Abstract
The timing of many biological events, including butterfly imago emergence, has advanced under climate change, with the rate of these phenological changes often differing among taxonomic groups. Such inter-taxa variability can lead to phenological mismatches. For example, the timing of a butterfly’s flight period may become misaligned with a key nectar resource, potentially increasing the extinction risk to both species. Here we fit statistical models to field data to determine how the phenology of the marbled white butterfly, Melanargia galathea, and its main nectar source, greater knapweed, Centaurea scabiosa, have changed over recent years at three sites across the UK. We also consider whether topographical diversity affects C. scabiosa’s flowering period. At our focal site, on the species’ northern range limit, we find that over a 13-year period the onset of C. scabiosa’s flowering period has become later whilst there is no obvious trend over time in the onset of M. galathea’s flight period. In recent years, butterflies have started to emerge before their key nectar source was available across most of the site. This raises the intriguing possibility that phenological mismatch could be an unrecognised determinant of range limits for some species. However, the presence of topographical diversity within the site decreased the chance of a mismatch occurring by increasing the length of the flowering period by up to 14 days. We suggest that topographical diversity could be an important component in minimising phenological mismatches under future climate change.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Recent climate change has seen the advancement of biological events across many taxonomic groups, including flowering in plants (Fitter and Fitter 2002; Menzel and Fabian 1999), egg laying in birds (Crick et al. 1997; Crick and Sparks 1999) and the migration of a wide range of taxonomic groups (Cherry et al. 2013; Marra et al. 2005). Whilst phenological responses to climate change are common, the rates of change can differ across taxonomic groups (Parmesan 2007; Thackeray et al. 2010). Differences in the rate or direction of change between interacting species can lead to phenological mismatches, with potential consequences for the abundance, distribution and extinction risk of the interacting species (Fabina et al. 2010; Memmott et al. 2007; Parmesan 2007; van Asch and Visser 2007). Interacting species must be temporally, as well as spatially, correlated for interactions to occur. Therefore, it is necessary to consider the effects of changing conditions on inter-dependent species, including pollinators and their food plants (Visser and Both 2005), when predicting the impacts of climate change and planning appropriate conservation measures.
Despite the important roles insects play in ecosystem functioning and crop pollination there has been little research on potential phenological mismatches in this taxa, and the wider ecosystem impacts these could have (though see Bartomeus et al. 2011; Kudo and Ida 2013; Rafferty and Ives 2011). In butterflies, earlier and longer flight periods have been recorded across the United Kingdom (UK) and Europe (Diamond et al. 2011; Roy and Sparks 2000; Stefanescu et al. 2003) with evidence of correlations between temperature and appearance date (Diamond et al. 2011; Roy and Sparks 2000). A warming of 1 °C could advance the first and the peak appearances of most British butterfly species by 2–10 days (Roy and Sparks 2000), whilst warming of 3 °C could advance their appearance by as much as 2 or 3 weeks (Sparks and Yates 1997).
Many adult butterflies are dependent on a restricted number of plant species for nectar (Asher et al. 2001). Whilst the flowering periods of many plants have become earlier under climate change (Calinger et al. 2013; Fitter and Fitter 2002; Primack et al. 2004), there is evidence that butterflies′ flight periods are showing more rapid rates of advancement (Gordo and Sanz 2005; Parmesan 2007). Little is known about the physiological mechanisms controlling the phenology of imago emergence in butterflies; however, there is evidence in some species that the climatic cues used by butterflies differ from those used by plants (Doi et al. 2008), which may increase the chance of phenological mismatches. The phenology of sessile organisms such as plants will be affected by microclimate as well as larger scale climate changes. Plant phenologies, therefore, may be especially affected by topography (Pellerin et al. 2012) and, as a result, topographically heterogeneous sites may have longer site-wide flowering periods than homogeneous sites. This heterogeneity may help to minimise phenological mismatches between butterflies and their nectar sources, especially when the caterpillar food plant and the nectar plants for the imago occur in different microhabitats, as can be the case with Melanargia galathea (Linnaeus 1758) (Asher et al. 2001; Stace 1991; Wilson 1985).
Here we examine the flight period of M. galathea, a generalist butterfly, over the period 2001–2013 at three sites across the species’ UK range. We relate the timing of M. galathea’s flight period to the flowering period of one of its main nectar sources, Centaurea scabiosa (Linnaeus 1758) (Asher et al. 2001). In particular, we explore the possibility that M. galathea’s flight period could be advancing more quickly than C. scabiosa’s flowering period, with the potential for a phenological mismatch to occur. Finally, we consider whether the presence of topographical diversity within a site alters the flowering period of this key nectar source, by comparing flowering phenology across multiple aspects at a site on the species’ northern range margin.
