Abstract
Phengaris (=Maculinea) arion is an endangered social parasite of Myrmica ants, and for a very long time was considered as specific to Myrmica sabuleti. Previous studies carried out in Poland suggested some discrepancies within this assumption, and therefore a much more intensive survey was undertaken. The host ant use of P. arion was studied at five sites in different types of biotopes in Poland, i.e. xerothermal grasslands where Thymus pulegioides was used as a larval food plant by the butterfly, and more or less sandy biotopes with Thymus serpyllum. Altogether nine Myrmica species were recorded, and considerable variation in species composition and density of nests was recorded. At four localities M. sabuleti proved to be the most common ant. A total of 529 Myrmica nests were examined, and only 20 of them contained larvae and pupae of P. arion. Host ants belonged to five different species, i.e. M. sabuleti, Myrmica scabrinodis, Myrmica schencki, Myrmica lobicornis and Myrmica hellenica. Only at one site (NE Poland) was a significant heterogeneity in parasitation rates among Myrmica species detected. M. lobicornis was the most often infested ant there, which may suggest local specialisation of the butterfly. Overall low parasitism rates may explain the vulnerability of P. arion in Central Europe but further studies are also necessary.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Large Blue Phengaris arion (Linnaeus, 1758) is an endangered habitat specialist, which in its larval stage depends on two different types of resources, both of which are essential to its successful development (Thomas et al. 2009). Caterpillars leave their food plant (Thymus Linnaeus, 1753 or Origanum vulgare Linnaeus, 1753, depending on locality), after their third (and final) moult, to be transported by Myrmica workers to their nests where they prey upon the host brood. These socially parasitic relationships are considered to be not only obligatory for the butterfly but also specific i.e. one single species of Myrmica is much more tolerant towards caterpillars than other species (Thomas 1995). Studies concerning another species, Phengaris Doherty, 1891, show that the caterpillar is taken equally willingly by any foraging Myrmica worker, and specificity occurs only at the post-adoption stage in an ant colony (Elmes et al. 2004; Schönrogge et al. 2004).
Earlier detailed studies on hosts of P. arion have in fact been carried out at four sites in Britain, France and Sweden (Thomas et al. 1989; Elmes et al. 1998). The studies showed that over ninety percent of butterflies emerged from the nests of Myrmica sabuleti Meinert, 1861, and of the other Myrmica species presented, only Myrmica scabrinodis Nylander, 1846 was successfully exploited as a minor host. A significant difference in the survival rate of P. arion larvae among ant species was the most important biological factor in the butterfly’s decline in Britain. The more thermophilous M. sabuleti was replaced by the less demanding M. scabrinodis when vegetation became higher at abandoned sites (Thomas et al. 2009). However, it is also hypothesised that nests of secondary hosts may play an important role in population persistence in seasons with extreme events, e.g. droughts (Thomas et al. 2005). Finally the colony structure is also a potential factor affecting survival, i.e. mortality of P. arion caterpillars in M. sabuleti nests is higher in queenright nests (Thomas and Wardlaw 1990).
The complex life history of the Large Blue has triggered enormous scientific and conservational interest in the whole genus Phengaris (=Maculinea Van Eecke, 1915) (Als et al. 2004; Thomas and Settele 2004; Settele et al. 2005; Pecsenye et al. 2007; Fric et al. 2007; Nash et al. 2008; Barbero et al. 2009). Several recent papers explore the host ant specificity of European relatives of P. arion (Pech et al. 2007 and references therein; Tartally and Varga 2008; Tartally et al. 2008; Witek et al. 2008). Surprisingly, such detailed knowledge on the ecology of P. arion on a larger geographical scale is lacking. There is an anecdotal record of a pupa of P. arion found in a Myrmica lobicornis Nylander, 1846 nest in NE Poland (Sielezniew et al. 2003). However, a survey performed on a different site in the same region proves that,at least locally, the butterfly does not depend on its ‘classic’ host M. sabuleti. P. arion was found in nests of three different Myrmica species i.e. Myrmica rugulosa Nylander, 1849, Myrmica hellenica Finzi, 1926 and Myrmica schencki Emery, 1895, whereas M. sabuleti was very rare and not infested (Sielezniew and Stankiewicz 2008). These data indicate that extensive studies are still necessary for a better understanding of the ecological complexity of the butterfly, which is endangered in many European countries (van Swaay and Warren 1999). The present paper explores the relationships of P. arion with ants at five Polish sites representing different regions, which are also differentiated in terms of biotope and management.
