Abstract
During the past 150 years forest management has dramatically altered in Central European woodlands, with severe consequences for biodiversity. Light forests that fulfilled variable human demands were replaced by dark high forests that function solely as wood plantations. In the Alps, by contrast, open woodlands are still present because the traditional land use as wood pasture has remained and physiographical conditions favour natural dynamics. The aim of our study was to investigate the effects of succession on the Orthoptera communities of alluvial pine woodlands in the northern Alps. Orthoptera showed a clear response to succession, with each successional stage harbouring a unique assemblage. The influence of succession on species richness and abundance were identical: The values were highest in the intermediate and lowest in the late seral stage. The diversity and abundance peak in the mid-successional stage probably reflects a trade-off between favourable ambient temperatures for optimal development and sufficient food, oviposition sites and shelter against predators. Food shortage and easy access for predators seemed to be limiting factors in the early successional stage. In contrast, in the late successional stage adverse microclimatic conditions probably limit Orthoptera occurrence. Although all three successional stages of the pine woodlands are relevant for conservation, the early and mid-successional stages are the most important ones. Conservation management for Orthoptera in this woodland type should aim at the reintroduction of cattle grazing and the restoration of the natural discharge and bedload-transport regimes of the alpine rivers.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
In the past, European woodlands were used intensively by humans (Bergmeier et al. 2010; Ellenberg and Leuschner 2010). They served as a source of litter, firewood and timber and were grazed by livestock. As a consequence they had a light and heterogeneous structure containing a high level of biodiversity (Peterken 1996; Vera 2000). During the past 150 years the total area of woodlands increased continuously in Central Europe; currently 30 % of the land surface is covered by woods (Steinecke and Venzke 2003). Nevertheless, species richness is declining (Benes et al. 2006; Vodka et al. 2009). This species loss is mainly driven by changes in forest management (Peterken 1996; Simberloff 1999). With the introduction of modern forestry, traditionally used light forests like coppiced woodlands or wood pastures were increasingly replaced by dark high forests, which solely function as plantations to provide wood (Benes et al. 2006).
In the European Alps, by contrast, the wood pasture tradition is still in place (Sachteleben 1995; Mayer and Huovinen 2007; Gimmi et al. 2008; Garbarino et al. 2011) and physiographical conditions favour natural dynamics (Hölzel 1996; Lederbogen et al. 2004). Landslides, avalanches and bedload transport in the floodplains slow succession down and, hence, enhance the continuity of light woodlands. At the northern edge of the Alps on dry, calcareous soils most of these open woodlands are pine forests (Calamagrostio-Pinetum) (Hölzel 1996). In the past, these pine woodlands benefited from grazing at the expense of mixed forests. Today, however, they are threatened by succession. Depending on the intensity of grazing and natural dynamics the pine forests usually form mosaics of different successional stages, from very light stands without a real tree layer up to woodlands with a relatively dense canopy.
The high value of the pine woodlands on dry, calcareous soils in the northern Alps for plant conservation is well-known and studied in detail (Hölzel 1996; Schmitt et al. 2010). In contrast, studies concerning the relevance of this woodland type for animal conservation are largely missing (Hölzel 1996). This also applies to Orthoptera, although Schlumprecht and Waeber (2003) point out the high relevance of these pine woodlands for this insect group.
Orthoptera are considered sensitive indicators of environmental changes (Bazelet and Samways 2011; Schirmel et al. 2011; Fartmann et al. 2012) since habitat selection depends on a complex combination of different and often interrelated environmental factors (see review in Ingrisch and Köhler 1998). The main drivers are vegetation structure (Gardiner et al. 2002; Poniatowski and Fartmann 2008) and microclimate (Willott and Hassall 1998; Gardiner and Dover 2008; Weiss et al. 2012). Predation and food supply are partly interrelated with the aforementioned environmental factors and may also be important, particularly in habitats with sparse vegetation (Belovsky and Slade 1993; Wünsch et al. 2012).
