of the habitat requirements and biology of early developmental stages within a single European country and habitat type. In this study, we used field observations and breeding experiments under field conditions to identify environmental parameters responsible for larval and oviposition microhabitat choice in three habitat types differing in soil humidity (wet vs. dry) and soil reaction (wet habitat: alkaline vs. acid). Then we compared three habitat types to determine common key features leading to the selection of larval and oviposition sites in C. oedippus across a large spatial scale.
Therefore, we hypothesized that (1) C. oedippus, as a grass-feeding species, is not very selective concerning oviposition substratum and host-plant species range within the contrasting habitat types; that (2) vegetation structure in larval/egg-laying microhabitats should be a more important factor in microhabitat selection than species composition of the vegetation; that (3) if differences in microhabitat structure between habitat types exist the required structure of larval/egg-laying microhabitat linking with microclimatic conditions most suitable for development of premature stages is achieved by adjusting micro-site selection across the macro-environmental gradient, meaning that larval/egg-laying habitat plasticity exists in the study species.
Based on the results from field observations and breeding experiments the implications for habitat management of the endangered C. oedippus are discussed.
Materials and methods
Study species
The False Ringlet is distributed from the Pyrenees in the west to Northeast-China, Korea and Japan in the east. At present, it is still very widespread and abundant in SE Transbaikalia and Altai (Gorbunov and Kosterin 2007). All populations east of the Ural Mountains are considered as different subspecies. In Europe, mostly isolated populations are still present in France, Germany, Liechtenstein, Austria, Italy, Slovenia, Croatia, Hungary, Poland, Belarus, Ukraine and Russia. Italy plays a central role in the conservation of C. oedippus in Europe as the species is still abundant in the north of the country, where over 100 populations are known and only 9 were documented to be extinct in recent times, mainly following habitat loss due to human activities (Balletto et al. 2007, 2010, 2014). In Slovenia, the species has a disjunct distribution, with two main centres: central (wet habitats) and south-western (dry habitats) part of the country. In central Slovenia only one metapopulation (ca. 2,000 individuals) and five small neighbouring populations (each of them <500 individuals) have been known within the last 15 years. During this period, three of the smaller populations have become extinct and total population size decreased by about 80 %. Main reasons are regular mowing during or before flight season and destruction of the habitat by conversion into arable land. In south-western Slovenia, the species is more widespread but population densities are much lower than the density of the strongest wet subpopulation estimated 15 years ago (Čelik, own observation). In Germany only a few sites (wet habitats) are documented historically. They are all in the southern part of Bavaria mainly along the Isar river valley. At present, one of these sites still harbours C. oedippus, consisting of three habitat patches in close vicinity to each other. Before its rediscovery in 1996, the species was thought to be extinct in Germany (Bräu and Schwibinger 2013).
Coenonympha oedippus inhabits late successional habitats, which are not dominated by woody plants. According to Osthelder (cit. in Kolar 1929), C. oedippus may already have immigrated from its core territory in Asia to central and western Europe during the last interstadial (Allerød) of the Weichselian glaciation about 10,000 years ago. Hence, primary habitats of the species may have been places where woodland could not completely close, but had larger open spaces because of very wet (e.g. in spring fens with continuously very wet soil or periodically flooded areas along rivers and brooks) or dry conditions, possibly combined with grazing of wild animals. This may be the reason why the species is also found, apart from wetlands, in dry abandoned grasslands of the karst regions of northern Italy (only old data exist which are not recently confirmed) and south-western Slovenia, and in folds of dry steppes on southern slopes with narrow strips of mesoxerophilous meadow vegetation and shrub-lands in southern Siberia (Gorbunov and Kosterin 2007). Specific ecological needs of C. oedippus together with a very low dispersal capacity (Čelik 2003; Örvössy et al. 2013) could explain the very scattered (presumably relict) distribution of the species in its western range. Many of the remaining habitats have changed due to human activities, such as lowering the water table for intensification of land use, peat ditching, and due to abandonment of extensive grazing.
Adults fly in one generation from June to July. Females lay single eggs on different substrata. Larvae are heliophilous and thermophilous: they feed during the day and bask in the sun in the warmest part of the day in late autumn and early spring (own observation). Many food plant records (of grasses and sedges) have been published (e.g. Chrétien 1886; Weidemann 1995; Lhonore 1996; Lhonore and Lagarde 1999; Lafranchis 2000; Tshikolovets 2003; Dierks 2006) but most of them refer to breeding experiments. Nevertheless, some of them are very likely to be used in the field as well [e.g. Deschampsia caespitosa (L.) P. Beauv.], while others were not confirmed in recent breeding experiments (e.g. Schoenus nigricans L., Dierks 2006, own observation).
Study sites
Between 2008 and 2012, we studied larval and egg-laying habitats of C. oedippus in three countries (Germany—DE, Italy—IT, Slovenia—SLO) that represent the main three habitat types occupied by this species in Europe: wet grasslands on alkaline soil (DE), wet grasslands on acid soil (IT) and dry grasslands (SLO).
In DE, the only remaining population was studied, which inhabits a small and isolated area (ca. 1.1 ha, altitude 490 m) in open wetlands partially enclosed by hedges (Bräu and Schwibinger 2013). The wetter parts of the habitat patches can be classified as Schoenetum ferruginei Du Rietz 1925, with interspersed clusters of Cladietum marisci Allorge 1922. However, the Schoenetum is highly dominated by Carex panicea L. Outside depressions created by peat ditching in former times, vegetation is transient to Allio suaveolentis-Molinietum caeruleae Görs in Oberd. ex Oberd. 1983 merging with Cirsio tuberosi-Molinietum arundinaceae Oberd. et Philippi ex Görs 1974 in drier parts. The habitat patches have been abandoned for decades. While in some parts of the site bushes (mainly Frangula alnus Mill.) are highly abundant, they are rare in areas with high densities of C. oedippus.
In IT one site within the “Baraggia” Regional Oriented Reserve was studied (WGS 84: 45°31′39.6″N 8°09′17.4″E, altitude 300 m). The Reserve protects fragments of natural areas, surrounded by human-modified habitats, mainly rice fields. It is characterised by scattered woods (Quercus robur L., Betula pendula Roth, Carpinus betulus L.), interrupted by large clearings, which are dominated by Calluna vulgaris (L.) Hull and Molinia caerulea (L.) Moench. The study site is composed of three isolated (1600–5500 m apart) patches (1.0, 1.2, 1.7 ha). All are M. caerulea meadows, partially covered by C. vulgaris and with a shrubby perimeter (B. pendula, Populus tremula L., F. alnus). One patch is regularly mown in its central part once per year; the second is extensively grazed; the third is unmanaged, but regularly used by tourists for recreational activities. All three patches are periodically used for military exercises.
In SLO one site on the Kras plateau (WGS 84: 45°52′12.17″N 13°36′27.32″E, altitude 260 m) was investigated. It consists of three adjoining (350–1,000 m apart) patches (1.7, 1.5, 0.8 ha) characterised by abandoned, floristically poor, and overgrown submediterranean–illyrian dry grasslands (associations of Danthonio–Scorzoneretum villosae Ht. and Ht-ić in Ht-ić 1963 and Carici humilis–Centaureetum rupestris Ht. and Ht-ić 1934 on deeper soils). It is characteristic for these successional stages that sedges and grasses dominate the vegetation, while non-graminoid herbs are less abundant. Characteristic is also a high structural heterogeneity, which is composed of dense swards of Sesleria autumnalis (Scop.) F. W. Schultz scattered over a predominant cover of mainly lower and sparse herb layer consisting of other grasses and sedges. Individual islands of shrubs (Cotinus coggygria Scop., Prunus mahaleb L., Ligustrum vulgare L.) and young thermophylic low trees (Fraxinus ornus L., Ailanthus altissima (Mill.) Swingle, Pinus nigra Arnold) grow scattered over the entire abandoned grasslands and surround the study patches.