Methods
Study species and sites
M. galathea is found primarily on calcareous grasslands; within the UK it is found predominantly in the South, naturally occurring as far north as Yorkshire (Asher et al. 2001; Fox et al. 2006). It has undergone rapid range expansions at its northern range boundary; a survey in 1995–1999 found it present in 66 % more 10 km grid squares than in 1970–1982 (Asher et al. 2001; Fox et al. 2006). C. scabiosa is one of the species’ main nectar sources although Centaurea nigra (Linneaus 1753) and other purple flowers such as Scabiosa spp. are also used (Asher et al. 2001). C. scabiosa is a perennial and flowers between June and September in the UK. It is common within our study sites and across the natural range of M. galathea in the UK. At our northernmost study site, at the species northern range limit, it is the dominant nectar source during M. galathea’s flight period.
Data were collected at three study sites (Fig. 1): Totternhoe Chalk Quarry Reserve, Bedfordshire (Totternhoe; OS grid reference SP9722), Brockadale Nature Reserve, North Yorkshire (Brockadale; OS grid reference SE5117) and Wingate Quarry Local Nature Reserve, Co. Durham (Wingate; OS grid reference NZ3737). Totternhoe and Wingate are the sites of disused limestone quarries, whilst Brockadale is a narrow valley created by the River Went. All three sites have been classified as Sites of Special Scientific Interest (SSSI) by Natural England. As well as being topographically diverse they contain a variety of habitats, including calcareous grasslands where M. galathea is found. Totternhoe is in the core of M. galathea’s UK range, Brockadale is close to M. galathea’s natural northern range boundary and Wingate is beyond M. galathea’s natural northern range limit. The Wingate study population was introduced in 2000 as part of an assisted colonisation experiment (Willis et al. 2009).
Flight period phenology
Data on the abundance of M. galathea adults were collected using transects, following the standard UK Butterfly Monitoring Scheme (UKBMS) guidelines (Pollard and Yates 1993). The transects at each site run through calcareous grassland with diverse topographies. Transect data were collected at Wingate every 2–3 days from a 1,550 m long and 5 m wide transect, for 9 years between 2001 and 2013. Weekly transect data from Totternhoe and Brockadale were collected by the Yorkshire Wildlife Trust and the UKBMS respectively. 8 and 5 years of data were available from Totternhoe and Brockadale respectively.
Flowering phenology
Data on the flowering phenology of C. scabiosa were collected by counting the number of developing (flower buds), flowering (bright inflorescences and pollen present) and spent (withering flowers, and developing or spent seed capsules) flowers on each plant. A minimum of 30 plants were surveyed on ground of varying topography approximately every 3–4 days during M. galathea’s flight period for nine non-consecutive years between 2001 and 2013 at Wingate and in 2011 at Brockadale and Totternhoe. Plants were selected randomly from across the site.
In 2013, additional data on the effect of topography on the flowering phenology of C. scabiosa were collected at Wingate. Four aspects were studied [north east (NE), south east (SE), south west (SW) and south (S)]. Data were also collected on two types of flat area: flat sheltered (FS) sites were in low-lying quarry bottoms and were sheltered on all sides; whereas, flat exposed (FE) sites were on the quarry tops, though still within the nature reserve, and were not sheltered on any side. 30–40 randomly selected individuals were surveyed on each of the six microhabitat types every 3–4 days.
Statistical analysis of butterfly flight period phenology
The expected number of M. galathea individuals seen on a transect on Julian day t was modelled as
where the peak abundance (y max) is reached on day \(\bar{t}\), and σ is positively related to the expected duration of the flight period. When fitting this general model to the data we investigated possible year and site effects on adult abundance by comparing fits for versions of the model that either fixed or varied y max, \(\bar{t}\), or σ with respect to year and site, respectively (Table 1).
Observed variation about the expected counts was assumed to have a negative-binomial distribution as there was evidence of overdispersion in the data (i.e. the variance was higher than the mean). The probability of observing i butterflies when µ were expected is:
where ϕ is a variance parameter, \(a = \mu /\phi\) and \(b = 1/\phi\). We assumed that ϕ was independent of site and year. We performed model selection using AIC (Burnham and Anderson 2002). Models were considered parsimonious if (1) they were associated with an AIC value within 6 of the minimum calculated from the set, and (2) there were no simpler nested models having a lower AIC value. This pair of selection rules prevents overly complex models from being selected when applying AIC (Richards 2008). The flight period was defined as the number of days during which more than one adult butterfly is expected to be observed alive during a transect survey, and it was calculated using the best AIC model (lowest AIC value).