The aim of this study was to analyse the Orthoptera communities along a successional gradient in alluvial pine woodlands, which with respect to its area is the most important type of dry pine woodlands in the Bavarian Alps (Hölzel 1996). Fieldwork was done in the Upper Isar Valley (southern Bavaria, Germany), one of the last remnants of the old braided river systems of the Alps and the part of the river Isar that is most like a natural floodplain (Reich 1991; Hering et al. 2004). In particular we addressed the following research questions:
-
1.
Do Orthoptera species richness and abundance differ between successional stages?
-
2.
Do all and threatened species show different patterns?
-
3.
How should pine woodlands be managed to promote Orthoptera?
Materials and methods
Study area
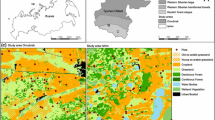
The study was conducted in the Upper Isar Valley in southern Bavaria (Germany) between “Wallgau” and the confluence of the “Rißbach” tributary at an altitude of 780–860 m a.s.l (47°31′N/11°18′E; area: 860 ha). The bottom of the valley consists of lime gravel (Karl et al. 1977). Due to its location at the northern edge of the Alps, the study area is characterized by a wet (annual precipitation 1,300–1,600 mm) and cool mountain climate (annual temperature 6.7 °C) (Krün/Vorderriß and Mittenwald meteorological stations, respectively; DWD 1952, 2013).
Although the Isar Valley is one of the last remaining near-natural floodplains in the Alps, it has been altered by the diversion of water for hydroelectricity (Homes et al. 1999). Since 1923 the complete discharge of the river Isar, except at peak flows, has been diverted to lake “Walchensee” for hydroelectric power generation (Hering et al. 2004). As a consequence the riverbed is dry most times of the year. However, the speed of succession was low due to the lack of water in the well-drained floodplain soils. Hence, areas with open gravel bars and open vegetation were still widespread. In 1990 a residual flow of the Isar water (3 m3/s in winter, 4.8 m3/s in summer) was established favouring late-successional plant communities due to better water and nutrient supplies (Schauer 1998). Today the floodplain is characterized by a mosaic of open gravel bars and gravel bars with pioneer vegetation dominated by Calamagrostis pseudophragmites (Calamagrostietum pseudophragmites) and willow shrubberies (Salicetum eleagno-purpureae) (Schauer 1998). Pine forests (Calamagrostio-Pinetum) dominate in those parts of the floodplain currently rarely flooded or no longer flooded (Hölzel 1996). Only the pine woodlands in the outermost southwest of the study area are currently grazed by cattle (Kraus pers. comm.)
The study area is part of a Natura 2000 site (“Karwendel mit Isar”) and one of the largest German nature reserves (“Karwendel und Karwendelvorgebirge”) (BfN 2013). Moreover, it belongs to one of the 30 national biodiversity hotspots (Ackermann et al. 2012).
Sampling design
The pine woodlands of the study area were divided according to Sänger (1977) into sections with homogenous vegetation structure. To avoid edge effects (Bieringer and Zulka 2003; Schirmel et al. 2010) only those sections with a size of at least 500 m2 were chosen as plots (Poniatowski and Fartmann 2008; Fartmann et al. 2012). This was the case for 50 sites that represented all successional stages of the pine woodlands across the study area.
Orthoptera sampling took place once per plot in August 2010. Densities were recorded with a box quadrat, which is among the best sampling methods to ascertain Orthoptera abundance (Gardiner and Hill 2006). The box quadrat had an area of 2 m2 (1.41 × 1.41 m), with gauze-covered sides 0.8 m in height. It was randomly dropped over the vegetation at 10 different points per plot; i.e., in total an area of 20 m2 was studied on each plot (Fartmann et al. 2008; Poniatowski and Fartmann 2010). Orthoptera species were identified in the field using Bellmann (2006) and Schulte (2003) and then released. Scientific nomenclature follows Coray and Lehmann (1998).