Larval microhabitat
To obtain data on host-plants and habitat preferences of larvae after hibernation, field surveys were carried out from April to May (DE: 2008, 2009; IT: 2009, SLO: 2010, 2011). For each detected larva we recorded the host-plant species (if feeding was observed) or plant species with feeding traces near the caterpillar, and the percent cover of main structural parameters within a radius of 50 cm around the larva: bare ground, rocks, mosses, litter, shrubs, herbs and known host-plants (HPs). Additional structural parameters were assessed for each country: percent cover of grass-like herbs (GLH, i.e. herbs with plant structure, that do not shade the lower parts of the herb layer; e.g. Allium spp., Anthericum ramosum L., Genista sylvestris Scop.; DE, SLO), percent cover of plants from the families Poaceace, Juncaceae, Cyperaceae (PJC, i.e. plants with erectophile leaf orientation; DE, SLO), percent cover of C. vulgaris (IT), percent cover of each species from the group PJC (SLO), average vegetation height (i.e. prevailing height of herb layer in cm; SLO) and maximum vegetation height (SLO), the lattermost being the tallest plant in the microhabitat. Those plants corresponded mainly to Chrysopogon gryllus (L.) Trin., Bromopsis erecta (Huds.) Fourr. s.str., B. condensata (Hack.) Holub, B. transsilvanica (Steud.) Holub and Stipa sp.; they deviated considerably from average vegetation height. In Germany and Italy, the percent cover of main and additional structural parameters were also recorded for random microlocations (May 2009) which were selected by a randomly thrown stick (Anthes et al. 2003), and represented the spectrum of available microlocations within the studied habitat type. In total, we recorded 49 random microhabitats (DE: 39, IT: 10) and 76 larval microhabitats (DE: 31, IT: 34, SLO: 11).
Oviposition microhabitat
We tracked egg-laying females on sunny days from June to July (DE: 2008, 2010 and some additional observations in 2011 and 2012; IT: 2009; SLO: 2010, 2011). Each female was chosen randomly and then followed for a maximum of ten ovipositions. If no egg-laying occurred within 20 min, we selected another female. Females followed for multiple ovipositions clearly switched plant species during consecutive ovipositions, meaning that repeated sampling of the same female did not represent pseudo-replication and did not bias the result on oviposition electivity. The same main and additional structural parameters were recorded for oviposition (DE: 76, IT: 101, SLO: 55) and random (DE: 35, IT: 150, SLO: 30) microlocations (DE, SLO: July 2010; IT: June 2009) as for the larval microhabitats (see above). Random microlocations again reflect the spectrum of available structures of the site. Additionally, we collected data on the oviposition substratum: plant species, plant part (leaf, steam, bud, other), support freshness (vital, dead), and oviposition height above ground.
Breeding experiments
Breeding of five generations of C. oedippus under field conditions (ex situ) was performed in Germany from 2009 to 2014. Several vivaria of different size (minimum 20 × 30 × 30 cm) were planted with sods taken from the habitat, containing mainly M. caerulea and C. panicea. The top of the vivaria was covered with gauze which was fixed with an elastic rubber band, allowing high air circulation. The vivaria were always kept outdoors, in the same region where the butterfly population lives. They received direct sunlight for several hours a day and were sheltered from rain and snow. Humidity was held at a high level by regular watering. Temperature and humidity were measured with a digital thermo-hygrometer over a long period under different weather conditions. Both were similar to the values occasionally measured at the species’ habitat. For oviposition within the vivaria, some female butterflies were taken from the field (after observation of oviposition in the field, to ensure that they already started ovipositing) and some that emerged and mated in captivity could be used for maintaining the breeding stock as well. Depending on the year, between 26 and 92 pupae were achieved.
In Slovenia, only one young caterpillar was transferred from the field (i.e. study site) in October 2011 to a glass vivarium (50 × 50 × 50 cm) covered with gauze and planted with the sod taken from the location of the caterpillar. The vivarium was exposed to outdoor temperature and sunlight but sheltered from rain and snow. Its inside was humidified with water spraying through the mesh every time it rained or snowed out. The caterpillar was bred until the butterfly hatched (in June 2012).
In both experiments, the behaviour of caterpillars was observed and noted in short intervals.
Statistical analyses
Larval/oviposition microhabitat electivity within each habitat type was evaluated by comparison between larval/oviposition and random microlocations using multiple stepwise forward logistic regression. We fitted a presence-absence logistic model to our presence-only data (i.e. random microlocations represent pseudo-absences, cf. Ward et al. 2009) as we anticipate that the probability of selecting the random microhabitat with the presence of eggs/larvae must be very low given the high density of oviposition substrata (considering non-selectivity of egg-laying females) and host-plants (considering host-plants growth form and larval polyphagy) compared with the density of C. oedippus in each study site. Further, in the case that some of selected random microlocations contained the eggs/larvae, the difference between oviposition/larval and random microlocations were even underestimated (cf. Eilers et al. 2013), meaning that our estimations are conservative. Before regression analysis, all explanatory variables (i.e. structural parameters of microlocations) were tested for intercorrelations by calculating Spearman’s rho correlation coefficients. We defined two types of explanatory variables, i.e. “basic” and “derived” (see Tables 2, 4). Basic variables are main structural parameters of the microhabitat, while derived variables are structural parameters which represent only a part of the coverage of corresponding basic variable. Within both types, we distingushed between “composed” and “simple” variables. Composed variables can be substituted by more simple variables, e.g. Herbs can be substituted by HPs and Herbs_without HPs, or PJC, GLH and NGLH. Simple variables can not be replaced by a set of other variables. In the case of strong correlation (Spearman Rho ≥ |0.9|) between two variables within pairs “basic versus basic” or “basic versus derived”, the simple variable was maintained for entering in regression analysis. If two derived variables were strongly correlated, the one which correlated strongly with selected basic variables was excluded from further regression analysis. The last criterium was considered also in the case of strong correlation between two simple variables.
For identifying the differences between three habitat types for each structural parameter separately, the Kruskal–Wallis Chi test (KW) and Mann–Whitney U tests (MWU) were applied on all possible comparisons as post hoc procedures with Bonferroni correction.
To find out whether the difference in oviposition height between three habitat types depends on height of prevailing egg-laying support, the Jonckheere–Terpstra test was applied. Four categories of egg-laying supports were coded according to plant height (Lauber and Wagner 1996): 1 = Carex humilis (3–11 cm), 2 = C. vulgaris (10–50 cm), 3 = C. panicea (20–40 cm), 4 = M. caerulea agg. (30–250 cm).
To detect the relationships between the type of oviposition support and most abundant structural parameter in egg-laying microhabitats, Chi Square tests using Likelihood ratio statistic were applied because of small sample sizes, which resulted in expected frequencies lower than one in some cases. For assessing the strength of association between both variables, Cramer’s V was used. Standardized residuals were used to define the significant contributors to the overall Chi square value. For the purpose of Chi square testing, the egg-laying supports and most abundant structural parameters were arranged in the following categories: DE—M. caerulea, C. panicea, other herbs, litter, shrub; IT—M. caerulea, Carex sp., C. vulgaris, other herbs; SLO—Carex humilis, Poaceae, other plants. As litter was the most abundant structural parameter in 76 % of the Slovenian egg-laying microhabitats, the possible relationships between the type of oviposition support and the abundance of structural parameters was analyzed using the second most abundant structure parameter in the microhabitats.
All statistical analyses were performed using SPSS 13.0 (SPSS Inc. 1989–2004).