Materials and methods
Phengaris arion has disappeared from western parts of Poland within the last few decades but is a widely distributed species in the south and east of the country (Sielezniew et al. 2005). However, it is not really common anywhere, especially in comparison with other Phengaris butterflies, in spite of the abundance of its larval food plants. Studies were performed at five sites in five different regions of distribution (Fig. 1). The main reasons for this selection were the relative abundance of the butterfly there, as reported by local observers, as well as the variation of biotopes and larval food plants (Table 1).
Localisation of study sites in Poland. The broken line shows the border of the present range of distribution of P. arion in the country according to Sielezniew et al. (2005)
Thymus serpyllum Linnaeus, 1753 was used as a larval food plant at three more or less sandy sites. The study at Gugny site in Biebrza (NE Poland) was a continuation of observations whose first anecdotal results were published by Sielezniew et al. (2003). The biotope of P. arion encompassed raised sandy land surrounded by fens (Fig. 2a, b). Small, dry hills were sparsely covered by bushes and trees, mainly oaks, and were lightly grazed by cattle and wild game. At Wola Uhruska (E Poland), P. arion inhabited a rather plain and mostly sheltered area neighbouring a disused railway line (Fig. 2c). The only form of management was the occasional ploughing of lines along the edge of the forest against fire. At Hutki-Kanki (Kraków-Częstochowa Upland), P. arion was encountered in a clearing under an electricity line (Fig. 2d), which was flat or of slight SW inclination. The biotope was created in the 1970 s and managed by occasional and rotational removal of regrowing trees and bushes.
Study sites of P. arion in Poland: a, b Gugny, c Wola Uhruska, d Hutki-Kanki, e Babice, f Kluszkowce (see also Table 1 and text for details)
Thymus pulegioides Linnaeus, 1753 was used as a larval food plant at two sites which were both on S exposed slopes. At Babice, P. arion inhabited an abandoned old field where regeneration of xerothermal grassland was observed (Fig. 2e). However, it was also becoming overgrown by tall grasses and Prunus spinosa bushes. At Kluszkowce, P. arion populated the S, SW and SE slopes of Mt. Wdżar (Fig. 2f), which at the moment are extensively grazed by sheep and trampled only by tourists. A very characteristic feature of the biotope was the Juniperus bush. Some parts were overgrown with ferns.
Studies were performed in 2002–2008, and Myrmica nests were examined in the period between mid May and mid June, i.e. when full-grown larvae or pupae were expected to be found. It is known that surveys made in autumn or early spring may result in erroneous determination of the host ant specificity of Phengaris butterflies, because it is uncertain whether they would be able to complete their development (Thomas et al. 1989). Areas of turf within 2 m of Thymus plants, the distance which is considered as the radius of the foraging zone of Myrmica plants (Elmes et al. 1998), were carefully inspected. A survey was made in patches where adult butterflies were observed.
Biotopes of P. arion at Gugny (1.3 ha) and Kluszkowce (1.5 ha) were isolated, i.e. surrounded by unfavourable types of vegetation where the butterfly was not observed. As thyme is the main nectar source and the exclusive larval-food plant of P. arion there, areas overgrown by thyme can be regarded, according to a resource-based approach (Dennis et al. 2006) as the habitat of the butterfly at those two sites. At the three remaining sites, the boundaries of areas occupied by populations were not clear, and we studied only the selected patches of biotopes (about 1 ha at Babice, 2.5 ha at Hutki-Kanki and 0.3 ha at Wola Uhruska) which had the highest density of adults, as recorded by local entomologists. The Hutki-Kanki and Wola Uhruska sites were located in areas where imagoes were observed within the space of many kilometres.
Unfortunately it is virtually impossible to determine exact oviposition places, as eggshells or caterpillars are relatively difficult to spot compared to other Phengaris butterflies. Special female preferences were not observed, but we are aware that they are guided by the phenological state of the larval food-plant and therefore possibly affected by some local microclimatic conditions, However, taking into consideration the extended flight period of the butterfly at Polish sites (about 4–6 weeks from mid June to early August depending on region and season) an assumption was made that eggs were distributed all over the examined patches.