Measurement of environmental parameters took place after quantitative sampling of Orthoptera in a randomly selected undisturbed part (size: 3 × 3 m; for the tree layer 10 × 10 m) of the plot (Table 1). We recorded the following parameters of the horizontal structure (in 5 % steps): cover of total vegetation, tree layer, shrub layer, herb layer, herbs, grasses, mosses/lichens, litter, gravel/stones and bare soil. In cases where cover was above 95 % or below 5, 2.5 % steps were used. The average turf depth was measured to an accuracy of 2.5 cm. Vegetation density was estimated using a 50 cm wide and 30 cm deep wire-framed box, which was open on all sides except the back. Horizontal wires on the front side of the box divided it into six layers (0–5, 5–10, etc. up to 25–30 cm). The cover of each layer was viewed horizontally against the bright back of the box, using the same classes as for the horizontal structure (Poniatowski and Fartmann 2008; Fartmann et al. 2012). Soil depth was classified into four categories using a metal rod with spacer marks (Table 1): 1: 0–3 cm, 2: 4–10 cm, 3: 11–20 cm and 4: >20 cm.
Statistical analysis
Plots with similar vegetation structure were classified using Ward’s method of agglomerative clustering to successional stages (Poniatowski and Fartmann 2008; Fartmann et al. 2012). The variables cover of tree layer and stones/gravel were used for clustering. Values were z-transformed prior to analysis.
The threat status of each Orthoptera species in Bavaria was gathered from BayLfU (2003). To evaluate if metric environmental variables as well as the species number and abundance of Orthoptera (all and threatened species) differ between successional stages, a Kruskal–Wallis H test followed by a Dunn’s test was used (Table 1). If the data were normally distributed and variances were homogeneous (‘vegetation density’) an ANOVA with Tukey test as a post hoc test was applied. For categorical variables (‘soil depth’) Fisher’s exact test was used.
To assess which environmental factors explain species number and abundance of Orthoptera (all and threatened species) generalized linear models (GLMs) were conducted (Crawley 2007) (Table 1). Non-significant predictors were excluded by stepwise backward selection by AIC values from the final model (step function). To deal with multicollinearity, we summarized some variables prior to GLM analysis and ordination (see below) (Table 1, cf. Poniatowski and Fartmann 2011).
Due to a gradient length of <2, the influence of environmental parameters on Orthoptera species composition was analysed using principal component analysis (PCA) (Leps and Smilauer 2003). Data were square root transformed and only Orthoptera species that occurred in at least 3 plots (6 % of all plots) were included in the analysis.
The analyses were performed using the Canoco 4.5, R 2.13.0 (R Development Core Team 2013), SigmaPlot 11.0 and SPSS 19.0 statistical packages.
Results
Environmental conditions
Based on the results of the cluster analysis, the 50 plots were grouped into three successional stages following a gradient from early (EARLY) to late stages (LATE) (Table 2; Fig. 1). The intermediate seral stage (INTER) clearly dominated, with 31 plots in the study area (62 % of all plots); 11 plots (22 %) belonged to LATE and 8 plots (16 %) to EARLY. Except for the cover of the shrub layer, mosses/lichens and bare soil all the environmental parameters differed significantly among the successional stages (Tables 2, 3). Along the successional gradient from EARLY to LATE the cover of total vegetation, the tree and herb layer, grasses, herbs and litter as well as soil depth and vegetation density increased. In contrast, the cover of stones/gravel decreased. In most cases the differences were particularly pronounced between EARLY on the one hand and INTER/LATE on the other.
Orthoptera
In total, we recorded 17 Orthoptera species (2 Ensifera, 15 Caelifera) on the 50 plots (“Appendix”). Eight of these species are threatened in Bavaria. The most widespread and abundant species were Metrioptera brachyptera and Chorthippus biguttulus; they occurred on 62 % (90 caught individuals) and 58 % (96 individuals) of the plots, respectively. Chorthippus parallelus and Stenobothrus lineatus followed with an occurrence of 42 % (62 and 57 individuals, respectively).
Orthoptera and successional stages
Both Orthoptera species number and abundance differed significantly among the successional stages (Fig. 2). The observed patterns were identical for all and threatened species: species number and abundance peaked in INTER and differed significantly from LATE. The figures for EARLY were intermediate with respect to those of the other two seral stages.