Results
Larval preferences
In our three habitat types, a total of 85 feeding observations were detected on six plant substrata: C. panicea, C. davalliana Sm., C. humilis, Carex sp., M. caerulea and Festuca rupicola (Table 1). Sedges represented the majority of the larval diet after winter in all three habitat types (DE: 71 %, IT: 94 %, SLO: 77 %), while observed alternative host-plants were M. caerulea in both wet grassland types and F. rupicola in dry habitat.
Larval microhabitats differed between three habitat types not only in the presence of main structural parameters (Table 2), but also in the coverage of those they had in common: litter (KW χ2 = 22.67, df = 2, p = 0.0001), herbs (KW χ2 = 11.94, df = 2, p = 0.003) and shrubs (KW χ2 = 60.34, df = 2, p = 0.0001) (Fig. 1a). Wet larval microhabitats were characterized by a higher coverage of litter and by lower abundances of herbs than dry microhabitats. Shrub cover was significantly higher in Italian microhabitats than in the other two countries. In Italy, the majority of shrub cover was formed by C. vulgaris (Table 2). If we assume that this perennial dwarf shrub has a similar function as litter for overwintering larvae and add its coverage to litter, the larval microhabitats still significantly differed between Italy and Slovenia, but not between both wet habitat types (Fig. 1a). The cover of host-plants (Table 2) was significantly higher in German (median = 30 %) than in Slovenian (median = 12 %) larval microhabitats (DE vs. SLO, MWU Z = −4.23, p = 0.0001; respective data not available for IT). If we assume that the other grass species present in dry habitats are also used as larval food-plants (PJC in Table 2), the host-plants abundances differ only slightly between both habitat types (MWU Z = −2.15, p = 0.032).
Coverage of main structural parameters in a larval and b oviposition microhabitats of C. oedippus in Germany (DE), Italy (IT) and Slovenia (SLO). Mann–Whitney tests: a litter, DE > IT: p = 0.0001, DE > SLO: p = 0.0001, IT ≈ SLO: p = 0.831; herbs, DE ≈ IT: p = 0.297, DE < SLO: p = 0.005, IT < SLO: p = 0.001; shrubs, DE < IT: p = 0.0001, DE < SLO: p = 0.0001, IT > SLO: p = 0.0001; litter + C. vulgaris, DE ≈ IT: p = 0.724, IT > SLO: p = 0.001. b litter, DE ≈ SLO: p = 0.577, DE > IT: p = 0.0001, SLO > IT: p = 0.0001; shrubs, DE ≈ SLO: p = 0.545; DE < IT: p = 0.0001, SLO < IT: p = 0.0001; Herbs without hostplants, SLO > DE: p = 0.0001; SLO > IT: p = 0.0001; DE > IT: p = 0.0001; hostplants, DE ≈ IT: p = 0.360; DE > SLO: p = 0.0001; IT > SLO: p = 0.0001; litter + C. vulgaris, DE ≈ IT: p = 0.053, IT > SLO: p = 0.021
In Germany, occupied microhabitats had a significantly higher coverage of litter and of the preferred host-plant C. panicea, and lower abundances of shrubs, M. caerulea and non grassy-like herbs than available sites (Table 2). Due to lower coverage of M. caerulea and herbs with planophile leaf orientation, larval microhabitats were characterized by lower abundances of host-plants, graminoids (PJC), all herbs and herbs other than host-plants. Larval preferences for microlocations with high coverage of C. panicea revealed that this preferred host-plant has additional characteristics, which enable better survival of caterpillars after hibernation compared to the alternative host M. caerulea (see section “Ex situ breeding observations”). In Italy, preferences of overwintering larvae showed a similar pattern as in Germany: they preferentially occurred on sites with higher coverage of litter or litter + C. vulgaris and lower abundance of herbs compared to the available microlocations (Table 2). Besides, larval microhabitats had lower coverage of bare ground. No information was available to assess the effect of host-plants on larval microhabitat selection in Italy. However, in both wet habitat types, the coverage of litter already explained most of the variation in microhabitat selection by overwintering larvae (Table 3). The likelihood of a microlocation being occupied by larvae after hibernation increased with litter coverage.
Preferences of egg-laying females
During this study we found out that females apply two oviposition modes in the pre-alighting phase of oviposition site selection, (1) “normal mode”—female lands on plants parts in the upper layer of the herb vegetation and remains there for post-alighting phase (egg-laying), and (2) “dropdown behaviour”—female lands on plants parts in the upper layer of the herb vegetation, then crawls or drops down to the ground, and walks on the ground searching for suitable egg-laying support. The second mode is less frequently used and it was observed only in Germany.
The eggs were deposited on plants growing at the edge of gaps in the herb layer. In three habitat types, a total of 236 eggs were laid on 27 different egg-laying supports (Table 1), including herbs (71 %), shrubs (19 %), litter (2 %) and undetermined plants (8 %). All eggs were deposited singly on leaves (89 %), stems (10 %) or buds (1 %). Three quarters (76 %) of the eggs were attached on vital parts of plants, the remaining on dry parts or litter (20 %) and undetermined substrata (4 %).
The above described wide range indicates a rather unselective egg-laying behaviour. Nevertheless, some plant species dominate the plant spectrum used for ovipositon. The prevailing supports were M. caerulea (55 %) and C. panicea (28 %) in Germany (χ2 = 40.35, df = 1, p < 0.001), C. vulgaris (41 %) and M. caerulea (34 %) in Italy (χ2 = 34.21, df = 1, p < 0.001), and Carex humilis (65 %) in Slovenia (χ2 = 5.27, df = 1, p < 0.05). The plant species used for oviposition and the most abundant structural parameter in the egg-laying microhabitat show a significant positive association (DE, LR = 40.90, df = 16, p = 0.001; Cramer’s V = 0.560, p = 0.0001; IT, LR = 43.47, df = 15, p = 0.0001; Cramer’s V = 0.406, p = 0.0001; SLO, LR = 13.03, df = 4, p = 0.020; Cramer’s V = 0.306, p = 0.032). Eggs are simply deposited on the most abundant plant species, which explains the regular use of the non-host C. vulgaris in Italy.
The vertical positions of eggs on substrata ranged from 0 to 44 cm and oviposition height increased with the height of prevailing egg-laying support (Jonckheere–Terpstra z = 5.12, No. of levels = 4, p = 0.0001). The eggs were deposited significantly higher in the most often applied (“normal”) oviposition mode (N = 67) than in dropdown mode (N = 9; MWU Z = −4.37, p = 0.0001).
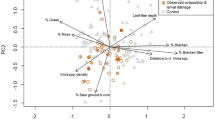
Oviposition sites differed between the three habitat types not only in vegetation composition (due to different vegetation grassland communities) but also in microhabitat structure considering the presence and abundances of main structural parameters (Table 4). German and Slovenian microhabitats were denoted by a higher cover of litter and a lower cover of shrubs than Italian ones (KW, litter: χ2 = 38.36, df = 2, p = 0.0001; shrubs: χ2 = 81.15, df = 2, p = 0.0001; Fig. 1b). The coverage of herbs other than host-plants was significantly higher in dry than in wet microhabitats (KW χ2 = 146.09, df = 2, p = 0.0001; Fig. 1b). Consequently, host-plants were more abundant in wet than in dry oviposition spots (KW χ2 = 65.39, df = 2, p = 0.0001; Fig. 1b). But in dry habitats several further grass species occurred (PJC in Table 4); if they are taken into consideration as potential host-plants, the difference is equalled out (KW χ2 = 5.91, df = 2, p = 0.052). Litter is an important structure in oviposition microhabitats, because it creates a vegetation structure (herb layer) rich in gaps. Eggs are usually deposited at the edges of these gaps. As it was one of the most variable structural parameter in Italian oviposition microhabitats, and its median cover was much lower than in the other two countries (Fig. 1b), we presumed that other structures take over its role when its cover is very low, e.g. perennial C. vulgaris according to its significant negative correlation with litter cover (Spearman rho = −0.399, p = 0.0001). Indeed, the sum of litter and C. vulgaris in Italian oviposition microhabitats was only slightly higher than litter cover in Germany and Slovenia (Fig. 1b).
