Abstract
Variable retention is an alternative silvicultural approach to timber forest management, which consist in a regeneration treatment with different degrees and patterns of stand retention. It has been proposed to mitigate harmful effects of harvesting, but effectiveness in insect conservation remains unknown in southern Patagonian Nothofagus pumilio forests. Here, the objectives were to: (1) define a baseline of insect diversity in old-growth forests along a site quality gradient (high, medium and low, associated to the forest productivity of each site); (2) evaluate stands with different retention treatments [aggregated (AR) surrounded by dispersed (DR) retention, and aggregated retention surrounded by clear-cut (CC)] and to compare with old-growth unmanaged forests (OGF); and (3) assess temporal changes during the first 4 years after harvesting (YAH). In a long term forest research plot, mobile epigean insect richness and relative abundance were characterized and classified in seven response type groups, using a wide spectrum sampling set. Data analyses included parametric and permutational ANOVAs, multivariate classification and ordinations. There were found 79 species before harvesting, and that richness was not related to site quality. After harvesting, 84 new species were added considering all treatments along the first four sampled YAH, of which 65 % were added to OGF, while in harvested sites richness and abundance directly diminished with retention degree (OGF > AR > DR > CC) due to incoming species cannot compensate the lost of them. However, fluctuations in diversity were observed along the YAH. Therefore, harvesting reduces insect richness in N. pumilio forests independently of the treatment, but the original insect assemblage significantly changes due to loss of sensitive species and introduction of others from surrounding environments. Despite this, inclusion of aggregates greatly diminished harvesting impacts because insect assemblage is favoured when structural complexity is preserved, conserving richness and abundance at similar levels than in old-growth forests. However, more studies are necessary to evaluate effects of different aggregate size, shape and distribution into harvested forests, as well as their fragmentation and connectivity at landscape level.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Insects are widely recognized to play a key role in ecosystem processes; therefore they are frequently used to evaluate the effects of human activities on biodiversity and environment quality (Kim 1993; Niemelä 2001; Gerlach et al. 2013). Their potential as indicators depends of their sensitiveness to local resource variations, which produce changes in abundance and richness (Werner and Raffa 2000; Gerlach et al. 2013). Boreal forest insect communities are rather well-known (Martikainen et al. 2000; Niemelä 2001; Matveinen-Huju et al. 2006) in contrast to the austral forest ecosystems (Lanfranco 1977; Stary 1994; Spagarino et al. 2001; Lencinas et al. 2008a). In southern Patagonia, Nothofagus pumilio forests have an endemic entomofauna, which includes unique, rare and relictual species (Lanfranco 1977; McQuillan 1993) of great importance to define biogeographic regions (Niemelä 1990; Massaccesi et al. 2008). Few works analyzed the impact of timber forest management in the austral temperate forests over insect communities (e.g., Spagarino et al. 2001 in Tierra del Fuego, and Bashford et al. 2001, Baker et al. 2004, 2009, 2013, and Grove 2010 in Tasmania). Improving our knowledge of insects in the context of forest management is necessary for an effective conservation (Kim 1993).
Variable retention is an alternative silvicultural approach to timber forest management, which consist in a regeneration treatment with different degrees and patterns of stand retention. The degree of retention after harvesting is crucial to determine the magnitude of impacts on forest biodiversity and natural ecological cycles (Kohm and Franklin 1997; Lindenmayer et al. 2012). The impact of traditional silvicultural practices (e.g., clear-cuts and shelterwood cuts) on insect diversity has been world-wide analyzed (e.g., Michaels and McQuillan 1995; Kaila et al. 1997; Lewis and Whitfield 1999; Werner and Raffa 2000; Baker 2006; Baker et al. 2004, 2009; Huber and Baumgarten 2005). Recently, the variable retention approach to timber harvesting emerged as an alternative silvicultural management proposal to mitigate harmful effects of traditional practices on forest ecosystems, where the major objectives are: create refuges for species and processes over the regeneration phase; increase structural variation in managed stands; and enhance connectivity at the landscape level (Franklin et al. 1997; Gustafsson et al. 2012). Variable retention benefits for insect conservation have been reported in North American, northern Europe and Australian temperate forests (Hammond et al. 2004; Lemieux and Lindgren 2004; Hyvärinen et al. 2005, 2006; Martikainen et al. 2006; Matveinen-Huju et al. 2006; Baker et al. 2009). However, there is a lack of information about the effectiveness of variable retention practices to improve insect conservation in other forest types including Patagonian forests. Beside this, few studies include medium- and long-term research to assess variable retention effects (Arnott and Beese 1997; Hickey et al. 2001; Spence et al. 2002; Aubry et al. 2004; Martínez Pastur et al. 2010) or compare diversity structure before and after harvesting (Before-After-Control-Impact or BACI approach) as a way to determine the extent of variation in biodiversity prior to the implementation of silvicultural treatments, especially on insect diversity (Grove 2010). Consequently, the objectives of this work were: (1) to define a base-line of insect diversity (relative abundance and richness) in unmanaged old-growth southern Patagonian N. pumilio forests (Argentina) along a site quality gradient of the stands; (2) to evaluate insect conservation in variable retention harvested stands, compared with old-growth unmanaged forests; and (3) to assess temporal changes over the first 4-years after harvesting. We expect that: (1) greater diversity occurs at better site quality stands; (2) the inclusion of different retention patterns in harvested stands improves the conservation of original insect diversity; and (3) the stability of insect diversity over time is related to the retention degree in the stands.
Materials and methods
Nothofagus pumilio forests
Tierra del Fuego Island, shared between Chile and Argentina, is at the austral extreme of South America and hosts the world’s southernmost forested ecosystems, which are also found in one of the least disturbed eco-regions on the planet (Mittermeier et al. 2003). N. pumilio is the main tree component of these forests. This species has a wide natural distribution from 36°50′ to 55°02′S (Dimitri 1972). Among the three species of Nothofagus found in southern Patagonia, N. pumilio forests are mainly used for timber harvesting activities due to good yield characteristics (Martínez Pastur et al. 2009). The understory of these timber quality stands in southern Patagonian N. pumilio forests comprises low vascular plant diversity (Lencinas et al. 2008b), but a rich bryophyte flora (Matteri and Schiavone 2002). A number of exotic plant and mammal species are also present, deliberately or accidentally introduced (Moore and Goodall 1977; Collantes and Anchorena 1993; Lizarralde and Escobar 2000).
Studied sites and forest structure characterization
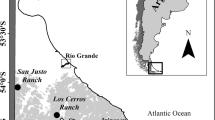
The study was conducted in a long-term permanent plot (61 ha) of pure old-growth N. pumilio forest in San Justo Ranch (54°06′S, 68°37′W), where variable retention harvesting was applied for the first time in Tierra del Fuego, Argentina (Martínez Pastur et al. 2010; Gustafsson et al. 2012). This forest presented a full range of site qualities associated to the forest productivity of each site (higher trees present greater volumes and productivities), for which the site index at base age of 60 years (SI60) varied between less than 9.8–23.2 m height (Martínez Pastur et al. 1997). Stands growing on high-quality sites (SI60 > 16.5 m) have total volume (including timber and non timber volume of trees) over 900 m3 ha−1 and trees with a total height over 24 m. On medium-quality sites (SI60 = 13.1–16.5 m), stands have a total volume of 700–900 m3 ha−1 and trees with a total height between 20.5 and 24 m, while stands growing on low-quality sites (SI60 < 13.1 m) have a total volume of less than 700 m3 ha−1 and trees with a total height less than 20.5 m (Martínez Pastur et al. 2009). These forests were undisturbed by forestry practices before silvicultural regeneration systems were in place.