Mean values (±SE) of species richness (a, c) and abundance (b, d) of all and threatened Orthoptera species, respectively, in the three successional stages of the pine woodlands. Differences between successional stages were tested using the Kruskal–Wallis H test (P < 0.05). Different letters indicate significant differences (Dunn’s test; P < 0.05)
Orthoptera and environmental conditions
Except for the number of threatened species, all other GLMs revealed a significant influence of environmental parameters on Orthoptera (number of all species, abundance of all and threatened species) (Table 4). The cover of the tree layer was the most important parameter. It had a negative impact on the total number of species as well as on the abundance of all and threatened species. Moreover, the number and abundance of all species was negatively correlated with soil depth and positively with horizontal vegetation. In addition, the abundance of threatened species decreased with vertical vegetation. The explanatory power of the models was generally high (Pseudo R2 [Nagelkerke]: 0.24–0.71).
The PCA confirmed the grouping of the plots into three successional stages (Fig. 3; Table 5). EARLY was characterized by a high cover of stones/gravel, mosses/lichens and bare soil. Typical species were Psophus stridulus and the two ground hoppers, Tetrix bipunctata and T. tenuicornis. Exactly opposed were the conditions in LATE: stones/gravel, mosses/lichens and bare soil hardly played a role. In contrast, soils were deeper, well-covered by a tall herb layer. Gomphocerippus rufus, Euthystira brachyptera and Metrioptera brachyptera were characteristic of this stage. INTER mediated between these two stages concerning the environmental conditions. Chorthippus biguttulus, C. dorsatus, C. parallelus and Stenobothrus lineatus preferred this stage.
PCA plot based on the densities of the most frequent Orthoptera species (constancy > 6 %) and sampled environmental parameters. For further explanations see section ‘sampling design’ and ‘statistical analysis’. Threat status (BayLfU 2003): filled circle = threatened species, open circle = secure species. For abbreviations of species see “Appendix”, for abbreviations of environmental parameters see Table 5
Discussion
Orthoptera of the alluvial pine woodlands in the northern Alps showed a clear response to succession, with each seral stage harbouring a unique assemblage. The influence of succession on Orthoptera species richness and abundance was identical: the values were highest in the intermediate and lowest in the late successional stage. The cover of the tree layer was the most important parameter explaining diversity and abundance of Orthoptera. Species richness (all species) and abundance (all and threatened species) were negatively correlated with tree cover. Moreover, species number and abundance of all species were negatively associated with soil depth but positively with horizontal vegetation. Abundance of threatened species decreased with vertical vegetation.
For open habitats like grasslands (Fartmann et al. 2012) and heathlands (Schirmel et al. 2011) some recent studies have addressed the influence of succession on Orthoptera. In contrast, comparable studies from woodland habitats have so far been lacking. Solely the great importance of clear-cuts in woodlands as Orthopteran habitats has already been shown (Sliacka et al. 2013a, b). In our study we found a negative impact of tree cover on Orthoptera. An increasing canopy closure results in more shade and, hence, lower maximum temperatures near the ground (Stoutjesdijk and Barkman 1992). Orthoptera are ectothermic organisms, whose egg and nymphal development, egg production and life span are decisively correlated with temperature (Chappell and Whitman 1990; Willott and Hassall 1998). Accordingly, we assume that the decline in Orthoptera species richness and abundance was caused by adverse microclimatic conditions due to shading. Comparable negative effects of shading on Orthoptera have been detected in dry grasslands (Bieringer and Zulka 2003) and woodland clearings (Theuerkauf and Rouys 2006) by adjacent woodland stands.
Soil depth was a further predictor in our study, showing a negative relationship with species richness and abundance of all Orthoptera species. Soil depth is directly correlated with water and nutrient availability for plants and, hence, promotes vegetation development in the alluvial pine woodlands (Hölzel 1996). As a result ground vegetation is denser and taller (cf. Fig. 3), leading to lower temperatures near the soil surface during the day compared with more open vegetation (Lemke et al. 2010) and most likely explaining the negative effect on Orthoptera. At first this seems to be a contradiction to the positive correlation between horizontal vegetation structure and the number and abundance of all Orthoptera species. However, we have to take into account that the early successional stage was characterized by a relatively low cover of herb layer. Here both shelter for predators and food supply were limited and probably explain the low species richness and abundance (Wünsch et al. 2012).