In all three habitat types, the shrub cover was significantly lower in oviposition than in available sites (Table 4). In Germany, this was the only difference between both types of microlocation. Italian egg-laying microhabitats had significantly higher abundances of litter and M. caerulea than available sites. In Slovenia, oviposition microhabitats were characterized by a higher coverage of litter and maximum vegetation height than were in available microlocations, and also by lower abundances of all herbs and herbs other than observed host-plants. Thus, German oviposition microhabitats were the standard structure of the locality inhabited by C. oedippus as the difference in shrub cover between oviposition and available sites was not enough to improve a constant-only model. In both other countries, oviposition pattern/site selection was best explained by a combination of litter cover and abundances of shrubs and non-host plants. In Italy, the likelihood of a spot being accepted for oviposition increased with the litter coverage and decreased with the cover of shrubs other than C. vulgaris and herbs other than host-plants (Table 5). In Slovenia, the presence of high plant stems of C. gryllus, Bromopsis sp., Stipa sp. which deviated from prevailing/average vegetation height positively influenced the selection of oviposition microhabitat, but high covers of shrubs and other herbs than host plants decreased the likelihood of a spot to be chosen by an egg-laying female (Table 5). Presence of high grass species indicates microlocations with higher coverage of C. humilis (Spearman rho, C.h. vs. Bromopsis sp. = 0.268, p = 0.014, C.h. vs. C. gryllus = 0.249, p = 0.022, C.h. vs. Stipa sp. = 0.269, p = 0.013) and litter (Spearman rho, Bromopsis sp. vs. litter = 0.233, p = 0.041), and lower abundance of Sesleria autumnalis (Spearman rho, S. a. vs. C. humilis = −0.363, p = 0.001; S. a. vs. Bromopsis sp. = −0.368, p = 0.001; S. a. vs. C. gryllus = −0.386, p = 0.0001; S. a. vs. Stipa sp. = −0.282, p = 0.009) which creates a very homogeneous and dense sward. Furthermore, coverage of herbs with planophile leaf orientation was lower in occupied than in available microlocations although the difference was only close to significance (Table 4: herbs without PJC).
Oviposition microhabitats were characterised by a clearly lower proportion of litter and a higher cover of herbs than larval spots (Tables 2, 4; MWU: litter, DE (40 vs. 70 %): Z = −6.71, p = 0.0001; IT (10 vs. 45 %): Z = −5.01, p = 0.0001; SLO (40 vs. 45 %): Z = −2.70, p = 0.007; herbs, DE (60 vs. 30 %): Z = −6.43, p = 0.0001; IT (50 vs. 30 %): Z = −2.70, p = 0.007; SLO (56 vs. 50 %): Z = −0.98, p = 0.327), which is largely a seasonal effect.
Ex situ breeding observations
Our observations from breeding under field conditions showed that caterpillars concentrate on the sun-facing side of tufts while feeding or resting, except during search for a place to pupate and during hibernation. From late October onwards, most caterpillars begin to retreat for hibernation: some overwinter at the base of sedges or grasses or even on upper parts of their food-plants hidden from the sun, presumably to avoid awakening too early on sunny winter days.
During breeding experiments we observed that caterpillars which hibernate in their third instar usually awake in spring when temperatures rise to about 20 °C for several consecutive days. At that time not all host-plants are available. In Germany, the time lag between larval awakening and the start of M. caerulea growth varied from about 10–30 days (Table 6). In contrast, C. panicea which remains green during winter is always available for larvae as food resource. Hibernated caterpillars were observed feeding on it shortly after the start of activity. Moreover, caterpillars do not feed on Molinia leaves shorter than about 5 cm, thus even enlarging the time lag. In Slovenia, comparing awaking time with observations of host-plants growth status in the field and in ex situ showed (Table 6) that besides known host-plants, C. humilis and F. rupicola which overwintered green, some other grass species, e.g. Brachypodium rupestre, Sesleria autumnalis, are also available.
Discussion
Habitat requirements of ovipositing females and larvae
Egg-laying females of C. oedippus are not selective regarding oviposition substratum, e.g. plant species or exact position on the plant. Eggs were mainly deposited on the most abundant structure parameter (plant/plant group) available in the microhabitat. This wide range is also reflected by previously known egg-laying plants from the species’ European range (Table 7).
Such absence of electivity is in contrast to many butterfly species, who’s females carefully choose the oviposition plant, e.g. Phengaris (Maculinea) butterflies (Dolek et al. 1998; Thomas and Elmes 2001; Kassai and Peregovits 2005), Lycaena alciphron (Dolek and Geyer 2001), Boloria aquilonaris (Turlure et al. 2013), Euphydrays desfontainii (Pennekamp et al. 2013), E. maturna (Dolek et al. 2013), Colias myrmidone (Dolek et al. 2005, Szentirmai et al. 2014). This contrast very likely relates to the wide host-plant range of C. oedippus larvae (i.e. different species from Poaceae and Cyperaceae), the growth form of host-plants (i.e. dense ground-covering plants growing mostly in tufts) and their relatively high stability in terms of growing period and abundance (e.g. due to possibility of vegetative reproduction).
Nevertheless, an important factor for suitable reproduction habitats is the availability of host-plants in close vicinity to the oviposition substratum, i.e. within reach of young caterpillars. The coverage of host-plants in oviposition sites was always high: between 45 and 50 % in wet, and 18 % (only observed host-plant species) or 41 % (including potential host-plant species) in dry habitats. The finding that coverage of bare ground was highest in Italian microlocations, and only there larval microhabitats had significantly lower bare ground cover than random sites indicates that microlocations with higher amounts of bare ground are less suitable for overwintering larvae, possibly because bare ground can restrict larval movements and expose them to predation (e.g. Doak 2000). Alternatively, it could simply reduce the coverage of host-plants and connectivity around the caterpillar, which could increase the time larvae spend searching for a new host. We indentified six food-plant species in the field. Feeding on M. caerulea in the field was also observed in France (Dierks 2006) and Poland (Selezniew et al. 2010). In former publications many additional grass and sedge species have been listed, but probably all of these refer to breeding experiments. Nevertheless, we are convinced that many more grass and sedge species are used, if they are present in the habitat.
Non-specificity for oviposition substratum, together with a high proportion of eggs (DE: 55 %, IT: 34 %) deposited on less frequently used host-plants (M. caerulea, DE: 27 %, IT: 6 %) or even on non-host plant material (Table 1) points to other crucial factors used by egg-laying females to maximize offspring performance (e.g. Mayhew 1997; Janz 2002). The models which best explain the oviposition and larval preferences of C. oedippus showed that vegetation structure of microlocation is an important parameter in oviposition site selection. High cover of litter and/or dwarf shrubs, such as C. vulgaris, in selected microhabitats (median, larval: 45–70 %, oviposition: 40–50 %) creates a herb layer rich in gaps. Egg deposition mostly occurred at the edges of these gaps in order to allow helio- and thermo-philous larvae to be adequately sun-exposed. Dry plant biomass provides warmer environments (WallisDeVries and van Swaay 2006) which is of particular importance for overwintering larvae in early spring to enable them to reach optimal body temperature, and may also function as a microclimatic buffer (e.g. Turlure et al. 2010; Weking et al. 2013). In the field, most caterpillars could be found on sun exposed edges of tufts which is in agreement with the observations from ex situ breeding. The lower abundances of shrubs in egg-laying microlocations compared with random sites in all three habitat types also points to the importance of vegetation architecture, with high solar insolation and low shading in oviposition/larval microhabitat selection.