Statistical analysis of flowering phenology
Next, we sought evidence that the timing and length of the flowering period of C. scabiosa differed across sites and years by modelling the expected cumulative proportion of flowers open by Julian day x as
where \(\bar{x}\) is the day by which 50 % of the flowers are expected to be open and β is negatively related to the length of the flowering period. We compared the fit of the model that fixed \(\bar{x}\) and β, with the model that allowed these two parameters to differ among years (2001–2013) and across the three sites (Table 2). We also used this general model formulation to determine whether the timing \((\bar{x})\) and length (β) of the flowering period were affected by topography at Wingate in 2013 (Table 3). In this case we compared model fits where \(\bar{x}\) and β were allowed to differ amongst the four aspects and two types of flat area.
For both of the flowering analyses we assumed that the variation in open flowers about the expected proportion was described by a beta-binomial distribution, which accounts for variation among plants caused by unknown covariates. The probability that n of the N flowers on a plant had opened, given that fraction p were expected to have opened is
where a = p/ϕ and b = (1 − p)/ϕ (Richards 2008). The data suggested that the degree of variation among plants, quantified by ϕ, was similar between sites, years and microhabitats; as a result, this term was kept constant in all of the models. Model selection was again performed using AIC as above. The most parsimonious model (lowest AIC value) was then used to calculate the flowering period, which was defined as the number of days during which between 5 and 95 % of the flowers had opened.
Results
Flight period phenology
Model selection suggested that the day of peak abundance, the peak abundance, and the length of the flight period differed among years and sites (Table 1). Wingate had the latest predicted day of peak abundance and the shortest predicted flight period (Table 4). At Wingate, the start (Linear regression; R 2 < 0.01, F 1,7 < 0.01, p = 0.96) and the length (Linear regression; R 2 < 0.01, F 1,7 = 0.03, p = 0.86) of the flight period have not changed significantly with year. Whilst the model generally appeared to fit the observed data well, this was not the case for all site-year combinations, particularly in 2009 and 2012 in Totternhoe and in 2008 in Brockadale (Table 4).
Flowering phenology
There was evidence that the timing and the length of the flowering period differed among years and between study sites (Table 2). Where data were available from all three sites (2011), the flowering period was earliest at the southernmost site (Totternhoe) and latest at the most northern site (Wingate; Table 5). At Wingate, where multiple years of data were available, over time the start of the flowering period generally shifted to later in the year (Linear regression; β = 2.72, R 2 = 0.52, F 1,7 = 7.68, p = 0.03). There was also a general decrease in the length of the flowering period over time (Linear regression; β = −3.20, R 2 = 0.59, F 1,7 = 9.95, p = 0.02).
There was evidence that the period between the start of C. scabiosa flowering and the start of M. galathea imago emergence had decreased over time (Fig. 2; Linear regression; β = −2.73, R 2 = 0.59, F 1,7 = 9.96, p = 0.02). There was no significant relationship between the length of C. scabiosa’s flowering period and M. galathea’s flight period (Linear regression; R 2 = 0.12, F 1,7 = 0.93, p = 0.37).
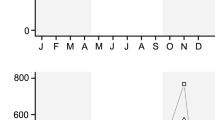
There was also evidence that at Wingate the timing, but not the length of the flowering period, varied among microhabitats (Table 3). Flowering was predicted to be earliest on south and south west facing slopes, where it occurred 14 days earlier than on the flat sheltered areas (Fig. 3). The flowering period in each microhabitat was estimated to be 54 days. Thus, the presence of topographic diversity within the site lengthened the site-wide flowering period by around 25 %, to 68 days across all six microhabitats. M. galathea’s flight period was estimated to start at day 187 in Wingate in 2013, which is after C. scabiosa′s flowering period was estimated to start on three of the six microhabitats studied (Fig. 3). The M. galathea flight period was predicted to start on the same day as the C. scabiosa flowering period on flat exposed areas and before the flowering period began on north-east slopes and in flat sheltered areas.