A certain cover by the herb layer is necessary for high Orthoptera species richness and abundance (see also Schirmel et al. 2011; Fartmann et al. 2012). However, too much dense vegetation has negative impacts on Orthoptera (see above, Fartmann and Mattes 1997). This is especially true for the threatened species found in this study. All eight species oviposit into the ground (Fartmann and Mattes 1997; Detzel 1998) and are more or less thermophilous (Detzel 1998; Schlumprecht and Waeber 2003). Accordingly, they are dependent on sufficient bare soil that is well-exposed to the sun. In line with this, their abundance decreased with an increase in the vertical vegetation shading the ground.
The intermediate successional stage, mostly belonging to the Calamagrostio-Pinetum thesietosum plant community (Hölzel 1996), had the highest Orthoptera species richness and abundance. The very light woodlands had a heterogenous and well-developed but thin herb layer best fulfilling the partially opposing requirements concerning microclimate, supply of oviposition sites, food availability and shelter from predators. The early successional stage, which largely relates to the Calamagrostio-Pinetum dryadetosum plant community (Hölzel 1996), is rich in bare soil and gravel/stones, having a dry and warm microclimate (Hölzel 1996; Lemke et al. 2010), conditions that favour Orthoptera in general (Gardiner and Dover 2008; Fartmann et al. 2012) and thermophilous species that oviposit in the ground in particular. However, both the food supply and shelter from predators were low. As a consequence, the richness and abundance of Orthoptera species are lower compared to the intermediate seral stage. In the late successional stage the environmental conditions for Orthoptera were worse. The tree layer covered about half the ground and the homogenous herb layer was dense and tall, leading to adverse microclimatic conditions for Orthoptera.
Implications for conservation
Our study clearly showed that alluvial pine forests are important habitats for Orthoptera in general and refuges for threatened species. Hence, as has already been shown for plants (Hölzel 1996), we can confirm the special relevance of this habitat type for biodiversity conservation. All three successional stages are relevant for the maintenance of Orthoptera diversity, as each stage had characteristic species. However, the intermediate and early seral stages are the most important. They exhibited the highest species richness and abundance of both all and threatened species.
Except for a few primary stands of Calamagrostio-Pinetum on very shallow south-facing slopes, most of the secondary stands in the northern Alps are currently threatened by succession (Hölzel 1996). In the long run, without management all secondary stands will be replaced by mixed forests (Hölzel 1996). The main reasons for this are the decline in forest grazing (Sachteleben 1995; Hölzel 1996; Lederbogen et al. 2004) and hydrological engineering measures in alpine rivers resulting in reduced dynamics (Kuhn 1993; Hölzel 1996; Schauer 1998). At present the vast majority of the pine forest stands in the study area belong to the intermediate successional stage, providing favourable conditions for Orthoptera. However, further succession would result in a high proportion of the late successional, which has a poor habitat quality.
One way to counteract succession and improve habitat quality for Orthoptera in the pine woodlands of the northern Alps is the reintroduction of grazing (Sachteleben 1995; Vera 2000). Forest grazing by cattle controls the growth of coarse grasses, like Brachypodium rupestre, Calamagrostis varia and Molinia arundinacea (Hölzel 1996), reduces litter accumulation and creates heterogeneous habitat structures with bare ground. Grazing is also known to be beneficial for threatened plant (Schmitt et al. 2010) and butterfly species (Streitberger et al. 2012) occurring in these pine woodlands.
At least as important would be the restoration of the natural discharge and bedload-transport regimes of the river Isar in particular and alpine rivers in general. Natural flooding dynamics would routinely create early successional stages in the floodplain. This would not only be beneficial for the establishment of early successional stages of the pine woodlands but also for the highly threatened communities of open gravel bars. For Chorthippus pullus and Bryodemella tuberculata, two of the rarest Orthoptera species in Central Europe (Maas et al. 2002) that occur in the early successional stage of pine woodlands, these dynamics are of vital importance. Both species depend on sparsely vegetated gravel bars (Reich 1991; Schlumprecht and Waeber 2003; Lemke et al. 2010).