The structure of the host-plant/oviposition substratum and surrounding vegetation directly influences the microclimate, e.g. humidity, temperature and solar exposure (Beyer and Schultz 2010; O’Connor et al. 2014). Considering that there was a positive association between the type of oviposition support and the most abundant structural parameter in egg-laying microhabitat in all three habitat types, the height of prevailing egg-laying support could be used as an indicator for average vegetation height in the egg-laying microhabitat. Thus, a positive correlation between oviposition height and the height of the prevailing egg-laying support across three habitat types [from min to max height: C. humilis (SLO)–C. vulgaris (IT)–C. panicea (IT, DE)–M. caerulea (IT, DE)] suggests that females try to oviposit as high as possible on the selected substratum and within the vegetation (cf. Obermaier et al. 2006), meaning that eggs are deposited at positions that receive high solar radiation. The adjusting of oviposition height to the height of the local radiation surface to maximise heat absorption was also observed in two other satyrinae species which attach the eggs to a substratum (not simply drop them between the grasses), Hipparchia fagi (Möllenbeck et al. 2009) and Coenonympha tullia (Weking et al. 2013). However, in normal oviposition mode, females of C. oedippus laid eggs just below the top of the surrounding herb vegetation, but despite a limited number of field observations, it seems that dropdown mode occurs when air temperature unexpectedly decreases due to clouds temporarily covering the sun. In such cases, egg-laying females climbed down and deposited eggs on the surface of the litter cover, probably as dry plant biomass provides warmer environment than green plants (WallisDeVries and van Swaay 2006). Furthermore, the lower coverage of herbs with planophile leaf orientation in oviposition (SLO) and larval (DE) than in random sites indicates that shading of the lower herb layer is not favourable for the development of eggs and caterpillars. It seems that oviposition site selection in C. oedippus is influenced by the thermal requirements of eggs and larvae. This is additionally supported by the female’s avoidance of dense tufts of S. autumnalis in dry habitats, which do not offer adequate sun-exposure. These results are also in agreement with a previous study on within-patch movements of C. oedippus adults in Slovenian wet habitat (Čelik et al. 2009a) which showed that spatial and temporal patterns of female micro-distribution is affected by vegetation height, the homogeneity of host plant stands and the shading of the the lowest parts of the herb layer.
Preferences of overwintering larvae and egg-laying females for microlocations with high amounts of host-plants and litter and/or dwarf shrubs, and low amounts of bare ground, shrubs and herbs other than graminoids, together with adjusting the oviposition height as high as possible within the vegetation across all three habitat types revealed the high importance of vegetation structure in C. oedippus larval/oviposition microhabitat selection. At first sight, this is a relatively general pattern of habitat use across the macro-environmental gradient. The utilization of host-plants specific to the habitat type, and differences of preferred microlocations in presence and relative abundances of structural parameters between the habitat types showed that such patterns are a result of microhabitat selection tailored to local environmental conditions.
Winter green food-plants as key factor for larval survival
Butterfly host-plant synchronisation is a known phenomenon (e.g. review in Munguira et al. 2009), which directly influences larval growth and survival, and ultimately population fitness. A perfect synchronisation is of crucial importance for monophagous and oligophagous species overwintering as egg (e.g. de Vries et al. 2011) or as young caterpillars, as with C. oedippus (e.g. Gradl 1946). Based on breeding experiments Gradl (1946) pointed out that a temporal mismatch exists between larval awakening and availability of the “prime” host-plant, M. caerulea. He reported that caterpillars awoke from hibernation very early due to enduring foehn weather in spring on March 20th, while their “normal” food resource Molinia was not yet available. Our breeding data and field observations after hibernation showed that caterpillars of C. oedippus awake from hibernation at a time period when only some host-plants are available (Table 6). At that time the larvae are still quite small (about 1.3 cm) and not able to move very far to search for food. Thus, they have to find host-plants in their immediate vicinity within a few days as their need for food and liquid is urgent after the long period of starvation during hibernation. Significant differences between larval and random spots in cover of the winter-green C. panicea support the assumption that the likelihood of larval survival is strongly influenced by the availability of this plant in German wetland habitats. Molinia starts to grow relatively late in spring, so in Molinia-dominated meadows C. panicea or other winter-green Carex species with soft leaves are needed as interim food. For C. oedippus habitats near Bordeaux, Dierks (2006) discussed the role of Pseudoarrhenaterum longifolium (Thore) Rouy which starts to grow earlier than Molinia and could serve as food for early awakening caterpillars. It seems that synchronisation between host-plants growing and larval diet requirements after hibernation is better harmonized in dry habitats of C. oedippus as the preferred food plant C. humilis overwinters green and sprouts already in early spring (March).
We hypothesize that winter-green host-plants may also play a substantial role in the survival of overwintering caterpillars of several other butterfly species occurring in habitats with highly dominating non-winter green host-plants; a potential relationship that is so far not adequately taken into account. Namely, field observations on two satyrinae species, Coenonympha hero (Wagner 2010) and Minois dryas (Sachteleben and Winterholler 2013), living in Molinia-dominated habitats also showed that winter-green grasses (e.g. Festuca spp.) or sedges (Carex spp.) were used as interim food source immediately after larval awakening.
Late successional habitats
It is well known and demonstrated in detail for many butterfly species that their caterpillars need early successional stages, short turf, or otherwise hot and open habitats for their development (e.g. Pyrgus malvae Krämer et al. 2012; Hesperia comma Hermann and Steiner 1997; Parnassius apollo Geyer and Dolek 1995; L. alciphron Dolek and Geyer 2001; Scolitantides baton Konvička et al. 2008; Phengaris arion Thomas 1980, Pauler et al. 1995, Fartmann 2005; Polyommatus bellargus Thomas 1983; Chazara briseis Königsdorfer 1997, Leopold 2001). The present study shows a contrasting habitat choice of C. oedippus, a species being restricted to largely unmanaged grassland with a dominating litter layer, but no substantial growth of woody plants. Our results on larval and oviposition preferences are in accordance with the findings from a population study of C. oedippus in Hungary (Örvössy et al. 2013) which reveals that large amounts of grass litter and structured vegetation with tussocks positively affect population size and density. The importance of late successional habitats, mainly characterised by pronounced litter layer, relatively nutrient-poor conditions, no considerable growth of shrubs and direct sun exposition, were also demonstrated for some other butterflies, e.g. C. hero (Steiner and Hermann 1999; Dolek 2011; Bräu and Dolek 2013), Lopinga achine (Geyer and Dolek 2013), Erebia medusa (Stuhldreher and Fartmann 2014) and Lycaena helle (Nunner 1995).