The baseline was defined prior to harvesting (2001) and sampling was conducted during nine summer days in high (HSQ), medium (MSQ) and low (LSQ) homogeneous site quality stands. Then, harvesting was conducted at autumn 2001, and at the following 4 years (YAH; 2002–2005), these treatments were studied: 10.7 ha of a combined practice, including aggregated retention (AR) and dispersed retention (DR), with one aggregate per hectare (30 m radius) and 10–15 m2 ha−1 basal area of remaining trees among them, representing 40–50 % of total retention; and 18.5 ha of pure aggregated retention surrounded by clear-cut (CC), with one aggregate per hectare (30 m radius), representing 28 % of retention. For a control, 8.6 ha of old-growth unmanaged forests (OGF) was left without harvesting (22.9 m total height, 528 trees ha−1, 40.6 cm diameter at breast height-DBH, 65.0 m2 ha−1 basal area-BA and 727.8 m3 ha−1 total over bark volume-TOBV). After harvesting, samples were taken in the geographic center of two plots during 12 days in each previously described treatments (AR, DR and CC), as well as in control stands (OGF). Location of each sampling was obtained from geographic information system (GIS), and then placed in field by global positioning system (GPS).
In the study area, climate was measured with two weather stations (Davis Weather Wizard III and accessories, USA) placed in old-growth and harvested stands from 2002 to 2005 (Martínez Pastur et al. 2007). Weather conditions were characterized by short, cool summers and long, snowy and frozen winters. Mean monthly temperatures (2 m above the forest floor) varied from −0.2 to 10.4 °C (extreme minimum and maximum from −9.6 °C in July to 24.9 °C in February) in the old-growth forest, while in the harvested stand temperature varied from −1.0 to 10.6 °C (extremes from −11.3 °C in July to 25.9 °C in February). Only 3 months per year did not have mean monthly temperatures under 0 °C, and the growing season was approximately 5 months. Soil temperatures at 30 cm deep were never below freezing in the old-growth forest, but soil freezing was observed in the harvested stand (−0.2 to −0.6 °C during June-July). Rainfall including snowfall (2 m above the forest floor) was 382 mm year−1 inside the old-growth forest, while it was 639 mm year−1 in the harvested stand. Annual average wind speed outside forests was 8 km h−1, reaching up to 100 km h−1 during storms.
Insect sampling methodology
Adult mobile insects were collected during the summer season (January–February) of five consecutive years (2001–2005), before and after harvesting. The sampling of the insect assemblage was done using a wide spectrum trap system with 13 trap types (Lencinas et al. 2008a). This included pit-fall traps (100 × 15 × 8 cm) to collect at leaf litter level; and smell ethanol attractive traps (20 cm diameter), black and white cold fluorescent light traps (20 cm diameter with 4 W lamps) and coloured pans (10 × 10 × 5 cm, using yellow, white and sky-blue colours), to collect at the understory (0.20–1.00 m height) and canopy levels (3/4 total height of tree overstory, which was 16–20 m height). Water was used as a retention agent and commercial detergent was employed to diminish surface tension. Traps were active during 1-day (24 h) sampling periods, which were demonstrated to be appropriated for sampling of insect communities in Nothofagus forests (Spagarino et al. 2001; Lencinas et al. 2008a). Collections were carried out under equivalent climatic conditions, discarding days of strong winds or heavy rain.
After trapping, individuals were quantified and classified under a binocular dissecting scope (×10–×20) at order and family levels (except for Lepidoptera and Psocoptera), following the classifications proposed by Richards and Davies (1984), and Romoser and Stoffolano (1998). Coleoptera were determined at genus or species level when possible (Ross 1973; Roig-Juñent 2000; Roig-Juñent and Domínguez 2001; Marvaldi and Lanteri 2005; Posadas 2012), using standard keys in collaboration with specialists (see “Acknowledgements”). In those orders for which species or genus cannot be determined, because Patagonian insect systematic is still incomplete, the recognizable taxonomic unit or morphospecies concept was employed (Oliver and Beattie 1993; Gerlach et al. 2013). The use of morphospecies instead of formal taxonomic species may be sufficiently close to estimate species richness with average errors below 15 % in assessment of biodiversity inventories, monitoring or preliminary ecological studies (Oliver and Beattie 1993). Likewise, morphospecies have been demonstrated to be a good tool for insect diversity studies in Nothofagus forests (Spagarino et al. 2001; Lencinas et al. 2008a). For convenience, the term “species” was used here to refer to both species and morphospecies. Specimens were deposited in the permanent reference collection at Centro Austral de Investigaciones Científicas (CADIC-CONICET) in Ushuaia, Argentina.
Different functional groups may respond differently to the presence of residual trees (Matveinen-Huju et al. 2006), therefore complementary analysis were carry out by sorting and quantification of species according to pre-defined functional groups, based in their response to environmental change (in this case, harvesting treatments). Two main response types were identified: detectors, which are sensitive to environmental change and decrease with added environmental stress, and exploiters, which increase in abundance in response to environmental stress (Gerlach et al. 2013). For more detailed analysis, a sub-classification was utilized, by which detectors were sub-classified as (1) R-OGF: old growth forest species sensitive to any kind of harvesting; (2) R-AR: old growth forest species better conserved in aggregated retention; (3) R-DR: old growth forest species better conserved in dispersed retention; (4) S-CC: species exclusively sensitive to clear-cut; (5) S-AR: species exclusively sensitive to aggregated retention; (6) S-DR: species exclusively sensitive to dispersed retention. Likewise, exploiters were sub-classified as (7) H-AR: species mainly favoured by aggregated retentions; (8) H-DR: species mainly favoured by dispersed retentions; (9) H-CC: species mainly favoured by clear-cuts. Moreover, another category was considered: (10) NS: non-sensitive species to environmental changes. R-OGF, R-AR and S-CC corresponded to species affiliated with mature forest structures, while H-AR, H-DR and H-CC corresponded to species affiliated with disturbed areas. The assignment of each species to each category was defined by their average abundance in the treatments, standardized by the maximum observed abundance. Thus, a species was considered R-OGF when their standardized abundance is maximum in OGF, and lesser than 50 % in the other treatments; R-AR when standardized abundance was 50 % or greater in AR than in OGF, and lesser than 50 % in the other treatments; S-CC: when standardized abundance was lesser than 50 % in CC and greater than 50 % in the other treatments; H-AR: when standardized abundance was lesser than 50 % in OGF stands and have maximum values in AR; H-DR: when standardized abundance was lesser than 50 % in OGF stands and have maximum values in DR; H-CC: when standardized abundance was lesser than 50 % in OGF stands and have maximum values in CC; and NS: when standardized abundance was greater than 50 % and similar between themselves in OGF and CC. The response types S-AR and S-DR were not detected in this study.
Data analysis
Richness calculations were made at plot, treatment, years and whole sampling levels. Species accumulation curves for each treatment were calculated by rarefaction using EstimateS software (Colwell 2005). Species rarity was analyzed (Willott 2001; Novotný and Basset 2000) considering as “common species” to those with abundance higher than two individuals, and “rare species” to doubletons and singletons. Rare species for the whole catching were excluded in the following analyses.
For the baseline characterization, one-way ANOVAs were conducted with site quality as main factor (three levels, N = 18). For comparison after harvesting, two-way ANOVAs were used, with treatments and YAH (four levels each, N = 48) as main factors. Averages were tested for significant differences by Tukey test (p < 0.05). The response variables in all the analyses were average species richness and abundance per trap system and day (species × trap system × day and individuals × trap system × day, respectively), both for the whole sampling and the four main orders (Diptera, Hymenoptera, Coleoptera and Lepidoptera). Statgraphics (Statistical Graphics Corp., USA) software was used for these analyses.
DCA (detrended correspondence analysis; Hill 1979) was conducted to evaluate the changing magnitude and direction of insect community composition among baseline, first and fourth YAH (Manly 1994). This ordination utilized species average abundance data of the main insect orders (excluding rare species), and was developed with rescaling of axes and without down-weighting for rare species in PC-Ord software (McCune and Mefford 1999).