References
Ackermann W, Balzer S, Ellwanger G, Gnittke I, Kruess A, May R, Riecken U, Sachteleben J, Schröder E (2012) Hot Spots der biologischen Vielfalt in Deutschland. Auswahl und Abgrenzung als Grundlage für das Bundesförderprogramm zur Umsetzung der Nationalen Strategie zur biologischen Vielfalt Nat Landsch 87:289–297
BayLfU (Bayrisches Landesamt für Umweltschutz) (2003) Rote Liste gefährdeter Tiere Bayerns. Schriftenr Bayer Landesamt Umweltschutz 166:68–72
Bazelet CS, Samways MJ (2011) Identifying grasshopper bioindicators for habitat quality assessment of ecological networks. Ecol Indic 11:1259–1269
Bellmann H (2006) Der Kosmos Heuschreckenführer: Die Arten Mitteleuropas sicher bestimmen. Kosmos, Stuttgart
Belovsky GE, Slade JB (1993) The role of vertebrate and invertebrate predators in a grasshopper community. Oikos 68:193–201
Benes J, Cizek O, Konvička M (2006) Intensive game keeping, coppicing and butterflies: the story of Milovicky Wood, Czech Republic. For Ecol Manage 237:353–365
Bergmeier E, Petermann J, Schröder E (2010) Geobotanical survey of wood-pasture habitats in Europe: diversity, threats and conservation. Biodivers Conserv 19:2995–3014
BfN (Bundesamt für Naturschutz) (2013) Naturschutzgebiete. http://www.bfn.de. Accessed 13 June 2013
Bieringer G, Zulka KP (2003) Shading out species richness: edge effect of a pine plantation on the Orthoptera (Tettigoniidae and Acrididae) assemblage of an adjacent dry grassland. Biodivers Conserv 12:1481–1495
Chappell MA, Whitman DW (1990) Grasshopper thermoregulation. In: Chapman RF, Joern A (eds) Biology of grasshoppers. Wiley, New York
Coray A, Lehmann AW (1998) Taxonomie der Heuschrecken Deutschlands (Orthoptera): Formale Aspekte der wissenschaftlichen Namen. Articulata Beih 7:63–152
Crawley MJ (2007) The R book. Wiley, Chichester
Detzel P (1998) Die Heuschrecken Baden-Württembergs. Ulmer, Stuttgart
DWD (Deutscher Wetterdienst), (1952) Klimaatlas von Bayern. DWD, Bad Kissingen
DWD (Deutscher Wetterdienst) (2013) Klimadaten ausgewählter deutscher Stationen. http://www.dwd.de. Accessed 13 June 2013
Ellenberg H, Leuschner C (2010) Vegetation Mitteleuropas mit den Alpen, 6th edn. Eugen Ulmer, Stuttgart
Fartmann T, Mattes H (1997) Heuschreckenfauna und Grünland. Bewirtschaftungsmaßnahmen und Biotopmanagement. In: Mattes H (ed) Ökologische Untersuchungen zur Heuschreckenfauna in Brandenburg und Westfalen. Arb Inst Landschaftsökol Münster 3, pp 179–188
Fartmann T, Behrens M, Loritz H (2008) Orthopteran communities in the conifer-broadleaved woodland zone of the Russian Far East. Eur J Entomol 105:673–680
Fartmann T, Krämer B, Stelzner F, Poniatowski D (2012) Orthoptera as ecological indicators for succession in steppe grassland. Ecol Indic 20:337–344
Garbarino M, Lingua E, Martinez Subirà M, Motta R (2011) The larch wood pasture: structure and dynamics of a cultural landscape. Eur J For Res 130:491–502
Gardiner T, Dover J (2008) Is microclimate important for Orthoptera in open landscapes?. J Insect Conserv 12:705–709
Gardiner T, Hill J (2006) A comparison of three sampling techniques used to estimate population density and assemblage diversity of Orthoptera. J Orthoptera Res 15:45–51
Gardiner T, Pye M, Field R, Hill J (2002) The influence of sward height and vegetation composition in determining the habitat preferences of three Chorthippus species (Orthoptera: Acrididae) in Chelmsford, Essex, UK. J Orthoptera Res 11:207–213