Implications for conservation
The results of the present study showed that besides the availability of fresh-green host-plants in the vicinity of hibernated larvae, mainly the vegetation structure and microclimate of the herb layer are essential factors for oviposition site selection and successful development of premature stages of C. oedippus. The herb layer has to be rich in gaps (but not bare ground), usually created by large amount of litter or alternatively by dwarf shrubs. Such vegetation structure enables egg-laying females to adapt the oviposition height to the local radiation surface with high heat absorption. In a gap-rich herb vegetation, the ectotherm and heliophilous caterpillar dependent on direct sunlight (i.e. when basking for elevation of body temperature) can select thermally favourable microclimates by behavioural thermoregulation (c.f. Stevenson 1985; Turlure et al. 2011), i.e. translocation of its position between the top of herbaceous plants, the litter surface (warmer than upper green vegetation during colder/cloudy days) and the more balanced microclimate inside the litter in terms of temperature and humidity. A dense litter layer can also slow down the further secondary succession in the habitat as it prevents germination of shrubs (Ellenberg 2009; Ruprecht and Szabo 2012), and consequently affects the structure, diversity and dynamics of grassland plant communities (Ruprecht et al. 2010; Loydi et al. 2013). As litter in dry grasslands persists for longer periods than litter from wet areas due to higher lignin concentration (Fortunel et al. 2009), the site management in C. oedippus habitats must be diversified and adapted to meet the special needs of this highly endangered species across its range. Hence, in most of C. oedippus habitats some kind of management has become essential to avoid overgrowth with bushes and trees or, in wet habitats, with reed. Manual removal of shrubs should be preferred. Regular mowing or forms of grazing which create a uniform vegetation structure (i.e. close homogeneous sward) are thought to be detrimental. Furthermore, direct losses of larvae can be caused by cutting, as caterpillars partially feed on their food-plants until the onset of November and some keep sitting on upper parts of their food-plants even in winter (observations from breeding). In habitats where the reduction of bushes is not sufficient, only patchy mowing in the winter period (December–February) can be recommended to keep the habitat open without harming the population too much. Overgrowing with dense reed can be a problem in some wet habitats. Mowing experiments since 2009 (Bräu and Völkl unpublished.) have already shown promising results on reduction of reed if cutting is done during the flight period with a cutter bar at a high level of about 30 cm above ground. However, this kind of mowing should be restricted just to parts of the habitat with dense reed to avoid emigration of butterflies and should mainly be used for habitat restoration.
References
Anthes N, Fartmann T, Hermann G, Kaule G (2003) Combining larval habitat quality and metapopulation structure—the key for successful management of pre-alpine Euphydryas aurinia colonies. J Insect Conserv 7:175–185
Anthes N, Fartmann T, Hermann G (2008) The Duke of Burgundy butterfly and its dukedom: larval niche variation in Hamearis lucina across Central Europe. J Insect Conserv 12:3–14
Awmack CS, Leather SR (2002) Host plant quality and fecunditiy in herbivorous insects. Annu Rev Entomol 47:817–844
Balletto E, Bonelli S, Cassulo L (2007) Insecta Lepidoptera Papilionoidea. In: Ruffo S, Stoch F (eds) Checklist and Distribution of the Italian Fauna. 10.000 terrestrial and inland water species, 2nd and revised edition, Verona, pp 257–261
Balletto E, Bonelli S, Zilli A (2014) Lepidotteri. In: Genovesi P, Angelini P, Bianchi E, Dupré E, Ercole S, Giacanelli V, Ronchi F, Stoch F Specie e habitat di interesse comunitario in Italia: distribuzione, stato di conservazione e trend. ISPRA, Serie Rapporti, 194/2014
Beyer LJ, Schultz CB (2010) Oviposition selection by rare grass skipper Polites mardon in montane habitats: advancing ecological understanding to develop conservation strategies. Biol Conserv 143:862–872
Bischof A (1968) Coenonympha oedippus Fabricius, eine kleine Chorographie (Lepidoptera, Satyridae). Mitt Ent Ges Basel 18:41–63
Bonelli S, Canterino S, Balletto E (2010) Ecology of Coenonympha oedippus (Fabricius, 1787) (Lepidoptera: Nymphalidae) in Italy. Oedippus 26:25–30
Bräu M, Dolek M (2013) Wald-Wiesenvögelchen Coenonympha hero (Linnaeus, 1758). In: Bräu M, Bolz R, Kolbeck H, Nunner A, Voith J, Wolf W (eds) Tagfalter in Bayern. Eugen Ulmer, Stuttgart, pp 472–475
Bräu M, Schwibinger M (2013) Moor-Wiesenvögelchen Coenonympha oedippus (Fabricius, 1787). In: Bräu M, Bolz R, Kolbeck H, Nunner A, Voith J, Wolf W (eds) Tagfalter in Bayern. Eugen Ulmer, Stuttgart, pp 460–463
Bräu M, Dolek M, Stettmer C (2010) Habitat requirements, larval development and food preferences of the German population of the False Ringlet Coenonympha oedippus (Fabricius, 1787) (Lepidoptera: Nymphalidae)—research on the ecological needs to develop management tools. Oedippus 26:41–51
Čelik T (1997) Ecological researches of endangered species Coenonympha oedippus Fabricius, 1787 (Lepidoptera: Satyridae) on the Ljubljansko barje. M.Sc. thesis; University of Ljubljana
Čelik T (2003) Population structure, migration and conservation of Coenonympha oedippus Fabricius, 1787 (Lepidoptera: Satyridae) in a fragmented landscape. Ph.D. thesis; University of Ljubljana
Čelik T (2004) Population dynamics of endangered species Coenonympha oedippus Fabricius, 1787 (Lepidoptera: Satyridae) on the Ljubljansko barje. Acta Entomol Slov 12:99–114
Čelik T, Verovnik R (2010) Distribution, habitat preferences and population ecology of the False Ringlet Coenonympha oedippus (Fabricius, 1787) (Lepidoptera: Nymphalidae) in Slovenia. Oedippus 26:7–15
Čelik T, Vreš B, Seliškar A (2009a) Determinants of within-patch microdistribution and movements of endangered butterfly Coenonympha oedippus (Fabricius, 1787) Nymphalidae: Satyrinae). Hacquetia 8(2):115–128
Čelik T, Vreš B, Seliškar A (2009b) The status of populations and habitats and recommendations for monitoring of threatened species False Ringlet (Coenonympha oedippus), Snake’shead fritillary (Fritillaria meleagris) and Loesel’s twayblade (Liparis loeselii) on Ljubljansko barje. Final report (in Slovene with English Summary). Biološki inštitut ZRC SAZU, Ljubljana
Chrétien MP (1886) Une note sur les premiers ćtats du Coenonympha oedippus. In: Bourgeois MJ (ed) Séance du 13 octobre 1886 (Bulletin entomologique). Ann Soc Entomol Fr 6:638 (157)
De Vries HH, Ens SH, de Graaf G, Teunissen L, van der Velde R, Vogelaar L, Winterink A, Visser ME (2011) Synchronisation of egg hatching of brown hairstreak (Thecla betulae) and budburst of blackthorn (Prunus spinosa) in a warmer future. J Insect Conserv 15:311–319
Dennis RLH (2003) Towards a functional resource-based concept for habitat: a butterfly biology viewpoint. Oikos 102:417–426
Dennis RLH, Shreeve TG, Van Dyck H (2006) Habitats and resources: the need for a resource-based definition to conserve butterflies. Biodivers Conserv 15:1943–1966