Myrmica nests were localised by searching the turf. At the two sites with the lowest density of nests (see Table 2), sugar cubes were additionally placed down in the late afternoon i.e. the period of highest activity of Myrmica ants in P. arion habitats (Thomas 2002), to bait workers and therefore to facilitate localisation of their colonies. The encountered nests were carefully opened with a knife and examined for the presence/absence of P. arion individuals. Nests were inspected, progressing from the uppermost to the deepest chambers. After examination the ground and vegetation were restored to a state as close to the original conditions as possible in order to facilitate the colonies’ survival.
Physical examination of nests was the only possible method of determining host-ant relationships as far as Polish sites are concerned. Unfortunately, the method of nest netting to spot newly emerged adults can not be applied due to the danger of vandalism and/or disturbance by freely grazing animals, including livestock. Limited access to the sites and geographical distances (see the map) as well as the long flight period of P. arion, i.e. extended emergence from pupae, also rendered such a method impractical.
We believe that the possible negative effect of the applied methodology is insignificant. Myrmica ants are known for their habit of changing nesting places, even without disturbance (Elmes et al. 1998). They are also adapted to disturbances made by animals, especially wild boar whose traces are often observed at Phengaris sites in Poland (e.g. Babice and Gugny). Small scale disturbances are important for the thriving of early successional habitats, which is also vital for xerothermophilous Myrmica species. Biotopes of P. arion situated under electricity lines can be seriously altered during periodic clearing of trees and shrubs, which is usually followed by ploughing (e.g. Hutki-Kanki). Finally our experience from earlier studies (Sielezniew and Stankiewicz 2008) indicates that after examination Myrmica colonies usually form new nests in close proximity to, or even in exactly the same places (M. Sielezniew, unpublished).
Phengaris arion individuals, after examination, were usually left with the ants. Pupae and full grown larvae are able to complete their development without an ant host. Some caterpillars were taken to the lab and kept until completion of their metamorphosis, in artificial colonies as described in Sielezniew and Stankiewicz (2007). The emerged adults were released at their sites of origin or, on rare occasions, when this was impossible for logistic reasons, used for genetic studies.
Ants were preliminarily identified in the field with hand lenses, but voucher samples of 5–10 workers were collected to confirm this determination, according to Czechowski et al. (2002), in the laboratory. Additionally, at every site randomly chosen squares (1 m2 each) were surveyed (90 squares at Gugny, 80 at Wola Uhruska, 335 at Hutki-Kanki, 73 at Babice and 131 at Kluszkowce), to estimate the average density of Myrmica colonies.
The similarity of Myrmica assemblages at different study sites was calculated with the Renkonen index. The equation is: Re = ∑ min(p i; q i) where p i and q i mean the relative frequency of species number on sites p and q. Since they operate with relative frequencies, the captures are rescaled between 0 and 1. We also compared proportions of infested and uninfested Myrmica colonies on each site by using an extended version of the Fisher exact test, generalised to more than two compared samples. This heterogeneity is one of the factors which is helpful in the quantification of the specificity of Phengaris butterflies (Tartally et al. 2008).
Results
During our studies a total number of 529 colonies of Myrmica ants was examined. The most intensive research was performed at Gugny, where 224 nests were opened, while only 30 nests were excavated at Wola Uhruska (Table 2). They belonged to nine species i.e. M. sabuleti (251 nests, 5 sites), M. schencki (123, 5), M. scabrinodis (80, 3), M. lobicornis (37, 3), Myrmica rubra (Linnaeus, 1758) (10, 4), Myrmica ruginodis Nylander, 1846 (5, 2), Myrmica lonae Finzi, 1926 (10, 2), Myrmica rugulosa (8, 2), M. hellenica (4, 1).
Myrmica ant communities showed considerable variation. At every site we recorded 4–7 species. The sites which were most similar to each other were Kluszkowce and Babice (Re = 0.75), both were dominated by M. sabuleti. On the other hand the lowest value of similarity index was found for Babice and Wola Uhruska (0.16; Table 3). The average density of Myrmica nests also showed considerable variation from 0.03/m2 at Wola Uhruska to 0.58/m2 at Gugny (Table 2).
A total of 23 larvae and pupae of P. arion in 20 nests of five Myrmica species was found (Table 2). The overall percentage of infested nests was 3.8%. If particular sites are considered, proportions ranged from 2.0% at Babice to 6.7% at Wola Uhruska.