Response types were analyzed by DCAs, which were conducted with abundances averaged by the first four YAH to represent the relative distribution and affinities of each response type to the studied treatments. These DCAs were also developed with rescaling of axes and without down-weighting for rare species in PC-Ord software. Additionally, representation of response types at different site quality in the baseline before harvesting, among treatments and years after harvesting were summarized by ANOVAs analyzing richness and abundance. Statgraphics software was used for these analyses. Abundance values (Y) were log transformed by the equation W = log (Y + 1) prior to the analyses to achieve normality and homocedasticity assumptions (Basset 1999; Martikainen et al. 2000), but non-transformed data are shown.
Results
The whole sampled insect richness was 163 species, of which 42 species (26 %) were rare (doubletons and singletons), and 121 species (74 %) were common. In the baseline, 79 species were found in old-growth forests, of which only 6 species (8 %) were rare for the whole sampling, 28 species (35 %) were rare only in the baseline (and totalled more than two individuals after harvesting), and 45 species (57 %) were common species. After harvesting, 84 species were added in the 4 years following harvesting (36 rare and 48 common species).
The 23,236 collected individuals belonged to 11 orders (Table 1). Diptera, Hymenoptera, Coleoptera and Lepidoptera were the best represented orders, with 49, 46, 34 and 21 species respectively. Among these, Hymenoptera had the maximum quantity of rare species (17 species) and Lepidoptera the minimum (only one species). Richness varied along the years and treatments, increasing with time and reaching to the highest value at the fourth YAH (121 species) and in OGF treatment considering all the years (134 species). However, the lowest value was recorded during the third YAH (97 species) and in the CC treatment considering all the years (100 species). Shared species between OGF and harvesting treatments varied in time, diminishing the three first YAH (106–96–87 species, respectively) and increasing at the fourth YAH (107 species). However, shared species with OGF diminished proportionally to the intensity of harvesting treatments (from 104 sp in AR, to 92 in DR, to 89 in CC). Rarefaction indexes standardized to the same number of samples for each treatment showed greater similarity of species between OGF and AR, while DR and CC presented also similar but lower values (Fig. 1).
In the baseline characterization, significant differences for insect average richness and abundance per capture were not detected among different site quality stands, both for total sampling and for the main four orders (Table 2), with the exception of Diptera richness, which was significantly higher in HSQ (17.2 species × trap system × day) than in LSQ (13.8 species × trap system × day). Total richness for the baseline including the different site quality stands was 24.3 species × trap system × day, and the average abundance was 178.4 individuals × trap system × day. For the main orders, the average richness was 2.5 species × trap system × day for Hymenoptera, 0.7 for Coleoptera and 5.1 for Lepidoptera, while average abundance was 156.2 individuals × trap system × day for Diptera, 3.2 for Hymenoptera, 0.7 for Coleoptera and 18.1 for Lepidoptera.
After harvesting, there were significant differences for insect average richness and abundance among treatments and years after harvesting, as well as in the interaction between the main factors, both for total and the main four orders (Table 3). Among treatments, differences were found for Diptera and Lepidoptera richness, and Lepidoptera abundance, with greater values in OGF (23.6 species × trap system × day, 9.8 species × trap system × day and 165.1 individuals × trap system × day, respectively) than in DR (19.9 species × trap system × day, 6.6 species × trap system × day and 50.0 individuals × trap system × day). Significant differences among YAH were found for total, Diptera, Hymenoptera and Lepidoptera richness, and for Coleoptera abundance. Maximum and minimum values occurred at different YAH for each variable, but trend to increase with time (36.8–48.5 species × trap system × day for total 18.0–23.0 species × trap system × day for Diptera, and 5.5–9.4 species × trap system × day for Hymenoptera richness), except for Coleoptera abundance that was higher at the first and the fourth YAH (14.9 and 18.6 individuals × trap system × day, respectively) than in the second YAH (4.2 individuals × trap system × day), and for Lepidoptera richness which was higher at the first YAH (10.6 species × trap system × day) than at the third YAH (7.1 species × trap system × day). Interactions were significant only for Hymenoptera richness and abundance, which presented greater values for different treatments in different years. Particularly, both variables were higher in OGF than in the other treatments at the first YAH, maximum in CC at the second YAH, highest and similar in AR and DR at the third YAH, and greater and similar in OGF and AR than in DR and CC in the fourth YAH.
In the representation of changing in insect community composition among the first and the fourth YAH using multivariate DCA ordination, the greatest changing magnitudes (Fig. 2) were observed in AR and DR treatments, but changing direction was similar for OGF, AR and CC while it was different for DR. Moreover, all treatments slightly differed from the baseline at the first YAH, but DR presented greater distance from baseline than the others.
Changing magnitude and direction in insect composition obtained by DCA with species average abundances of the main insect orders in the base-line prior to harvesting (BL), and in the first (1) and the fourth (4) year after harvesting for each treatment (OGF old growth forests, AR aggregated retention, DR dispersed retention, CC clear cuts)
When response was analyzed (Fig. 3), the exploiter species, mainly those favoured by dispersed retention (H-DR, 25 species) included the greatest richness, followed by old growth forest species sensitive to any kind of harvesting (R-OGF, 24 species) and species mainly favoured by aggregated retentions (H-AR, 22 species). However, common species observed in the baseline mainly included non-sensitive species (NS, 11 species), R-OGF (10 species) and H-AR (8 species); the rare species detected during the base-line mostly included H-DR (8 species), R-OGF (6 species) and H-AR (5 species); and the species added after harvesting largely included H-DR (13 species) and species mainly favoured by clear-cuts (H-CC, 11 species). Likewise, almost all response types were observed for the four main insect orders (Fig. 4).
Number of species for each response type discriminated by the base-line (common and rare) and those added after variable retention harvesting. Response types: R-OGF old growth forest species sensitive to harvesting, R-AR old growth forest species conserved in aggregated retention, S-CC clear-cut sensitive species, NS non-sensitive species to harvesting, H-AR species favored for harvesting, mainly predominant in the aggregated retention, H-DR species favored for harvesting, mainly predominant in the dispersed retention, H-CC species favored for harvesting, mainly predominant in clear cuts
Examples of species response types to harvesting treatments (OGF old growth forests, AR aggregated retention, DR dispersed retention, CC clear cuts) for the main insect orders, based on average abundance for the first years after harvesting. Response types: R-OGF old growth forest species sensitive to harvesting, R-AR old growth forest species conserved in aggregated retention; S-CC = clear-cut sensitive species; NS = non-sensitive species to harvesting, H-AR species favored for harvesting, mainly predominant in the aggregated retention, H-DR species favored for harvesting, mainly predominant in the dispersed retention, H-CC species favored for harvesting, mainly predominant in clear cuts
The relative distribution of species presented in DCAs (Fig. 5), showed the clear association of response types with treatments. The R-AR response type was few represented in both DR and CC treatments, while the central part of the graphics was mainly occupied by NS and S-CC species. On the other hand, there were more species shared between DR and AR (7 species) than between AR and CC (5 species), as well as there were less species shared between CC and OGF (4 species) than between DR and OGF (6 species).
Relative distribution of species for each response types among treatments (R-OGF old growth forest species sensitive to harvesting, R-AR old growth forest species conserved in aggregated retention, S-CC clear-cut sensitive species, NS non-sensitive species to harvesting, H-AR species favored for harvesting, mainly predominant in the aggregated retention, H-DR species favored for harvesting, mainly predominant in the dispersed retention, H-CC species favored for harvesting, mainly predominant in clear cuts)
In the ANOVAs for response type species distribution at different site qualities, in the baseline prior to harvesting (Table 4), significant differences in richness were only detected in H-CC, with greater values in HSQ (2.0 species × trap system × day) and minimum ones in MSQ (1.0 species × trap system × day), while LSQ did not present differences with HSQ and MSQ. Response types with not significant differences presented at baseline a richness of 4.3 species × trap system × day for R-OGF, 3.1 for R-AR, 2.5 for S-CC, 6.1 for NS, 3.5 for H-AR and 3.3 for H-DR, combining the different site quality stands. On the other hand, significant differences were detected for H-DR and H-CC abundances. In H-DR, abundance was higher in MSQ (77.3 individuals × trap system × day) than in the others (12.1 individuals × trap system × day in average), while in H-CC abundance was higher in HSQ than in MSQ (3.7 individuals × trap system × day vs. 1.8 individuals × trap system × day, respectively) and LSQ did not present differences. Response types with not significant differences presented at baseline abundances of 12.2 individuals × trap system × day for R-OGF, 11.3 for R-AR, 14.9 for S-CC, 96.7 for NS and 7.0 for H-AR.