Dierks K (2006) Beobachtungen zur Larvalbiologie von Coenonympha oedippus (Ffabricius, 1787) im Südwesten Frankreichs (Lepidoptera: Satyridae). Entomol Z 116(4):186–188
Doak P (2000) Population consequences of restricted dispersal for an insect herbivore in a subdivided habitat. Ecology 81:1828–1841
Doak P, Kareiva P, Kingsolver J (2006) Fitness consequences of choosy oviposition for time-limited butterfly. Ecology 87:395–408
Dolek M (2011) Wald-Wiesenvögelchen Coenonympha hero (Linnaeus, 1758). Merkblatt Artenschutz 37, Bayerisches Landesamt für Umwelt, Augsburg
Dolek M, Geyer A (2001) Der Violette Feuerfalter (Lycaena alciphron): Artenhilfsprogramm für einen wenig bekannten Tagfalter. Schriftenreihe des Bayerischen LfU 156:341–354
Dolek M, Geyer A, Bolz R (1998) Distribution of Maculinea rebeli and host plant use along the river Danube. J Insect Conserv 2:85–89
Dolek M, Freese A, Geyer A, Stetter H (2005) The decline of Colias myrmidone at the western edge of its range and notes on its habitat requirements. Biologia 60:607–610
Dolek M, Freese-Hager A, Geyer A, Balletto E, Bonelli S (2013) Multiple oviposition and larval feeding strategies in Euphydryas maturna (Linné, 1758) (Nymphalidae) at two disjoint European sites. J Insect Conserv 17:357–366
Dušej G, Wemeille E, Carron G, Ziegler H (2010) Concerning the situation of the False Ringlet Coenonympha oedippus (Fabricius, 1787) (Lepidoptera: Nymphalidae) in Switzerland. Oedippus 26:38–40
Eilers S, Pettersson LB, Öckinger E (2013) Micro-climate determines oviposition site selection and abundance in the butterfly Pyrgus armoricanus at its northern range margin. Ecol Entomol 38:183–192
Ellenberg H (2009) Vegetation ecology of Central Europe. Cambridge University Press, Cambridge
Fartmann T (2005) Quendel-Ameisenbläuling Glaucopsyche arion (Linnaeus, 1758). Naturschutz und Biologische Vielfalt 20:175–180
Fortunel C, Garnier E, Joffre R, Kazakou E, Quested H, Grigulis K, Lavorel S, Ansquer P, Castro H, Cruz P (2009) Leaf traits capture the effects of land use changes and climate on litter decomposability in grasslands across Europe. Ecology 90:598–611
Geyer A, Dolek M (1995) Ökologie und Schutz des Apollofalters (Parnassius apollo) in der Frankenalb. Mitt Dt Gesell Allg Ang Ent 10:333–336
Geyer A, Dolek M (2013) Gelbringfalter Lopinga achine (Scopoli, 1763). In: Bräu M, Bolz R, Kolbeck H, Nunner A, Voith J, Wolf W (eds) Tagfalter in Bayern. Eugen Ulmer, Stuttgart, pp 452–455
Gorbunov P, Kosterin O (2007) The butterflies (Hesperioidea in et Papilionidea) of North Asia (assian part of Russia) in nature, vol 2. Rodina and Fodio, Moscow
Gradl F (1946) Coenonympha oedippus F. Z Wien Ent Ges 30:14–20
Gripenberg S, Mayhew PJ, Parnell M, Roslin T (2010) A meta-analysis of preference–performance relationships in phytophagous insects. Ecol Lett 13:383–393
Habeler H (1972) Zur Kenntnis der Lebensräume von Coenonympha oedippus F. (Lep. Satyridae). Nachr Bayer Ent 21(3):51–54
Hafner J (1910) Makrolepidopteren von Görz und Umgebung. Entomologischen Zeitschrift, Sonder-Abdruck, pp 1–40
Hermann G, Steiner R (1997) Eiablage und Larvalhabitat des Komma-Dickkopffalters (Hesperia comma Linnaeus 1758) in Baden-Württemberg (Lepidoptera, Hesperiidae). Carolinea 55:35–42
Janz N (2002) Evolutionary ecology of oviposition strategies. In: Hilker M, Meiners T (eds) Chemoecology of insect eggs and egg deposition. Blackwell, Berlin, pp 349–376
Kalarus K, Skórka P, Nowicki P (2013) Resource use in two contrasting habitat types raises different challenges for the conservation of the dryad butterfly Minois dryas. J Insect Conserv 17:777–786
Kassai F, Peregovits L (2005) Contrasting egg laying behaviour of the ecotypes of Maculinea alcon in Hungary. In: Settele J, Kühn E, Thomas JA (eds) Studies on the ecology and conservation of butterflies in Europe, Vol 2. Species ecology along a European gradient: Maculinea butterflies as a Model. Sofia-Moskau, Pensoft Publishers, p 73
Kolar H (1919) Über das Vorkommen von Coenonympha oedippus F. Z Österr Entomol Ver Wien 4:96
Kolar H (1929) Verbreitung von Coenonympha oedippus F. in Europa. Verh Zoo Bot Ver Wien 78:105–108
Königsdorfer M (1997) Die Berghexe (Chazara briseis L.) in Schwaben und angrenzenden Gebieten. Ber Nat Ver Schwaben 101:69–87
Konvička M, Dvořak L, Pavličko A, Fric Z (2008) The Baton blue (Pseudophilotes baton) (Lepidoptera, Lycaenidae) in south-western Bohemia: iron curtain, military ranges and endangered butterfly. Silva Gabreta 14:187–198
Krämer B, Kämpf I, Enderle J, Poniatowski D, Fartmann T (2012) Microhabitat selection in a grassland butterfly: a trade-off between microclimate and food availability. J Insect Conserv 16:857–865
Kudrna O, Harpke A, Lux K, Pennerstorfer J, Schweiger O, Settele J, Wiemers M (2011) Distribution Atlas of Butterflies in Europe. Gesellschaft für Schmetterlingsschutz e.V, Halle
Lafranchis T (2000) Les Papillions de jour de France. Collection Parthenope, editions Biotope, Meze, Belgique et Luxembourg et leurs chenilles
Lauber K, Wagner G (1996) Flora Helvetica. Paul Haupt, Bern
Lawson CR, Bennie J, Hodgson JA, Thomas CD, Wilson RJ (2014) Topographic microclimates drive microhabitat associations at the range margin of a butterfly. Ecography 37:732–740
Leopold P (2001) Schmetterlingszönosen ausgewählter Kalk-Magerrasen im Saale-Unstrut-Gebiet (Sachsen-Anhalt) unter besonderer Berücksichtigung der Habitate des Segelfalters und der Berghexe. Dipl.-Arb. Inst. F. Landschaftsökologie, Univ. Münster
Lhonore J (1996) Coenonympha oedippus. In: Helsdingen van PJ, Willemse L, Speight MCD. (eds) Background information on invertebrates of the habitats directive and the bern convention. Part I—Crustacea, Coleoptera and Lepidoptera. Council of Europe, Strasbourg, Nature and environment 79:98–104
Lhonore J, Lagarde M (1999) Biogeographie, ecologie et protection de Coenonympha oedippus (Fab., 1787) (Lepidoptera: Nymphalidae: Satyrinae). Ann Soc Entomol Fr 35(Suppl):299–307
Lindman L, Johansson B, Gotthard K, Tammmaru T (2013) Host plant relationships of an endangered butterfly, Lopinga achine (Lepidoptera: Nymphalidae) in northern Europe. J Insect Conserv 17:375–383
Loydi A, Eckstein RL, Otte A, Donath T (2013) Effects of litter on seedling establishment in natural and semi-natural grasslands: a meta-analysis. J Ecol 101:454–464
Mayhew PJ (1997) Adaptive patterns of host-plant selection by phytophagous insects. Oikos 79:417–428
Möllenbeck V, Hermann G, Fartmann T (2009) Does prescribed burning mean a threat to the rare satyrine butterfly Hipparchia fagi? Larval-habitat preferences give the answer. J Insect Conserv 13:77–87
Munguira ML, García-Barros E, Cano JM (2009) Butterfly herbivory and larval ecology. In: Settele J, Shreeve T, Konvička M, Van Dyck H (eds) Ecology of butterflies in Europe. Cambridge University Press, Cambridge, pp 43–54
Nunner A (1995) Zur Autökologie von Boloria eunomia (Esper 1799) und Lycaena helle (Denis & Schiffermueller 1775) (Lepidoptera: Rhopalocera) im bayerischen Alpenvorland. Dipl. Thesis, Universität Tübingen
Obermaier E, Heisswolf A, Randlkofer B, Meiners T (2006) Enemies in low places—insects avoid winter mortality and egg parasitism by modulating oviposition height. B Entomol Res 96:337–343