Mean cumulative proportion of flowers on each plant open in different microhabitats at Wingate each day during the flowering period in 2013. The error bars show standard error of the mean. The lines show the predicted number of open flowers according to the best model fit (M3(\(\bar{x}\)); Table 3). The solid horizontal line above the x-axis shows the estimated M. galathea flight period at Wingate in 2013
Discussion
Over recent years, the start of M. galathea’s flight period has become much closer to, or even preceded, the onset of flowering of a key nectar source, at least at its northernmost site in the UK. This has resulted in some butterflies emerging before the start of C. scabiosa’s peak flowering period. Whilst the majority of M. galathea’s flight period still overlaps with C. scabiosa’s flowering period, complete mismatches are not necessary for community effects to occur (Fabina et al. 2010). The observed mismatch, if it continues, could have pronounced effects for M. galathea, given that there appears to be a sexual phenological dimorphism, with males emerging several days before females (personal observation by SGW, 2001–2014), probably to maximise mating opportunities (Bulmer 1983; Wiklund and Solbreck 1982). Even slight phenological mismatch in such a situation could result in substantial population declines, if many males in the population fail to find sufficient nectar following emergence. This effect could be particularly acute at the population margin at Wingate, as other key nectar sources either do not exist (e.g. Origanum majorana), occur only in small numbers (e.g. Knautia arvensis, Scabiosa columbaria), or are not yet flowering at the time of M. galathea butterfly emergence (e.g. C. nigra).
The relatively poor model fit in some year and site combinations in the M. galathea model suggests that site and year are not the only factors affecting the abundance of butterflies and the timing of the flight period. Here, we do not link the phenology of either species directly to climate. Further studies are needed to explain why the phenologies of both species have not advanced as may be expected (Fitter and Fitter 2002; Roy et al. 2001), and such studies could also predict how phenological patterns may change under projected rates of climate change (Murphy et al. 2009). However, as the difference between the start of flowering period and flight period has changed over time, it appears that these two species may use different climatic cues, or possibly that one species may not be able to react as quickly to changes in the environment. This suggests that future predicted climate change could lead to further phenological mismatch between the species, probably to the greater detriment of M. galathea.
The increased length of C. scabiosa’s flowering period seen in the topographically diverse Wingate site could help to reduce the chance of a phenological mismatch occurring between these two species as their phenologies change under climate change. The phenology of individuals may be affected by genetics and biotic or abiotic environmental factors. However, here we suggest that the differences in C. scabiosa’s phenology within the site were most likely to be caused by an abiotic factor, namely the aspect and its effect on microclimate, rather than other factors. There is little chance of genetic differences in C. scabiosa between aspects given their proximity to one another. As microclimate will also be affected by habitat type, this could affect phenology (Altermatt 2012); however, as all of the aspects studied here were calcareous grassland, habitat variability is also an unlikely driver of the observed phenological patterns. Topography is likely to affect the phenology of sessile organisms, through changes to the microclimate. It seems likely that these are not the only species for which topographically diverse sites could help to ameliorate the potential for phenological mismatch under climate change, at least in the short-term.
Mismatches between obligate plant–pollinator systems will obviously have severe impacts. In the same way that pollinator biodiversity could buffer the effects of phenological asynchrony in plants (Bartomeus et al. 2013), diversity in potential nectar sources may reduce the effects of phenological asynchrony on butterfly abundance. Predicting whether generalist species will be affected by phenological mismatches requires detailed studies of interactions with all possible interacting species present, rather than simple paired interactions (Bartomeus et al. 2013). Whilst specialist species are therefore more vulnerable to the effects of phenological mismatches, impacts may still be pronounced for more generalist species that experience decreases in dietary breadth, possibly leading to population declines (Memmott et al. 2007).
Consistent phenological shifts within broad taxonomic groups but that differ among taxonomic groups (Parmesan 2007; Thackeray et al. 2010), e.g. insects versus flowering plants, could result from different cueing systems for e.g. growth/emergence/flowering operating. This could result in substantial changes in ecosystems, even among populations of e.g. generalist butterfly species, if flowering is largely synchronous among nectar provisioning plants. The general trend in flowering plants in the UK has been towards earlier flowering (Amano et al. 2010; Fitter and Fitter 2002), rather than the later flowering seen here in C. scabiosa. However, the current study was over a relatively restricted period, during the latter part of which there were several unusually late and wet summers. The decreasing length of flowering period seen in C. scabiosa may also increase the likelihood of a phenological mismatch occurring with M. galathea, and the effect of this may be exacerbated if shorter flowering periods occur in other nectar sources. Although the negative effect of phenological mismatches are likely to be larger for specialist species, which are unable to substitute resources, the impact of phenological mismatching among generalist species is a field that merits future research. The potential for multiple phenological mismatches in taxa such as Lepidoptera that use different plant resources and species at different life stages must render them especially vulnerable. The ability of butterflies to alter their flight period phenology under climate change may be affected by their larval diet (Altermatt 2010).
The impacts of a plant–pollinator phenological mismatch will depend on whether nectar sources are a limiting factor for that population. Despite one study suggesting that there is little evidence that nectar limitation is causing population declines (Thomas et al. 2011), others have found correlations between nectar abundance and butterfly density (e.g. Wallisdevries et al. 2012); nectar abundance may be more important for determining generalist species abundance, whilst specialist species are more dependent on larval host plant abundance (Kraemer et al. 2012). Specifically, in the case of M. galathea, several studies have shown correlations between the abundance of nectar sources such as C. scabiosa and this butterfly species (Lenda and Skorka 2010; Loertscher et al. 1995).