In the ANOVAs for response type species after harvesting, there were found many significant differences for richness and abundance among treatments and years, as well as some interaction between the main factors (Table 5). Among treatments, differences were found for R-OGF, R-AR, H-AR, H-DR and H-CC richness, and for R-OGF, R-AR, S-CC, H-AR and H-CC abundances. Detectors, or response types groups affiliated with mature forests structures (R-OGF, R-AR and S-CC) presented lower richness and abundance in DR and CC, while exploiters or species affiliated with disturbed areas (H-AR, H-DR and H-CC) showed minimum richness and abundance in OGF. On the other hand, significant differences among YAH were found for S-CC, H-AR, H-DR and H-CC richness, and for R-OGF, H-AR and H-CC abundance, with maximum and minimum values occurred at different YAH for each response type. Some of them trended to increase with time (S-CC richness, H-AR and H-CC richness and abundance), and other trended to decrease (R-OGF abundance). Interactions were significant for R-OGF, R-AR and S-CC richness, and for R-AR and H-CC abundance. In R-OGF richness, lowest values were observed for CC in YAH 1 and in DR in YAH 2–3–4, while highest values were observed in OGF in all YAH except in YAH 3, which had greatest richness in AR. Similar changes among minimum values in the YAH 1 and 2 were observed for R-AR richness and abundance (lowest values in CC at YAH 1, and in DR in YAH 2–3). Maximum values in S-CC richness varied among treatments along the YAH 4, but always was minima at CC, except in YAH 3, when was lowest in DR. Finally, interactions in H-CC abundance are explained by almost the same value in all treatments for YAH 1, with increasing and consistent differences in the following YAH (greatest values in CC compared to the other treatments).
Discussion
Insect communities in N. pumilio forests and baseline before harvesting
Total richness for insect species during the baseline in the OGF included only 79 species, despite the wide spectrum system trap employed captures 11 different orders. This richness is comparable to the observed in other studies in Nothofagus forests (Lanfranco 1991; Spagarino et al. 2001; Lencinas et al. 2008a). The general order dominance is similar among these studies too, as well as to those from other Patagonian forest types (Coscarón and Wygodzinsky 1962; Pérez et al. 1997), and independently of the trap system employed. Although there is not possible to compare diversity obtained from different sampling systems, comparison with richness observed in only one family for some orders in Northern Hemisphere primary temperate forests, denotes total baseline richness here is lower than in other temperate forests, e.g., 200 Carabidae species in Central Finland (Heliölä et al. 2001), or 93 Braconidae species in US forests (Lewis and Whitfield 1999). Low insect diversity in Tierra del Fuego forests follows the pattern of generally poorer faunal diversity in southern Patagonia (Guzmán et al. 1985–1986) compared to northern regions of similar latitudes (Martikainen et al. 2000). This low diversity is probably related to the short growing season (Massaccesi et al. 2008), low thermal amplitude between winter and summer, and low average summer temperatures (Ferreyra et al. 1998). Differences in richness compared with other primary Patagonian N. pumilio forests (55 species in Spagarino et al. 2001), which used similar broad sampling set, could be explained by latitudinal gradient differences in the study sites (Kusnezov 1957; Hillebrand 2004).
Rare species (singletons and doubletons) represent 43 % of richness in the baseline characterization of this study, but 85 % of rare species totalized more than two individuals in the samplings after harvesting. Probably, rare species in the OGF could be typical species from other environments that are eventually introduced in closed forests, and after harvesting found better conditions to establish in disturbed areas. Rare insect species in the baseline that never were sampled after harvesting were only 6 species. Likewise, rare species totalized 42 species (26 % of total richness) in the whole sampling, including before and after harvesting studies. Singletons usually could represent half or more of the richness in insect inventories (Lewis and Whitfield 1999; Novotný and Basset 2000). Lower singleton values in this study could be again related to the relatively low general diversity observed in Southern Patagonian forests. However, it is possible that some insect species of the Fuegian forests were not studied or captured yet, thus currently an underestimation of their real diversity likely exists.
In this study, order insect diversity was almost not correlated with site quality in OGF, contrary to the expected first hypothesis. The exception was Diptera richness, which was favoured by high site quality sites. This could be explained by better moisture conditions in HSQ than in MSQ and LSQ, likely explained by topography (HSQ usually occupy down slope zones in valleys, and LSQ are in the top of the hills) and presence of microenvironments (old decaying logs, accumulation of water in stumps, pits and mounds, woody debris in the forest floor) commonly observed in Nothofagus forests (Ramírez et al. 1985; Martínez Pastur et al. 2002). Habitat heterogeneity increases the variety of available niches for colonization, and consequently the richness that this could support (Ozanne et al. 2000). For example, the soil depressions left by the roots of wind-thrown trees and hollowed trunks can fill with rain water in timber forests, that makes them appropriated niches for Chironomidae (Diptera) aquatic larvae development (Pérez et al. 1997). Positive association of insect species abundance with site quality has been observed in other temperate forests (Safranyik 1985; De Somviele et al. 2004). In Tierra del Fuego, site quality of the stands is mainly determined by abiotic factors (e.g., soil nutrient contents, soil drainage and depth, slope, aspect, topography and wind exposure) (Martínez Pastur et al. 1997), which could influence both over overstory structure and understory plant diversity (Martínez Pastur et al. 2002).
The analysis by functional groups based on response types helped to understand changes in the assemblages of insect species communities (Matveinen-Huju et al. 2006; Baker et al. 2009). When response type insect species was analyzed for the baseline, these were mostly uniformly represented among different site quality stands. This was observed for more abundant types (NS, R-OGF and H-AR), as well as in low represented response types (R-AR and S-CC). Nonetheless, two response types significantly differed among site qualities: H-DR was more abundant in MSQ than in the other site qualities, while H-CC presented highest richness and abundance in HSQ, probably because the presence of greater canopy gaps in these stands (Lencinas et al. 2011) generates similar conditions and appropriate niches for response groups favored by more intensive harvesting.
Harvesting impacts on insect diversity
Total insect richness measured in harvested N. pumilio forests (163 species) was higher than in the baseline characterization (79 species), showing an increase in total insect diversity when harvesting occurs. Moreover, total richness was also higher than in other N. pumilio studies that only analyzed shelterwood-cuts (104 species, Spagarino et al. 2001), which could be interpreted as greater entrance of species under variable retention harvesting than under traditional silvicultural practices. However, greater richness and abundance were observed for OGF and AR, than for DR and CC, as was similarly observed by Spagarino et al. (2001) for old-growth forests compared with shelterwood harvested stands along the whole forest cycle. Therefore, harvesting reduce N. pumilio old-growth forest insect richness independently of the silvicultural regeneration systems applied, and the original insect community assemblage significantly changed in the harvested forests, due to the loss of sensitive species and the income of species from other surrounding environments.
The overall addition of 84 species in N. pumilio forests during the first post harvesting years occurred not only in the harvested stands, but also in the unharvested areas (65 % of the species added after harvesting were only observed in OGF), probably by the introduction of species from grasslands, peat-lands and N. antarctica forests, which possess a different insect species assemblage than the unharvested stands (Lencinas et al. 2008a), or by the eventual increase of rare species population, which allow their capture after harvesting. Location of sampling set in different aspects (east vs. west), as well as at relatively low distances from associated environments (N. antarctica patches in wetlands or edges), could result in higher presence of rare or less frequent species, especially when dispersion is favoured by wind (e.g., winged insect species).