O’Connor RS, Hails RS, Thomas JA (2014) Accounting for habitat when considering climate: has the niche of the Adonis blue butterfly changed in the UK? Oecologia 174:1463–1472
Örvössy N, Vozár Á, Kőrösi Á, Batáry P, Peregovits L (2010) Structure and size of a threatened population of the False Ringelt Coenonympha oedippus (Fabricius, 1787) (Lepidoptera: Nymphalidae) in Hungary. Oedippus 26:31–37
Örvössy N, Vozár Á, Kőrösi Á, Batáry P, Peregovits L (2013) Potential metapopulation structure and the effects of habitat quality on population size of the endangered False Ringlet butterfly. J Insect Conserv 17:537–547
Pauler R, Kaule G, Verhaagh M, Settele J (1995) Untersuchungen zur Autökologie des Schwarzgefleckten Ameisenbläulings, Maculinea arion (Linnaeus 1758) (Lepidoptera, Lycaenidae) in Südwestdeutschland. Nachr Entomol Ver Apollo 16:147–186
Pennekamp F, Monteiro E, Schmitt T (2013) The larval ecology of the butterfly Euphydryas desfontainii (Lepidoptera: Nymphalidae) in SW Portugal: food plant quantity and quality as main predictors of habitat quality. J Insect Conserv 17:195–206
Ruehl (1895) Die paläarktischen Großschmetterlinge und ihre Naturgeschichte. Lief. 55, Leipzig
Ruprecht E, Szabo A (2012) Grass litter is a natural seed trap in long-term undisturbed grassland. J Veg Sci 23:495–504
Ruprecht E, Enyedi MZ, Eckstein RL, Donath TW (2010) Restorative removal of plant litter and vegetation 40 years after abandonment enhances re-emergence of steppe grassland vegetation. Biol Conserv 143:449–456
Sachteleben J, Winterholler M (2013) Blaukernauge Minois dryas (Scopoli, 1763). In: Bräu M, Bolz R, Kolbeck H, Nunner A, Voith J, Wolf W (eds) Tagfalter in Bayern. Eugen Ulmer, Stuttgart, pp 527–529
Šašić M (2010) False Ringlet Coenonympha oedippus (Fabricius, 1787) (Lepidoptera: Nymphalidae) in Croatia: current status, population dynamics and conservation management. Oedippus 26:16–19
Selezniew M, Pałka K, Michalczuk W, Bystrowski C, Hołowiński M, Czerwiński M (2010) False Ringlet Coenonympha oedippus (Fabricius, 1787) (Lepidoptera: Nymphalidae) in Poland: state of knowledge and conservation prospects. Oedippus 26:20–24
Settele J, Kudrna O, Hharpke A, Kühn I, van Swaay C, Verovnik R, Warren M, Wiemers M, Hanspach J, Hickler T, Kühn E, van Halder I, Veling K, Vliegenthart A, Wynhoff I, Schweiger O (2008) Climatic risk atlas of European butterflies. Pensoft, Sofia-Moscow
SPSS Inc (1989–2004) SPSS for Windows. Release 13.0 (1 Sep 2004)
Steiner R, Hermann G (1999) Freilandbeobachtungen zu Eiablageverhalten und –habitat des Wald-Wiesenvögelchen, Coenonympha hero (Linnaeus, 1761), an einer Flugstelle in Baden-Württemberg. Nach Entomol Ver Apollo 20:111–118
Stevenson RD (1985) The relative importance of behavioral and physiological adjustments controlling temperature in terrestrial ecotherms. Am Nat 126:362–386
Stuhldreher G, Fartmann T (2014) When habitat management can be a bad thing: effects of habitat quality, isolation and climate on a declining grassland butterfly. J Insect Conserv 18:965–979
Szentirmai I, Mesterházy A, Varga I, Schubert Z, Sándor LC, Ábrahám L, Kőrösi Á (2014) Habitat use and population biology of the Danube Clouded Yellow butterfly Colias myrmidone (Lepidoptera: Pieridae) in Romania. J Insect Conserv 18:417–425
Thomas JA (1980) Why did the large blue become extinct in Britain? Oryx 15:243–247
Thomas JA (1983) The ecology and conservation of Lysandra bellargus (Lepidoptera, Lycaenidae) in Britain. J Appl Ecol 20:59–83
Thomas JA (1993) Holocene climate change and warm man-made refugia may explain why a sixth of british butterflies inhabit unnatural early successional habitats. Ecography 16:278–284
Thomas JA, Elmes GW (2001) Food-plant niche selection rather than the presence of ant nests explains oviposition patterns in the myrmecophilous butterfly genus Maculinea. Proc R Soc Lond B Biol Sci 268:471–477
Tshikolovets VV (2003) Butterflies of Eastern Europe. Urals and Caucasus, Brno
Turlure C, Choutt J, Baguette M, van Dyck H (2010) Microclimatic buffering and resource-based habitat in a glacial relict butterfly: significance for conservation under climate change. Glob Change Biol 16:1883–1893
Turlure C, Radchuk V, Baguette M, van Dyck H, Schtickzelle N (2011) On the significance of structural vegetation elements for caterpillar thermoregulation in two peat bog butterflies: boloria eunomia and B. aquilonaris. J Therm Biol 36:173–180
Turlure C, Radchuk V, Baguette M, Meijrink M, van den Burg A, WallisDeVries M, van Duinen GJ (2013) Plant quality and local adaptation undermine relocation in a bog specialist butterfly. Ecol Evol 3:244–254
Van Dyck H (2012) Changing organisms in rapidly changing anthropogenic landscapes: the significance of the “Umwelt”-concept and functional habitat for animal conservation. Evol Appl 5:144–153
Van Swaay CAM, Cuttelod A, Collins S, Maes D, Munguira López M, Šašić M, Settele J, Verovnik R, Verstrael T, Warren M, Wiemers M, Wynhoff I (2010) European red list of butterflies. Publications Office of the European Union, Luxembourg
Wagner W (2010) Europäische Schmetterlinge und ihre Ökologie. www.pyrgus.de
WallisDeVries MF, van Swaay CAM (2006) Global warming and excess nitrogen may induce butterfly decline by microclimate cooling. Glob Change Biol 12:1620–1626
Ward G, Hastie T, Barry S, Elith J, Leathwick JR (2009) Presence-only data and the EM algorithm. Biometrics 65:554–563
Weidemann HJ (1995) Tagfalter: beobachten, bestimmen. 2nd Ed., völlig neu bearb. Aufl. Naturbuch, Augsburg
Weking S, Hermann G, Fartmann T (2013) Effects of mire type, land use and climate on a strongly declining wetland butterfly. J Insect Conserv 17:1081–1091
Wiklund C (1984) Egg-laying patterns in butterflies in relation to their phenology and visual apparency and abundance of their host plants. Oecologia 63:23–29
Zalucki MP, Clarke AR, Malcolm SB (2002) Ecology and behaviour of first instar larval Lepidoptera. Annu Rev Entomol 47:361–393
Acknowledgments
Special thanks to all colleagues, who contributed to this study in providing information on other populations in Europe and beyond, and to two anonymous referees for suggestions that greatly improved the manuscript. We are grateful to R. Völkl and T. Hermann for support during field work, and to Giorgio Buffa for the determination of the Italian plant species. We also particularly thank the Bavarian Academy for Nature Conservation and Landscape Management (ANL) for funding this project. Many thanks also to the Bavarian State Office for Environment for financing the investigations in all previous years and to the Government of Upper Bavaria as well as local nature conservation authorities for their support. The Slovenian part of study was partly funded by the Slovenian Research Agency (P1-0236).
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Čelik, T., Bräu, M., Bonelli, S. et al. Winter-green host-plants, litter quantity and vegetation structure are key determinants of habitat quality for Coenonympha oedippus in Europe. J Insect Conserv 19, 359–375 (2015). https://doi.org/10.1007/s10841-014-9736-3
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10841-014-9736-3