Climate change is expected to significantly impact many species, communities and ecosystems (Parmesan and Yohe 2003), yet studies often focus on single species, without considering the effects of biotic interactions. Phenological mismatches have the potential to disrupt ecosystem functioning, with consequences not only for the species that have become asynchronous (Fabina et al. 2010; Nakazawa and Doi 2012). Whilst biotic interactions are slowly being included into models of climate impacts on species (Araujo and Luoto 2007; Cormont et al. 2013), and in some cases are considered to exceed the importance of climate in affecting distributions (Mason et al. 2014), there has been a lack of research on how the phenologies of insects, and the species they interact with, will be affected. Phenological change may affect the host plant preferences of generalist species, as growth rates on different host plants may be affected by the time of season (Audusseau et al. 2013).
We have demonstrated here how heterogeneous habitats can serve to ameliorate phenological mismatching, for species that can move between habitats/microclimates. We suggest that sites of topographic heterogeneity might be prioritised for conserving similar species under climate change. The diverse microclimates that such sites provide could also buffer populations against the impacts of the extreme climatic events that are forecast to be more frequent in future (Easterling et al. 2000; van Halder et al. 2011; Weiss et al. 1988). For sites with little topographical diversity, management actions that increase the breadth of microclimatic conditions could be encouraged (Hopkins et al. 2007).
As the distributions of the majority of European butterflies appear to be lagging behind climate change (Devictor et al. 2012; Warren et al. 2001), with specialist species particularly vulnerable, conservation strategies are necessary to prevent widespread extinctions. Management strategies such as increasing connectivity between protected areas may help to increase dispersal, whilst assisted colonisations could be used for those species that are unlikely to be able to remain within climatically suitable habitats (Loss et al. 2011; Vitt et al. 2009). Assisted colonisation remains a controversial strategy, with risks of species potentially becoming invasive, and its cost and feasibility questioned (Ricciardi and Simberloff 2009). However, it may be a necessary conservation strategy if rare species with limited dispersal ability are to be conserved (Hoegh-Guldberg et al. 2008; Thomas 2011) and could also be used to restore ecosystem function (Lunt et al. 2013). The M. galathea population at our main study site is an example of a successful translocation, and provides evidence that the distributions of even relatively mobile generalist species may lag behind changes in suitable climate (Willis et al. 2009). Here we emphasise the importance of considering species interactions before carrying out translocations, including the possibility of temporal mismatch. Paired or multispecies assisted colonisations may be necessary for specialist species that require particular host-plants (McLachlan et al. 2007); however, these approaches may also be necessary for more generalist species. At Wingate, where potential nectar sources from other parts of the range of M. galathea are absent, it may be beneficial to M. galathea to introduce such species that might not otherwise reach the site, to minimise the impact of phenological mismatch.
Here we demonstrate how the phenology of a generalist butterfly may lose synchronicity with the flowering period of a key nectar source. However, this asynchrony may be reduced by the presence of topographical diversity. The role of topography in egg-laying, caterpillar growth and imago emergence in M. galathea has not been studied. Spatial preferences of adult butterflies probably bear little relation to their natal area, apart from during egg-laying, and, as such, could mask the role of habitat heterogeneity on phenology. Microhabitat choice is of particular importance in ectothermic species such as butterflies, especially in the less mobile larval stages, and may affect egg hatching and the survival and growth of the larval stages (Bergman 2001; Bryant et al. 2002; Turlure and Van Dyck 2009; Weiss et al. 1988). Studies on caterpillar topographic preferences could further our understanding of the mechanisms explaining differential advancements in phenology of butterflies versus their nectar sources.