Income of insect species into the harvested stands (in our study, 11 species in DR and CC, 21 species in AR) was also observed for other authors in other temperate forests of the world, e.g., carabid beetles common to open habitats increased in abundance after cuttings in US and Finland forests (Lenski 1982; Niemelä et al. 1988; Werner and Raffa 2000). The assemblages observed in the first post-treatment year consist mostly of colonizers and is determined by the properties of the surrounding forest landscape (Hyvärinen et al. 2006). Income of insect species from associated surrounding environments was probably greater in N. pumilio harvested stands with aggregates, due to greater availability of microenvironments than in DR or CC. Old-growth structures (including dead wood, large old trees and other features) are well preserved in the aggregates, but also new environmental conditions are created in the edges (inner and outside) of the aggregates. Forest edges often host more species and individuals of different taxa than the interiors (Matveinen-Huju et al. 2006). Insect species introductions into variable retention treatments were also observed in other retention-harvested forests. For example, Matveinen-Huju et al. (2006) observed in Finland forests that individuals of many species of semi and totally open, and/or medium dry habitats increased over the first YAH 3 in all retention groups. We know that harvesting intensity is usually proportional to the changes in microclimatic factors and resource availability at the understory level (e.g., radiation and effective rainfall) (Chen et al. 1993, 1995; Promis et al. 2010; Martínez Pastur et al. 2011). Microsite differences in factors such as local moisture, humidity, or predation may affect insect catch (Werner and Raffa 2000). Likewise, light intensity and moisture are the primary driving factors for arthropod responses to microclimate changes (Thiele 1964; Huhta 1971). The use of BACI approach (Before-After-Control-Impact) is highly recommended to control for environmental variation between sites and the year-to-year fluctuation in several environmental conditions (Niemelä 2001), and in this study allowed us to avoid attributing insect community changes to pre-harvesting differences, as was criticized by North et al. (1996).
The loss of old-growth forest insect species in harvested stands is strongly related to harvesting intensity, from 30 species in AR, to 42 species in DR, and to 45 species in CC (including both common and rare species), and greatly affects forest specialist species (Lenski 1982; Niemelä et al. 1988; Werner and Raffa 2000). Similarly to these results, many forest species in Finland and/or species requiring moist habitats decrease over the three first years after logging in all retention-tree groups (Matveinen-Huju et al. 2006). Despite this, aggregated retention in N. pumilio forests conserves richness and abundance at high and similar levels that old-growth forests have, as observed by Baker et al. (2009) in Tasmania and in Finland tree-group retention harvested forests (Matveinen-Huju et al. 2006). Beside this, only common species favoured by more intensive harvesting (as dispersed retention and clear-cuts) presented lower richness and abundance in OGF and AR than in the other treatments. Insect community assemblage in AR was more similar to OGF than to the other treatments (DR and CC), which were also very similar between themselves. This result was confirmed by the ANOVAs for orders, but better highlighted by the ANOVAs for response type insect species, especially in R-OGF, R-AR, S-CC and H-AR types, which included mainly species associated with old-growthness of forests. Old-growth forests are less dynamic, but structurally more complex than harvested stands. Environmental heterogeneity and interspecific microhabitat preservation are thus important for the maintenance of local insect species diversity, not only for common species but also for infrequent species or functional groups. The low overall loss of OGF species during the first four YAH (only two rare species) could be explained by the good performance of aggregated retention as refuges for N. pumilio insect diversity.
Unexpectedly, richness in old-growth forests varied after harvesting to higher and lower values than in the baseline (92–67–60–87 species during the first 4 years after harvesting). These changes might be attributed to annual insect population fluctuations among years (Spitzer et al. 1984; Spitzer and Lepš 1988; McArdle and Gaston 1992; Martikainen and Kaila 2004). However, harvesting modifies microclimatic conditions not only inside harvested areas but also in the surrounding old-growth forests (e.g., in wind permeability), which facilitate dispersion of winged species (Grove and Forster 2011; Noreika and Kotze 2012).
The time after the harvesting differently influences richness and abundance of common species classified by order, as well as by response type, with greatest values mainly at the first or at the fourth YAH. This could be explained mainly by the differences originated for the annual fluctuations, and also by colonizer population stability reached in the second and the following years post-treatment (Hyvärinen et al. 2006). However, a clear changing trend was observed when the first and the fourth YAH were compared, which had the same direction for OGF, AR and CC, but not for DR (as was showed in DCA at Fig. 2). Also, the facilitation of the introduction of insect species was directly related to time since disturbance (7, 10, 10 and 14 species introduced from the first to the fourth YAH). However, the time-span considered in this study could not produce many detectable changes at the population levels for some insect species. A follow-up study would be needed to determine whether populations of forest species continue decreasing, and population of open habitat species continue increasing. Furthermore, some studies with arthropods demonstrate a time lag in the response to forest cutting (Huhta 1971; Niemelä et al. 1993; Koivula 2002).
As we stated in the 2nd hypothesis, the inclusion of retention in N. pumilio harvested stands improves the conservation of original insect diversity, because aggregates of retention act as insect community reserves (mainly for close forests and moist habitat insect species), as well as occurs with understory vegetation (Lencinas et al. 2011), allowing the survival of species sensitive to canopy openings or habitat loss. Due to the highest impact of clear cutting over the N. pumilio forest insect communities, the application of dispersed retention among the aggregates also contribute to diminish some of the negative impacts of harvesting. However, the 3rd hypothesis must be refuted, at least for the short time analyzed in this work, because stability of insect diversity over time cannot be reached in the first 4 years after harvesting at any harvesting practice assayed, although aggregated retention better preserve the composition, richness and abundance of the original insect communities (Baker et al. 2009). The real importance of retention-tree groups can only be assessed after long-term empirical studies at both stand and landscape level. Moreover, the biodiversity benefits generated by retention approach can vary by region, silvicultural system and taxonomic and functional group (Rosenvald and Lõhmus 2008).
Ecosystem management implications of variable retention
Several silvicultural methods based on natural regeneration of the natural forests have been proposed for southern Patagonia (Martínez Pastur et al. 2000, 2009; Martínez Pastur and Lencinas 2005; González et al. 2006; Rosenfeld et al. 2006). Initially, Nothofagus forests were harvested using clear-cuts (Gea et al. 2004), and then shelterwood cuts were recommended to improve re-growth (Martínez Pastur et al. 2000; Rosenfeld et al. 2006). But, this last method significantly affects the original diversity of N. pumilio forests (plants, mosses, birds and mammals) (Martínez Pastur et al. 2002; Pulido et al. 2000; Deferrari et al. 2001; Ducid et al. 2005). Specifically in insects, shelterwood cuts cause a large impact on insect diversity with one species lost every 11 years during the first silviculture cycle, and allow the introduction of species from other environments that quickly colonize the harvested stands (Spagarino et al. 2001). Biodiversity conservation in managed landscapes could be improved by maintaining the associated non-timber-quality stands (Lencinas et al. 2005, 2008a, b), where species could survive until the forest structure of the harvested timber-quality forest will be recovered. Associated environments or key habitats exclusion from harvesting (Gustafsson et al. 2010) creates retention patches in managed landscapes. However, this alternative does not offer a solution for insect conservation at the landscape level in N. pumilio forests, as we have found that many insect taxa only inhabited timber-quality stands (Lencinas et al. 2008a). Moreover, associated environment structural and functional characteristics greatly differ from old-growth timber quality stands in southern Patagonian forests (Lencinas et al. 2005, 2008a, b). So, preservation of timber stands as reserves or inclusion of retention aggregates in harvesting practices could conserve old-growth qualities and particular habitat characteristics present in quality stands. Managing landscapes for a greater range of habitat conditions may, therefore, be essential for some organisms (Mitchell and Beese 2002).