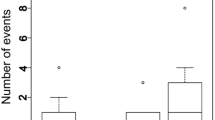
Myrmica sabuleti was observed as a host at Hutki-Kanki (2 of 22 nests were infested) and at Kluszkowce (4 of 120), M. scabrinodis at Gugny (2 of 66) and at Babice (1 of 10), M. schencki at Gugny (3 of 52; Fig. 3a) and at Wola Uhruska (1 of 16), M. lobicornis at Gugny (6 of 29; Fig. 3b) and M. hellenica at Wola Uhruska (1 of 4). Only at one site was a significant heterogeneity in parasitation rates among Myrmica species detected, i.e. at Gugny M. lobicornis proved to be a primary host (P = 0.007; Table 2).
The mean number of P. arion larvae and pupae per colony was 1.15. Only in two cases were more than one specimen recorded in a single colony i.e. two full-grown larvae at Gugny (M. lobicornis) and one pupa and two full-grown larvae at Hutki-Kanki (M. sabuleti).
Discussion
During the present studies P. arion was found in the nests of five different Myrmica species. Taking into consideration M. rugulosa, in whose colonies the butterfly has also been observed in Poland (Sielezniew and Stankiewicz 2008), the number of ants ever recorded as hosts rises to six (Table 4). Outside Poland, P. arion has been observed exclusively in colonies of M. sabuleti and M. scabrinodis. However, it is worth mentioning that only five Myrmica species have been recorded at sites in the United Kingdom, France and Sweden and it is also unclear what the proportions of infested and uninfested nests were (Thomas et al. 1989; Elmes et al. 1998).
Our results do not enable us to point to the best host ant of P. arion on a national scale. However, it is hypothesised that the ‘classic’ host M. sabuleti might be the most commonly exploited ant in the south of the country, where it was found to be by far the most common Myrmica species in turf. Differences in Myrmica ant communities reflect biotope variation. Two of the sites which were the most similar to each other in this respect i.e. Kluszkowce and Babice, were xerothermal grasslands on slopes with south-facing aspects. The observed preferences are also consistent with analyses made in Southwest Germany (Pauler-Fürste et al. 1996). The model of survival of a P. arion population developed by Thomas (1995) may probably be applied to all similar Central European sites. It states that a 51% co-occurrence of M. sabuleti and the host plant is necessary for intrinsic growth rate = 1. Therefore, while at a less intensively studied Polish site, Babice, a P. arion larva was found with M. scabrinodis, it is still possible that M. sabuleti is the primary host.
On the third site in Southern Poland (Hutki-Kanki), colonies of M. sabuleti constituted nearly half of all nests found and the butterfly was recorded in two of their colonies. However, the site differed from Kluszkowce and Babice in terms of biotope, larval food plant and its much lower density of Myrmica nests in the vicinity of the larval food plant. The last feature is especially important, because according to the model presented by Pauler-Fürste et al. (1996) the extinction probability of that population is 1 if we assume that M. sabuleti is the exclusive host.
A very low density of Myrmica nests was also recorded at Wola Uhruska in E Poland, but the site was quite distinct as far as Myrmica species composition is concerned. It was dominated by M. schencki and was also the only site of the occurrence of M. hellenica. It was found along a forest edge at a ploughed fire line in sandy soil, which may explain its supposed pioneer character (Czechowski et al. 2002). However, besides these, two xerothermophilous species, M. rubra and M. ruginodis, which are related to taller vegetation (Elmes et al. 1998), constituted 20% of observed Myrmica ants. This reflects unfavourable succession changes caused by the cessation of railway use. The anecdotal data on host ant use as well as observed deterioration of the habitat make any conclusions rather speculative. Nevertheless, it is not out of the question that M. schencki and M. hellenica were primary hosts at the site rather than M. sabuleti, and in this respect the population was similar to another one previously studied in NE Poland (Sielezniew and Stankiewicz 2008).
At the most intensively studied site, i.e. at Gugny, M. sabuleti was the commonest Myrmica species, with a relative proportion of nearly one-third. However, P. arion juveniles were observed in the nests of three other species. This was the only site in which it was possible to point to the primary host, as the parasitation rate of M. lobicornis was significantly higher than that of other Myrmica species. Therefore, it was confirmed that the first record of this host on the site (Sielezniew et al. 2003) was not accidental, as is sometimes observed with Phengaris butterflies (Thomas et al. 2005).