References
Altermatt F (2010) Tell me what you eat and I’ll tell you when you fly: diet can predict phenological changes in response to climate change. Ecol Lett 13:1475–1484. doi:10.1111/j.1461-0248.2010.01534.x
Altermatt F (2012) Temperature-related shifts in butterfly phenology depend on the habitat. Glob Change Biol 18:2429–2438. doi:10.1111/j.1365-2486.2012.02727.x
Amano T, Smithers RJ, Sparks TH, Sutherland WJ (2010) A 250-year index of first flowering dates and its response to temperature changes. Proc R Soc B-Biol Sci 277:2451–2457. doi:10.1098/rspb.2010.0291
Araujo MB, Luoto M (2007) The importance of biotic interactions for modelling species distributions under climate change. Glob Ecol Biogeogr 16:743–753. doi:10.1111/j.1466-8238.2007.00359.x
Asher J, Warren M, Fox R, Harding P, Jeffcoate G, Jeffcoate S (2001) Millenium atlas of butterflies in Britain and Ireland. Oxford University Press, Oxford
Audusseau H, Nylin S, Janz N (2013) Implications of a temperature increase for host plant range: predictions for a butterfly. Ecol Evol 3:3021–3029. doi:10.1002/ece3.696
Bartomeus I, Ascher JS, Wagner D, Danforth BN, Colla S, Kornbluth S, Winfree R (2011) Climate-associated phenological advances in bee pollinators and bee-pollinated plants. Proc Natl Acad Sci USA 108:20645–20649. doi:10.1073/pnas.1115559108
Bartomeus I, Park MG, Gibbs J, Danforth BN, Lakso AN, Winfree R (2013) Biodiversity ensures plant–pollinator phenological synchrony against climate change. Ecol Lett 16:1331–1338. doi:10.1111/ele.12170
Bergman KO (2001) Population dynamics and the importance of habitat management for conservation of the butterfly Lopinga achine. J Appl Ecol 38:1303–1313. doi:10.1046/j.0021-8901.2001.00672.x
Bryant SR, Thomas CD, Bale JS (2002) The influence of thermal ecology on the distribution of three nymphalid butterflies. J Appl Ecol 39:43–55. doi:10.1046/j.1365-2664.2002.00688.x
Bulmer MG (1983) Models for the evolution of protandry in insects. Theor Popul Biol 23:314–322. doi:10.1016/0040-5809(83)90021-7
Burnham KP, Anderson DR (2002) Model selection and multimodel inference: a practical information-theoretic approach. Springer, New York
Calinger KM, Queenborough S, Curtis PS (2013) Herbarium specimens reveal the footprint of climate change on flowering trends across north-central North America. Ecol Lett 16:1037–1044. doi:10.1111/ele.12135
Cherry SG, Derocher AE, Thiemann GW, Lunn NJ (2013) Migration phenology and seasonal fidelity of an Arctic marine predator in relation to sea ice dynamics. J Anim Ecol 82:912–921. doi:10.1111/1365-2656.12050
Cormont A, Wamelink GWW, Jochem R, WallisDeVries MF, Wegman RMA (2013) Host plant-mediated effects of climate change on the occurrence of the Alcon blue butterfly (Phengaris alcon). Ecol Model 250:329–337. doi:10.1016/j.ecolmodel.2012.11.022
Crick HQP, Sparks TH (1999) Climate change related to egg-laying trends. Nature 399:423–424. doi:10.1038/20839
Crick HQP, Dudley C, Glue DE, Thomson DL (1997) UK birds are laying eggs earlier. Nature 388:526–526. doi:10.1038/41453
Devictor V et al (2012) Differences in the climatic debts of birds and butterflies at a continental scale. Nat Clim Change 2:121–124. doi:10.1038/nclimate1347
Diamond SE, Frame AM, Martin RA, Buckley LB (2011) Species’ traits predict phenological responses to climate change in butterflies. Ecology 92:1005–1012
Doi H, Gordo O, Katano I (2008) Heterogeneous intra-annual climatic changes drive different phenological responses at two trophic levels. Clim Res 36:181–190. doi:10.3354/cr00741
Easterling DR, Meehl GA, Parmesan C, Changnon SA, Karl TR, Mearns LO (2000) Climate extremes: observations, modeling, and impacts. Science 289:2068–2074. doi:10.1126/science.289.5487.2068
Fabina NS, Abbott KC, Gilman RT (2010) Sensitivity of plant–pollinator-herbivore communities to changes in phenology. Ecol Model 221:453–458. doi:10.1016/j.ecolmodel.2009.10.020
Fitter AH, Fitter RSR (2002) Rapid Changes in Flowering Time in British plants. Science 296:1689–1691. doi:10.1126/science.1071617
Fox R, Asher J, Brereton T, Roy D, Warren M (2006) The state of butterflies in Britain and Ireland. NatureBureau i–viii, 1–112
Gordo O, Sanz JJ (2005) Phenology and climate change: a long-term study in a Mediterranean locality. Oecologia 146:484–495. doi:10.1007/s00442-005-0240-z