Alternative silvicultural methods (Franklin et al. 1997) have been proposed for harvesting N. pumilio forests, which conserve some of the original heterogeneity of the old-growth forest. Bava and López Bernal (2005) proposed to selectively cut groups affecting a small percentage of the forest area, but there is none evidence of the benefit of this practice in biodiversity conservation due to this proposal cutting all the trees along the silviculture cycle. During the last 10 years, the variable retention approach has been proposed as a new and more conservative silviculture for these forests (Gustafsson et al. 2012; Lindenmayer et al. 2012). It has been found to mostly conserve microclimatic and heterogeneity characteristics of the original forest structure (Martínez Pastur et al. 2010), while aggregated retention benefits birds (Lencinas et al. 2009), mosses (Lencinas et al. 2008c) and understory plants (Lencinas et al. 2011). The variable retention silvicultural system is best suited for areas where timber production is desired but maintenance of the structural complexity and biological legacies found in older forests is as important as, or even supersedes, yield and improvement of growing stock (Mitchell and Beese 2002). Old-growth forest insect diversity is better favoured when structural complexity is preserved; therefore, aggregated retention greatly diminished harvesting impacts on insect communities, although invasion by other species cannot be prevented. Aggregated retention are increasingly applied in Tierra del Fuego, and according to these results must be included in N. pumilio silvicultural practices to better achieve long-term insect diversity conservation, but more studies are necessary to evaluate effects of different size, shape and distribution of aggregates into harvested timber forests. Similarly to implications for understory plant species conservation (Lencinas et al. 2011), combination of aggregated and dispersed retention better diminished loss of insect species compared to the implementation of clear-cuts between the aggregates. However, dispersed retention alone cannot provide enough good habitat conditions to prevent the loss of the most sensitive insect species. On the other hand, aggregated retention facilitate the introduction of greater quantity of species, therefore long term studies are necessary to evaluate long term changes in the original community assemblages preserved inside aggregates.
References
Arnott JT, Beese WJ (1997) Alternatives to clearcutting in British Columbia coastal montane forests. For Chron 73:670–678
Aubry KB, Halpern CB, Maguire DA (2004) Ecological effects of variable-retention harvests in the north-western United States: the DEMO study. For Snow Lands Res 78:119–137
Baker SC (2006) A comparison of litter beetle assemblages (Coleoptera) in mature and recently clearfelled Eucalyptus obliqua forest. Aust J Entom 45:130–136
Baker SC, Richardson AMM, Seeman OD, Barmuta LA (2004) Does clearfell, burn and sow silviculture mimic the effect of wildfire? A field study and review using litter beetles. For Ecol Manag 199:433–448
Baker SC, Grove SJ, Forster L, Bonham KJ, Bashford D (2009) Short-term responses of ground-active beetles to alternative silvicultural systems in the Warra Silvicultural Systems Trial, Tasmania, Australia. For Ecol Manag 258:444–459
Baker SC, Spies TA, Wardlaw TJ, Balmer J, Franklin JF, Jordan GJ (2013) The harvested side of edges: effect of retained forests on the re-establishment of biodiversity in adjacent harvested areas. For Ecol Manag 302:107–121
Bashford R, Taylor R, Driessen M, Doran N, Richardson A (2001) Research on invertebrate assemblages at the Warra LTER site. Tasforests 13:109–118
Basset Y (1999) Diversity and abundance of insect herbivores collected on Castanopsis acuminatissima (Fagaceae) in New Guinea: relationships with leaf production and surrounding vegetation. Eur J Entomol 96:381–391
Bava J, López Bernal PM (2005) Cortas de selección en grupo en bosques de lenga. IDIA-XXI 5:39–42
Chen J, Franklin JF, Spies TA (1993) Contrasting microclimates among clear-cut, edge, and interior of old-growth Douglas-fir forest. Agric For Meteorol 63:219–237
Chen J, Franklin JF, Spies TA (1995) Growing season microclimatic gradients from clear-cut edges into old-growth Douglas-fir forests. Ecol Appl 5:74–86
Collantes MB, Anchorena J (1993) Las malezas exóticas y plantas escapadas de cultivo en la región de estepa de Tierra del Fuego. Parodiana 8:213–217
Colwell RK (2005) EstimateS: statistical estimation of species richness and shared species from samples. UConn. Version 7.5. http://viceroy.eeb.uconn.edu/estimates
Coscarón S, Wygodzinsky P (1962) Simuliidae (Diptera, Insecta) de Tierra del Fuego, Patagonia e Islas Juan Fernández. Acta Zool Lilloana XVII:281–333
De Somviele B, Lyytikäinen-Saarenmaa P, Niemelä P (2004) Sawfly (Hym., Diprionidae) outbreaks on Scots pine: effect of stand structure, site quality and relative tree position on defoliation intensity. For Ecol Manag 194:305–317
Deferrari G, Camilion C, Martínez Pastur G, Peri P (2001) Changes in Nothofagus pumilio forest biodiversity during the forest management cycle: 2. Birds. Biodiv Conserv 10:2093–2108
Dimitri M (1972) La región de los bosques andino patagónicos. Sinopsis general. INTA, Buenos Aires
Ducid MG, Murace M, Cellini JM (2005) Diversidad fúngica en el filoplano de Osmorhiza spp. relacionado con el sistema de regeneración empleado en bosques de Nothofagus pumilio en Tierra del Fuego, Argentina. Bosque 26(1):33–42
Ferreyra M, Cingolani A, Ezcurra C, Bran D (1998) High-Andean vegetation and environmental gradients in northwestern Patagonia, Argentina. J Veg Sci 9:307–316
Franklin J, Berg D, Thornburgh D, Tappeiner J (1997) Alternative silvicultural approaches to timber harvesting: variable retention harvest systems. In: Kohm K, Franklin J (eds) Creating a forestry for the 21st century. Island Press, New York, pp 111–140
Gea G, Martínez Pastur G, Cellini JM, Lencinas MV (2004) Forty years of silvicultural management in southern Nothofagus pumilio (Poepp. et Endl.) Krasser primary forests. For Ecol Manag 201:335–347
Gerlach J, Samways M, Pryke J (2013) Terrestrial invertebrates as bioindicators: and overview of available taxonomic groups. J Insect Conserv 17:831–850
González M, Donoso Zegers C, Ovalle P, Martínez Pastur G (2006) Nothofagus pumilio (Poepp. et Endl) Krasser - lenga, roble blanco, leñar, roble de Tierra del Fuego - Familia: Fagaceae. In: Donoso Zegers C (ed) Las Especies arbóreas de los Bosques Templados de Chile y Argentina: Autoecología. Marisa Cúneo, Valdivia, pp 486–500
Grove SJ (2010) Do wildlife habitat strips act as refuges for mature-forest carabid beetle assemblages? A case-study in Tasmanian wet eucalypt forest, Australia. For Ecol Manag 259:496–504
Grove SJ, Forster L (2011) A decade of change in the saproxylic beetle fauna of eucalypt logs in the Warra long-term log-decay experiment, Tasmania. 1. Description of the fauna and seasonality patterns. Biodiv Conserv 20:2149–2165
Gustafsson L, Kouki J, Sverdrup-Thygeson A (2010) Tree retention as a conservation measure in clear-cut forests of northern Europe: a review of ecological consequences. Scand J For Res 25:295–308