Our data suggests a local specialisation in the parasitism of this ant. The Gugny site is quite unusual because it is surrounded by fen communities, and therefore it has possibly undergone long term isolation from other populations. M. lobicornis is not a typical ant of xerothermal grasslands, and in Poland it is most often encountered in coniferous forests (Czechowski et al. 2002). According to Elmes et al. (1998), M. lobicornis prefers cooler habitats to M. sabuleti and also to M. schencki and M. scabrinodis, which were secondarily used hosts at the site. At Gugny, M. lobicornis was observed in lower and slightly shady parts of overgrown hills with eastern exposure. It may be the case that this population of P. arion is less thermophilous than expected, taking into consideration the generally assessed habitat fidelity of the butterfly (Thomas 1995). Detailed studies on female oviposition preferences are necessary to check whether host ant use is not related to some features of adult behaviour.
It would be also interesting to compare periodic variation in parasitisation of different ant species between seasons. However, because of the difficulties mentioned above, limitations related to the status of the butterfly and low infestations of colonies, such data are not easy to obtain. Alternatively, laboratory experiments with artificial nests may be considered in future research.
Present studies, like most of the research on Phengaris butterflies, do not allow us to draw ultimate conclusions about the survival of caterpillars in nests of different Myrmica, because the initial distribution of larvae in ant colonies remains unknown. This pattern can be affected by the foraging behaviour of workers. It is usually assumed, after Elmes et al. (1998), that the foraging zone of potential hosts of P. arion is about 2 m. However, it has been observed elsewhere that M. lobicornis workers came numerously to a sugar bait put more than 3 m away from their colony (Sielezniew unpublished), so detailed studies on this phenomena, as well as on coincidence in host plants and ant nests (some ant resources are possibly not studied when ‘standard’ foraging zone is assumed), are vital. Moreover, individual Myrmica species apply different foraging strategies (Putyatina 2007), which may also affect the initial distribution of P. arion caterpillars in ant nests.
In conclusion, although P. arion was observed in Poland with five different Myrmica species altogether, this hardly proves that it is a generalist species even on the scale of the country. Data from the Gugny site even indicates the existence of local specialisations, and therefore a geographical variation in host ant specificity. Taking into consideration our previous data (Sielezniew and Stankiewicz 2008) it is also plausible that some populations are more specialised than others. This was found to be the case for Phengaris ‘rebeli’ (xerothermophilous ecotype of P. alcon Denis & Shiffermüller, 1775), which forms clear-cut host races in some parts of the distribution range even though in others it seems to be less specific (Elmes et al. 1998; Stankiewicz et al. 2005; Sielezniew and Stankiewicz 2007; Tartally et al. 2008; Sielezniew and Dziekańska 2009). In contrast, populations of P. alcon which inhabit wet meadows show well-defined specialisations everywhere (Elmes et al. 1998; Sielezniew and Stankiewicz 2007; Nash et al. 2008; Tartally et al. 2008; Witek et al. 2008).
However, caterpillars of P. alcon and P. ‘rebeli’, which are mainly fed directly by workers are considered as more advanced social parasites, which require better chemical mimicry and therefore higher specificity. The low effectiveness of P. arion parasitism is reflected not only in low incidence rates in Myrmica colonies but also in a mean number of specimens developing in individual nests which is a few times lower than P. ‘rebeli’ (Thomas and Elmes 1998).
We also noticed that mean numbers of P. arion individuals per infested colony recorded in Poland were very similar to data collected in the UK, France and Sweden (1.3 and 1.2, respectively; see Table 4), but lower as far as other predatory hygrophilous Phengaris species are concerned. Both Phengaris teleius (Bergsträsser, 1779), which does not show specificity either on a local or regional scale across Central Europe, and Phengaris nausithous (Bergsträsser, 1779), which is specific to M. rubra almost everywhere, are usually found in higher numbers and also more frequently in examined colonies (compare Stankiewicz and Sielezniew 2002; Witek et al. 2008; Tartally and Varga 2008). Results of the present studies may therefore contribute to an explanation of the decline of P. arion, and its relative scarcity, even in suitable looking biotopes across Europe.
The low quality of host-ant resources recorded at two sites, i.e. low density of Myrmica nests, indicates that the butterfly requires relatively broad areas to survive. It also suggests that e.g. a small area studied at Wola Uhruska is probably part of network of a wider system of biotopes, which supports a population or a metapopulation of P. arion. Therefore, further research should also undoubtedly include studies of adult mobility, which are essential for gaining an understanding of the butterfly ecology especially in woodland areas where P. arion occupies ‘corridor’ types of biotopes as roadsides, railways or clearings under electricity lines. This knowledge is also important for successful conservation of the butterfly in Poland and Europe.