Hoegh-Guldberg O, Hughes L, McIntyre S, Lindenmayer DB, Parmesan C, Possingham HP, Thomas CD (2008) Assisted colonization and rapid climate change. Science 321:345–346. doi:10.1126/science.1157897
Hopkins JJ, Allison HM, Walmsley CA, Gaywood M, Thurgate G (2007) Conserving biodiversity in a changing climate: guidance on building capacity to adapt. Defra, London
Kraemer B, Poniatowski D, Fartmann T (2012) Effects of landscape and habitat quality on butterfly communities in pre-alpine calcareous grasslands. Biol Conserv 152:253–261
Kudo G, Ida TY (2013) Early onset of spring increases the phenological mismatch between plants and pollinators. Ecology 94:2311–2320. doi:10.1890/12-2003.1
Lenda M, Skorka P (2010) Patch occupancy, number of individuals and population density of the Marbled White in a changing agricultural landscape. Acta Oecol-Int J Ecol 36:497–506. doi:10.1016/j.actao.2010.07.002
Loertscher M, Erhardt A, Zettel J (1995) Microdistribution of butterflies in a mosaic-like habitat—the role of nectar sources. Ecography 18:15–26. doi:10.1111/j.1600-0587.1995.tb00115.x
Loss SR, Terwilliger LA, Peterson AC (2011) Assisted colonization: integrating conservation strategies in the face of climate change. Biol Conserv 144:92–100. doi:10.1016/j.biocon.2010.11.016
Lunt ID et al (2013) Using assisted colonisation to conserve biodiversity and restore ecosystem function under climate change. Biol Conserv 157:172–177. doi:10.1016/j.biocon.2012.08.034
Marra PP, Francis CM, Mulvihill RS, Moore FR (2005) The influence of climate on the timing and rate of spring bird migration. Oecologia 142:307–315. doi:10.1007/s00442-004-1725-x
Mason THE, Stephens PA, Apollonio M, Willis SG (2014) Predicting potential responses to future climate in an alpine ungulate: interspecific interactions exceed climate effects. Glob Change Biol. doi:10.1111/gcb.12641
McLachlan JS, Hellmann JJ, Schwartz MW (2007) A framework for debate of assisted migration in an era of climate change. Conserv Biol 21:297–302. doi:10.1111/j.1523-1739.2007.00676.x
Memmott J, Craze PG, Waser NM, Price MV (2007) Global warming and the disruption of plant–pollinator interactions. Ecol Lett 10:710–717. doi:10.1111/j.1461-0248.2007.01061.x
Menzel A, Fabian P (1999) Growing season extended in Europe. Nature 397:659–659. doi:10.1038/17709
Murphy JM et al (2009) UK climate projections science report: climate change projections. Met Office Hadley Centre, Exeter
Nakazawa T, Doi H (2012) A perspective on match/mismatch of phenology in community contexts. Oikos 121:489–495. doi:10.1111/j.1600-0706.2011.20171.x
Parmesan C (2007) Influences of species, latitudes and methodologies on estimates of phenological response to global warming. Glob Change Biol 13:1860–1872. doi:10.1111/j.1365-2486.2007.01404.x
Parmesan C, Yohe G (2003) A globally coherent fingerprint of climate change impacts across natural systems. Nature 421:37–42. doi:10.1038/nature01286
Pellerin M, Delestrade A, Mathieu G, Rigault O, Yoccoz NG (2012) Spring tree phenology in the Alps: effects of air temperature, altitude and local topography. Eur J For Res 131:1957–1965. doi:10.1007/s10342-012-0646-1
Pollard E, Yates TJ (1993) Monitoring butterflies for ecology and conservation. Chapman & Hall, London
Primack D, Imbres C, Primack RB, Miller-Rushing AJ, Del Tredici P (2004) Herbarium specimens demonstrate earlier flowering times in response to warming in Boston. Am J Bot 91:1260–1264. doi:10.3732/ajb.91.8.1260
Rafferty NE, Ives AR (2011) Effects of experimental shifts in flowering phenology on plant–pollinator interactions. Ecol Lett 14:69–74. doi:10.1111/j.1461-0248.2010.01557.x
Ricciardi A, Simberloff D (2009) Assisted colonization is not a viable conservation strategy. Trends Ecol Evol 24:248–253. doi:10.1016/j.tree.2008.12.006
Richards SA (2008) Dealing with overdispersed count data in applied ecology. J Appl Ecol 45:218–227. doi:10.1111/j.1365-2664.2007.01377.x
Roy DB, Sparks TH (2000) Phenology of British butterflies and climate change. Glob Change Biol 6:407–416. doi:10.1046/j.1365-2486.2000.00322.x
Roy DB, Rothery P, Moss D, Pollard E, Thomas JA (2001) Butterfly numbers and weather: predicting historical trends in abundance and the future effects of climate change. J Anim Ecol 70:201–217. doi:10.1046/j.1365-2656.2001.00480.x