Gimmi U, Bürgi M, Stuber M (2008) Reconstructing anthropogenic disturbance regimes in forest ecosystems: a case study from the Swiss Rhone Valley. Ecosyst 11:113–124
Hering D, Gerhard M, Manderbach R, Reich M (2004) Impact of a 100-year flood on vegetation, benthic invertebrates, riparian fauna and large woody debris standing stock in an alpine floodplain. Regul Rivers Res Manage 20:445–457
Hölzel N (1996) Schneeheide-Kiefernwälder in den mittleren Nördlichen Kalkalpen. Laufener Forschungsber 3:1–192
Homes V, Hering D, Reich M (1999) The distribution and macrofauna of ponds in stretches of an alpine floodplain differently impacted by hydrological engineering. Regul Rivers Res Manage 15:405–417
Ingrisch S, Köhler G (1998) Die Heuschrecken Mitteleuropas. Westarp Wissenschaften, Magdeburg
Karl JM, Mangelsdorf J, Scheurmann K (1977) Die Isar, ein Gebirgsfluß im Spannungsfeld zwischen Natur und Zivilisation. Jahrbuch Ver Schutz Bergwelt 42:175–214
Kuhn J (1993) Naturschutzprobleme einer Wildflußlandschaft: Anmerkungen zur Teilrückleitung der oberen Isar (Oberbayern). Nat Landsch 68:449–454
Lederbogen D, Rosenthal G, Scholle D, Trautner J, Zimmermann B, Kaule G (2004) Allmendweiden in Südbayern: Naturschutz durch landwirtschaftliche Nutzung. Schriftenr Angewandte Landschaftsökol 62:1–469
Lemke H, Löffler F, Fartmann T (2010) Habitat- und Nahrungspräferenzen des Kiesbank-Grashüpfers (Chorthippus pullus) in Südbayern. Articulata 25:133–149
Leps J, Smilauer P (2003) Multivariate analysis of ecological data using CANOCO. Cambridge University Press, Cambridge
Maas S, Detzel P, Staudt A (2002) Gefährdungsanalyse der Heuschrecken Deutschlands. Verbreitungsatlas, Gefährdungseinstufung und Schutzkonzepte. Bundesamt für Naturschutz, Bonn-Bad Godesberg
Mayer AC, Huovinen C (2007) Silvopastoralism in the Alps: native plant species selection under different grazing pressure. Ecol Eng 29:372–381
Peterken GF (1996) Natural woodland: ecology and conservation in northern temperate regions. Cambridge University Press, Cambridge
Poniatowski D, Fartmann T (2008) The classification of insect communities: lessons from Orthoptera assemblages of semi-dry grassland complexes in central Germany. Eur J Entomol 105:659–671
Poniatowski D, Fartmann T (2010) What determines the distribution of a flightless bush-cricket (Metrioptera brachyptera) in a fragmented landscape? J Insect Conserv 14:637–645
Poniatowski D, Fartmann T (2011) Weather-driven changes in population density determine wing dimorphism in a bush-cricket species. Agric Ecosyst Environ 145:5–9
R Development Core Team (2013) R: a language and environment for statistical computing. http://www.r-project.org. Accessed 13 June 2013
Reich M (1991) Grasshoppers (Orthoptera, Saltatoria) on alpine and dealpine riverbanks and their use as indicators for natural floodplain dynamics. Regul Rivers Res Manage 6:333–339
Sachteleben J (1995) Waldweide und Naturschutz—Vorschläge für die naturschutzfachliche Beurteilung der Trennung von Wald und Weide im bayerischen Alpenraum. Forstwiss Cbl 114:375–387
Sänger K (1977) Über die Beziehungen zwischen Heuschrecken (Orthoptera: Saltatoria) und der Raumstruktur ihrer Habitate. Zool Jb Syst 104:433–488
Schauer T (1998) Die Vegetationsverhältnisse an der Oberen Isar vor und nach der Teilrückleitung. Jahrbuch Ver Schutz Bergwelt 63:131–141