Gustafsson L, Baker SC, Bauhus J, Beese WJ, Brodie A, Kouki J, Lindenmayer DB, Lõhmus A, Martínez Pastur G, Messier C, Neyland M, Palik B, Sverdrup-Thygeson A, Volney WJA, Wayne A, Franklin JF (2012) Retention forestry to maintain multifunctional forests: a world perspectiva. BioScience 62:633–645
Guzmán L, Atalah A, Venegas C (1985–86) Composición específica y estructura de la comunidad de aves de verano en el complejo de la Tundra Magellánica. An Inst Pat, Serie Cs Nat 16:75–86
Hammond HEJ, Langor DW, Spence JR (2004) Saproxylic beetles (Coleoptera) using Populus in boreal aspen stands of western Canada: spatiotemporal variation and conservation of assemblages. Can J For Res 34(1):1–19
Heliölä J, Koivula M, Niemelä J (2001) Distribution of Carabid beetles (Coleoptera, Carabidae) across a boreal forest-clearcut ecotone. Conserv Biol 15(2):370–377
Hickey JE, Neyland MG, Bassett OD (2001) Rationale and design for the Warra silvicultural systems trial in wet Eucalyptus oblique forests in Tasmania. Tasforests 13(2):155–182
Hill MO (1979) DECORANA. A Fortran program for detrended correspondence analysis and reciprocal averaging. Publication of Section of Ecology and Systematics. Cornell Univ. Ithaca, New York
Hillebrand H (2004) On the generality of the latitudinal diversity gradient. Am Nat 163:192–211
Huber C, Baumgarten M (2005) Early effects of forest regeneration with selective and small scale clear-cutting on ground beetles (Coleoptera, Carabidae) in a Norway spruce stand in Southern Bavaria (Höglwald). Biodiv Conserv 14:1989–2007
Huhta V (1971) Succession in the spider communities of the forest floor after clear-cutting and prescribed buerning. Ann Zool Fennici 8:483–542
Hyvärinen E, Kouki J, Martikainen P, Lappalainen H (2005) Short-term effects of controlled burning and green-tree retention on beetle (Coleoptera) assemblages in managed boreal forests. For Ecol Manag 212:315–332
Hyvärinen E, Kouki J, Martikainen P (2006) Fire and green-tree retention in conservation of red-listed and rare deadwood-dependent beetles in Finnish boreal forests. Conserv Biol 20(6):1711–1719
Kaila L, Martikainen P, Punttila P (1997) Dead trees left in clear-cuts benefit saproxylic Coleoptera adapted to natural disturbances in boreal forest. Biodiv Conserv 18:1–18
Kim K (1993) Biodiversity, conservation and inventory: why insects matter. Biodiv Conserv 2:191–214
Kohm KA, Franklin JE (1997) Creating a forestry for the 21st century: the science of ecosystem management. Island Press, Chicago
Koivula M (2002) Alternative harvesting methods and boreal carabid beetles (Coleoptera, Carabidae). For Ecol Manag 167:103–121
Kusnezov M (1957) Numbers of species of ants in faunas of different latitudes. Evolution 11:298–299
Lanfranco D (1977) Entomofauna asociada a los bosques de Nothofagus pumilio en la región de Magallanes. 1º parte: Monte Alto (Río Rubens, Última Esperanza). An Inst Patagon 8:319–346
Lanfranco D (1991) Sinopsis de los insectos que atacan bosques de lenga (Nothofagus pumilio (Poepp. et Endl.) Krass.) en Magallanes. An Inst Patagonia, Serie Cs Nat 20:89–93
Lemieux JP, Lindgren BS (2004) Ground beetle responses to patch retention harvesting in high elevation forests of British Columbia. Ecography 27:557–566
Lencinas MV, Martínez Pastur G, Medina M, Busso C (2005) Richness and density of birds in timber Nothofagus pumilio forests and their unproductive associated environments. Biodiv Conserv 14:2299–2320
Lencinas MV, Martínez Pastur G, Anderson CB, Busso C (2008a) The value of timber quality forests for insect conservation on Tierra del Fuego Island compared to associated non-timber quality stands. J Ins Conserv 12:461–475
Lencinas MV, Martínez Pastur G, Rivero P, Busso C (2008b) Conservation value of timber quality versus associated non-timber quality stands for understory diversity in Nothofagus forests. Biodiv Conserv 17:2579–2597
Lencinas MV, Martínez Pastur G, Solán R, Gallo E, Cellini JM (2008c) Forest management with variable retention impact over bryophyte communities of Nothofagus pumilio understory. Forstarchiv 79:77–82
Lencinas MV, Martínez Pastur G, Gallo E, Cellini JM (2009) Alternative silvicultural practices with variable retention improve bird conservation in managed South Patagonian forests. For Ecol Manag 258:472–480
Lencinas MV, Martínez Pastur G, Gallo E, Cellini JM (2011) Alternative silvicultural practices with variable retention to improve understory plant diversity conservation in southern Patagonian forests. For Ecol Manag 262:1236–1250
Lenski FT (1982) The impact of forest cutting on the diversity of ground beetle (Coleoptera, Carabidae) in the southern Appalachians. Ecol Monogr 7:385–390
Lewis CN, Whitfield JB (1999) Braconid wasp (Hymenoptera: Braconidae) diversity in forest plots under different silvicultural methods. Environ Entom 28(6):986–997
Lindenmayer DB, Franklin JF, Lõhmus A, Baker SC, Bauhus J, Beese W, Brodie A, Kiehl B, Kouki J, Martínez Pastur G, Messier C, Neyland M, Palik B, Sverdrup-Thygeson A, Volney WJA, Wayne A, Gustafsson L (2012) A major shift to the retention approach for forestry can help resolve some global forest sustainability issues. Conserv Lett 5:421–431
Lizarralde MS, Escobar J (2000) Mamíferos exóticos en la Tierra del Fuego. Ciencia Hoy 10:52–63
Manly B (1994) Multivariate statistical methods. A primer. Chapman and Hall, London
Martikainen P, Kaila L (2004) Sampling saproxylic beetles: lessons from a 10-year monitoring study. Biol Conserv 120:171–181
Martikainen P, Siitonen J, Punttila P, Kaila L, Rauh J (2000) Species richness of Coleoptera in mature managed and old-growth boreal forests in southern Finland. Biol Conserv 94:199–209
Martikainen P, Kouki J, Heikkala O (2006) The effects of green tree retention and subsequent prescribed burning on ground beetles (Coleoptera: Carabidae) in boreal pine-dominated forests. Ecography 29:659–670
Martínez Pastur G, Lencinas MV (2005) El manejo forestal en los bosques de Nothofagus pumilio en Tierra del Fuego. IDIA-XXI 5(8):107–110
Martínez Pastur G, Peri P, Vukasovic R, Vaccaro S, Piriz Carrillo V (1997) Site index equation for Nothofagus pumilio Patagonian forest. Phyton 6(1/2):55–60
Martínez Pastur G, Cellini JM, Peri P, Vukasovic R, Fernández C (2000) Timber production of Nothofagus pumilio forests by a shelterwood system in Tierra del Fuego (Argentina). For Ecol Manag 134:153–162
Martínez Pastur G, Peri P, Fernández C, Staffieri G, Lencinas MV (2002) Changes in understory species diversity during the Nothofagus pumilio forest management cycle. J For Res 7(3):165–174
Martínez Pastur G, Lencinas MV, Peri P, Moretto A, Cellini JM, Mormeneo I, Vukasovic R (2007) Harvesting adaptation to biodiversity conservation in sawmill industry: technology innovation and monitoring program. J Technol Manag Innov 2(3):58–70
Martínez Pastur G, Lencinas MV, Cellini JM, Peri PL, Soler R (2009) Timber management with variable retention in Nothofagus pumilio forests of Southern Patagonia. For Ecol Manag 258:436–443
Martínez Pastur G, Cellini JM, Lencinas MV, Barrera M, Peri PL (2010) Environmental variables influencing regeneration of Nothofagus pumilio in system with combined aggregated and dispersed retention. For Ecol Manag 261:178–186
Martínez Pastur G, Cellini JM, Lencinas MV, Barrera M, Peri PL (2011) Environmental variables influencing regeneration of Nothofagus pumilio in system with combined aggregated and dispersed retention. For Ecol Manag 261:178–186