Sparks TH, Yates TJ (1997) The effect of spring temperature on the appearance dates of British butterflies 1883–1993. Ecography 20:368–374
Stace C (1991) New flora of the British Isles. Cambridge University Press, Cambridge
Stefanescu C, Penuelas J, Filella I (2003) Effects of climatic change on the phenology of butterflies in the northwest Mediterranean Basin. Glob Change Biol 9:1494–1506. doi:10.1046/j.1365-2486.2003.00682.x
Thackeray SJ et al (2010) Trophic level asynchrony in rates of phenological change for marine, freshwater and terrestrial environments. Glob Change Biol 16:3304–3313. doi:10.1111/j.1365-2486.2010.02165.x
Thomas CD (2011) Translocation of species, climate change, and the end of trying to recreate past ecological communities. Trends Ecol Evol 26:216–221. doi:10.1016/j.tree.2011.02.006
Thomas JA, Simcox DJ, Hovestadt T (2011) Evidence based conservation of butterflies. J Insect Conserv 15:241–258. doi:10.1007/s10841-010-9341-z
Turlure C, Van Dyck H (2009) On the consequences of aggressive male mate-locating behaviour and micro-climate for female host plant use in the butterfly Lycaena hippothoe. Behav Ecol Sociobiol 63:1581–1591. doi:10.1007/s00265-009-0753-2
van Asch M, Visser ME (2007) Phenology of forest caterpillars and their host trees: The importance of synchrony. In: Annual Review of Entomology, vol 52. Annual Review of Entomology. Annual Reviews, Palo Alto, pp 37–55. doi:10.1146/annurev.ento.52.110405.091418
van Halder I, Barbaro L, Jactel H (2011) Conserving butterflies in fragmented plantation forests: are edge and interior habitats equally important? J Insect Conserv 15:591–601. doi:10.1007/s10841-010-9360-9
Visser ME, Both C (2005) Shifts in phenology due to global climate change: the need for a yardstick. Proc R Soc B Biol Sci 272:2561–2569. doi:10.1098/rspb.2005.3356
Vitt P, Havens K, Hoegh-Guldberg O (2009) Assisted migration: part of an integrated conservation strategy. Trends Ecol Evol 24:473–474. doi:10.1016/j.tree.2009.05.007
Wallisdevries MF, Van Swaay CAM, Plate CL (2012) Changes in nectar supply: a possible cause of widespread butterfly decline. Curr Zool 58:384–391
Warren MS et al (2001) Rapid responses of British butterflies to opposing forces of climate and habitat change. Nature 414:65–69. doi:10.1038/35102054
Weiss SB, Murphy DD, White RR (1988) Sun, slope and butterflies—topographic determinants of habitat quality for Euphydryas-editha. Ecology 69:1486–1496. doi:10.2307/1941646
Wiklund C, Solbreck C (1982) Adaptive versus incidental explanations for the occurrence of protandry in a butterfly, Leptidea-sinapis L. Evolution 36:56–62. doi:10.2307/2407966
Willis SG, Hill JK, Thomas CD, Roy DB, Fox R, Blakeley DS, Huntley B (2009) Assisted colonization in a changing climate: a test-study using two U.K. butterflies. Conserv Lett 2:46–51. doi:10.1111/j.1755-263X.2008.00043.x
Wilson A (1985) Flavonoid pigments in marbled white butterfly (Melanargia-galathea) are dependent on flavonoid content of larval diet. J Chem Ecol 11:1161–1179. doi:10.1007/bf01024106
Acknowledgments
Funding was provided by Grevillea Trust studentships to B.J.H. and C.L.K., both supervised by S.G.W. and cosupervised by S.A.R. and Dr Robert Baxter respectively. Data were supplied by the UK Butterfly Monitoring Scheme (UKBMS) and Yorkshire Wildlife Trust. The UKBMS is operated by the Centre for Ecology & Hydrology and Butterfly Conservation and funded by a multi-agency consortium including the Countryside Council for Wales, Defra, the Joint Nature Conservation Committee, Forestry Commission, Natural England, the Natural Environment Research Council, and Scottish Natural Heritage. The UKBMS is indebted to all volunteers who contribute data to the scheme. We thank Yorkshire Wildlife Trust, Durham City Council and the Wildlife Trust for Bedfordshire, Cambridgeshire and Northamptonshire for access to their land and numerous field assistants for their help in collecting data. We are grateful to two anonymous reviewers for helpful comments on an earlier draft.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Hindle, B.J., Kerr, C.L., Richards, S.A. et al. Topographical variation reduces phenological mismatch between a butterfly and its nectar source. J Insect Conserv 19, 227–236 (2015). https://doi.org/10.1007/s10841-014-9713-x
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10841-014-9713-x