Schirmel J, Blindow I, Fartmann T (2010) The importance of habitat mosaics for Orthoptera (Caelifera and Ensifera) in dry heathlands. Eur J Entomol 107:129–132
Schirmel J, Mantilla-Contreras J, Blindow I, Fartmann T (2011) Impacts of succession and grass encroachment on Orthoptera in heathlands. J Insect Conserv 15:633–642
Schlumprecht H, Waeber G (2003) Heuschrecken in Bayern. Ulmer, Stuttgart
Schmitt B, Fartmann T, Hölzel N (2010) Vergesellschaftung und Ökologie der Sumpf-Siegwurz (Gladiolus palustris) in Südbayern. Tuexenia 30:105–127
Schulte AM (2003) Taxonomie, Verbreitung und Ökologie von Tetrix bipunctata (Linnaeus 1758) und Tetrix tenuicornis (Sahlberg 1893) (Saltatoria: Tetrigidae). Articulata Beih 10:1–226
Simberloff D (1999) The role of science in the preservation of forest biodiversity. For Ecol Manage 115:101–111
Sliacka A, Krištín A, Naďo L (2013a) Response of Orthoptera to clear-cuts in beech forests. Eur J Entomol 110:319–326
Sliacka A, Krištín A, Naďo L (2013b) Orthoptera assemblages of beech stand plots during early succession stages after clearcutting. J For Sci 59:93–100
Steinecke K, Venzke JF (2003) Wald und Forst heute. In: Leibniz-Institut für Länderkunde (eds) Nationalatlas Bundesrepublik Deutschland. Klima, Pflanzen und Tierwelt. Spektrum Akademischer Verlag, Heidelberg
Stoutjesdijk P, Barkman JJ (1992) Microclimate, vegetation and fauna. Opulus Press, Uppsala
Streitberger M, Hermann G, Kraus W, Fartmann T (2012) Modern forest management and the decline of the Woodland Brown (Lopinga achine) in Central Europe. For Ecol Manage 269:239–248
Theuerkauf J, Rouys S (2006) Do Orthoptera need human land use in central Europe? The role of habitat patch size and linear corridors in the Bialowieza Forest, Poland. Biodivers Conserv 15:1497–1508
Vera FWM (2000) Grazing ecology and forest history. CABI Publishing, Wallingford
Vodka S, Konvička M, Cizek L (2009) Habitat preferences of oak-feeding xylophagous beetles in a temperate woodland: implications for forest history and management. J Insect Conserv 13:553–562
Weiss N, Zucci H, Hochkirch A (2012) The effects of grassland management and aspect on Orthoptera diversity and abundance: site conditions are as important as management. Biodivers Conserv. doi:10.1007/s10531-012-0398-8
Willott SJ, Hassall M (1998) Life-history responses of British grasshoppers (Orthoptera: Acrididae) to temperature change. Funct Ecol 12:232–241
Wünsch Y, Schirmel J, Fartmann T (2012) Conservation management of coastal dunes for Orthoptera has to consider oviposition and nymphal preferences. J Insect Conserv 16:501–510
Acknowledgments
We are grateful to Wolfgang Kraus (Landratsamt Garmisch-Partenkirchen) for helpful information concerning the study area. Jan Beck and two anonymous reviewers made valuable comments on an earlier version of the manuscript. The Government of Upper Bavaria gave permission to conduct the study in the nature reserve “Karwendel und Karwendelvorgebirge”.
Author information
Authors and Affiliations
Corresponding author
Appendix
Appendix
See Table 6.
Rights and permissions
About this article
Cite this article
Helbing, F., Blaeser, T.P., Löffler, F. et al. Response of Orthoptera communities to succession in alluvial pine woodlands. J Insect Conserv 18, 215–224 (2014). https://doi.org/10.1007/s10841-014-9632-x
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10841-014-9632-x