Marvaldi AE, Lanteri AA (2005) Key to higher taxa of South American weevils based on adult characters (Coleoptera, Curculionoidea). Rev Chil Hist Nat 78:65–87
Massaccesi G, Roig FA, Martínez Pastur G, Barrera MD (2008) Growth patterns of Nothofagus pumilio trees along altitudinal gradients in Tierra del Fuego, Argentina. Trees 22:245–255
Matteri CM, Schiavone MM (2002) Catálogo de los musgos (Bryophyta) de la región Fueguina en Argentina y Chile. Rev Museo Arg Cs Nat 4:111–138
Matveinen-Huju K, Niemelä J, Rita H, O’Hara RB (2006) Retention-tree groups in clear-cuts: do they constitute ‘life-boats’ for spiders and carabids? For Ecol Manag 2006:119–135
McArdle B, Gaston K (1992) Comparing population variabilities. Oikos 64:610–612
McCune B, Mefford MJ (1999) Multivariate analysis of ecological data. Version 4.0. MjM software. Gleneden Beach, Oregon
McQuillan P (1993) Nothofagus (Fagaceae) and its invertebrate fauna—an overview and preliminary synthesis. Biol J Linn Soc 49:317–354
Michaels K, McQuillan PB (1995) Impact of commercial forest management on geophilous carabid beetles (Coleoptera: Carabidae) in tall, wet Eucalyptus obliqua forest in southern Tasmania. Aust J Ecol 20:316–323
Mitchell SJ, Beese WJ (2002) The retention system: reconciling variable retention with the principles of silvicultural systems. For Chron 78:397–403
Mittermeier RA, Mittermeier CG, Brooks TM, Pilgrim JD, Konstant WR, da Fonseca GAB, Kormos C (2003) Wilderness and biodiversity conservation. Proc Natl Acad Sci 100:10309–10313
Moore DM, Goodall RNP (1977) La flora adventicia de Tierra del Fuego. Ann Inst Patagon 8:263–274
Niemelä J (1990) Habitat distribution of carabid beetles in Tierra del Fuego, South America. Entom Fennica 29:3–16
Niemelä J (2001) Carabid beetles (Coleopterae: Carabidae) and habitat fragmentation: a review. Eur J Entomol 98:127–132
Niemelä J, Halme E, Lahti T, Pajunen T, Puntilla P (1988) The distribution of carabid beetles in fragments of old coniferous taiga and adjacent managed forest. Ann Zool Fennici 25:107–119
Niemelä J, Langor D, Spence J (1993) Effects of clear-cut harvesting on boreal ground-beetle assemblages (Coleoptera: Carabidae) in western Canada. Conserve Biol 7(3):551–561
Noreika N, Kotze DJ (2012) Forest edge contrasts have a predictable effect on the spatial distribution of carabid beetles in urban forests. J Ins Conserv 16:867–881
North M, Chen J, Smith G, Krakowiak L, Franklin J (1996) Initial response of understory plant diversity and overstory tree diameter growth to a green tree retention harvest. Northwest Sci 70:24–35
Novotný V, Basset Y (2000) Rare species in communities of tropical insect herbivores: pondering the mystery of singletons. Oikos 89:564–572
Oliver I, Beattie AJ (1993) A possible method for the rapid assessment of biodiversity. Conserv Biol 7(3):562–568
Ozanne CMP, Speight MR, Hambler C, Evans HF (2000) Isolated trees and forest patches: patterns in canopy arthropod abundance and diversity in Pinus sylvestris (Scots Pine). Forest Ecol Manag 137:53–63
Pérez V, Mutschke E, Vera M (1997) Hexápodos (Arthropoda: Parainsecta e Insecta) en territorios en proceso de desglaciación y revegetación en Fuego-Patagonia (Chile). An Inst Patagonia, Serie Cs Nat 25:57–76
Posadas P (2012) Species composition and geographic distribution of Fuegian Curculionidae (Coleoptera: Curculionoidea). Zootaxa 3303:1–36
Promis A, Caldentey J, Ibarra M (2010) Microclima en el interior de un bosque de Nothofagus pumilio y el efecto de una corta de regeneración. Bosque 31:129–139
Pulido F, Díaz B, Martínez Pastur G (2000) Incidencia del ramoneo del guanaco (Lama guanicoe) sobre la regeneración de lenga (Nothofagus pumilio) en bosques de Tierra del Fuego, Argentina. Inv Agr: Sist Rec For 9:381–394
Ramírez C, Correa M, Figueroa H, San Martín J (1985) Variación del hábito y hábitat de Nothofagus antarctica en el centro sur de Chile. Bosque 6:55–73
Richards OW, Davies RG (1984) Tratado de entomología Imms. Volumen 2: Clasificación y Biología. Omega, Barcelona
Roig-Juñent S (2000) The subtribes and genera of the Tribe Broscini (Coleoptera: Carabidae): cladistic analysis, taxonomic treatment, and biogeographical considerations. Bull Am Mus Nat Hist, Nueva York
Roig-Juñent S, Domínguez M (2001) Diversidad de la familia Carabidae (Coleopterae) en Chile. Rev Chil Hist Nat 74:549–571
Romoser WS, Stoffolano JG (1998) The science of entomology. WCB/McGraw-Hill, Boston
Rosenfeld JM, Navarro Cerrillo RM, Guzmán Álvarez JR (2006) Regeneration of Nothofagus pumilio (Poepp. et Endl.) Krasser forests after five years of seed tree cutting. J Environ Manag 78:44–51
Rosenvald R, Lõhmus A (2008) For what, when, and where is green-tree retention better than clear-cutting? A review of the biodiversity aspects. For Ecol Manag 255:1–15
Ross H (1973) Introducción a la entomología general y aplicada. Omega, Barcelona
Safranyik BL (1985) Infestation incidence and mortality in white spruce stands by Dendroctonus rufipennis Kirby (Coleoptera, Scolytidae) in central British Columbia. J Appl Entomol 99:86–93
Spagarino C, Martínez Pastur G, Peri P (2001) Changes in Nothofagus pumilio forest biodiversity during the forest management cycle: insects. Biodiv Conserv 10:2077–2092
Spence J, Volney WJA, Sidders D, Luchkow S, Vinge T, Oberle F, Gilmore D, Bielech JP, Wearmouth P, Edwards J, Bothwell P, Shorthouse D, Wilkinson D, Brais S (2002) The EMEND Experience. In: Veeman T (ed) Advances in forest management: from knowledge to practice, proceedings of SFMN conference, 13–15 Nov. SFMN Network, Edmonton, pp 40–44
Spitzer K, Lepš J (1988) Determinants of temporal variation in moth abundance. Oikos 53:31–36
Spitzer K, Regmánek M, Soldán T (1984) The fecundity and long-term variability in abundance of noctuid moths (Lepidoptera, Noctuidae). Oecologia 62:91–93
Stary P (1994) Aphid parasitoid fauna (Hymenoptera, Aphidiidae) of the southern beech (Nothofagus) forest. Stud Neotrop Fauna Environ 29:87–98
Thiele HU (1964) Carabid beetles in their environments. Springer, Berlin
Werner SM, Raffa KF (2000) Effects of forest management practices on the diversity of ground-occurring beetles in mixed northern hardwood forests of the Great Lakes Region. For Ecol Manag 139:135–155
Willott SJ (2001) Species accumulation curves and the measure of sampling effort. J Appl Ecol 38:484–486
Acknowledgments
The authors gratefully thank to the Centro Austral de Investigaciones Científicas, Servicios Forestales Consultancy, Los Castores sawmill, Lenga Patagonia S.A. and Dirección de Bosques—Secretaría de Ambiente y Desarrollo Sustentable of Argentina (PIARFON BIRF 4085-AR) for their support during the realization of this work. Also we especially thank to Dr. Sergio Roig-Juñent (Curculionida and Carabida), Dr. Gustavo Flores (Perymilopida), Dr. Andrew Schmidt (Scarabeida) for their collaboration in species identification, and to students who participate in the field work for their help, curiosity and willingness.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Lencinas, M.V., Martínez Pastur, G., Gallo, E. et al. Decreasing negative impacts of harvesting over insect communities using variable retention in southern Patagonian forests. J Insect Conserv 18, 479–495 (2014). https://doi.org/10.1007/s10841-014-9661-5
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10841-014-9661-5