References
Als TD, Vila R, Kandul NP, Nash DR, Yen SH, Hsu YF, Mignault AA, Boomsma JJ, Pierce NE (2004) The evolution of alternative parasitic life histories in large blue butterflies. Nature 432:386–390
Barbero F, Thomas JA, Bonelli S, Balletto E, Schönrogge K (2009) Queen ants make distinctive sounds that are mimicked by a butterfly social parasite. Science 323:782–785
Czechowski W, Radchenko A, Czechowska W (2002) The ants (Hymenoptera, Formicidae) of Poland. Museum and Institute of Zoology PAS, Warsaw
Dennis RLH, Shreeve TG, Van Dyck H (2006) Habitats and resources: the need for a resource-based definition to conserve butterflies. Biodiv Conserv 15:1943–1966
Elmes GW, Thomas JA, Wardlaw JC, Hochberg ME, Clarke RT, Simcox DJ (1998) The ecology of Myrmica ants in relation to the conservation of Maculinea butterflies. J Insect Conserv 2:67–78
Elmes GW, Wardlaw JC, Schönrogge K, Thomas JA, Clarke RT (2004) Food stress causes differential survival of socially parasitic caterpillars of Maculinea rebeli integrated in colonies of host and non-host Myrmica ant species. Entomol Exp Appl 110:53–63
Fric Z, Wahlberg N, Pech P, Zrzavý J (2007) Phylogeny and classification of the Phengaris-Maculinea clade (Lepidoptera: Lycaenidae): total evidence and phylogenetic species concepts. System Entomol 32:558–567
Nash DR, Als TD, Maile R, Jones GR, Boomsma JJ (2008) A mosaic of chemical coevolution in a large blue butterfly. Science 319:88–90
Pauler-Fürste R, Kaule G, Settele J (1996) Aspects of the population vulnerability of the large blue butterfly, Glaucopsyche (Maculinea) arion, in south-west Germany. In: Settele J, Margules C, Poschlod P, Henle K (eds) Species survival in fragmented landscapes. Kluwer, Dordrecht, pp 275–281
Pech P, Fric Z, Konvicka M (2007) Species-specificity of the Phengaris (Maculinea)–Myrmica host system: Fact or myth? (Lepidoptera: Lycaenidae; Hymenoptera: Formicidae). Sociobiology 50:983–1004
Pecsenye K, Bereczki J, Tihanyi B, Toth A, Peregovits L, Varga Z (2007) Genetic differentiation among the Maculinea species (Lepidoptera: Lycaenidae) in eastern Central Europe. Biol J Linn Soc 91:11–21
Putyatina TS (2007) The choice of foraging strategy as a mechanism for the coexistence of Myrmica species (Hymenoptera, Formicidae) in a multispecific ant association. Entomol Rev 87:650–657
Schönrogge K, Wardlaw JC, Peters AJ, Everett S, Thomas JA, Elmes GW (2004) Changes in chemical signature and host specificity from larval retrieval to full social integration in the myrmecophilous butterfly. J Chem Ecol 30:91–107
Settele J, Kühn E, Thomas JA (2005) Studies on the ecology and conservation of butterflies in Europe: species ecology along a European gradient: Maculinea butterflies as a model, vol 2. Pensoft, Sofia, Moscow
Sielezniew M, Dziekańska I (2009) Butterfly-ant relationships: host ant specificity of Phengaris ‘rebeli’ Hirschke (Lepidoptera: Lycaenidae) in Pieniny Mts (southern Poland). Pol J Ecol 57:403–409
Sielezniew M, Stankiewicz AM (2007) Differences in the development of the closely related myrmecophilous butterflies Maculinea alcon and M. rebeli (Lepidoptera: Lycaenidae). Eur J Entomol 104:433–444
Sielezniew M, Stankiewicz AM (2008) Myrmica sabuleti (Hymenoptera: Formicidae) not necessary for the survival of the population of Phengaris (Maculinea) arion (Lepidoptera: Lycaenidae) in eastern Poland: lower host-ant specificity or evidence for geographical variation of an endangered social parasite? Eur J Entomol 105:637–641
Sielezniew M, Stankiewicz A, Bystrowski C (2003) First observation of one Maculinea arion pupa in a Myrmica lobicornis nest in Poland. Nota lep 25:249–250
Sielezniew M, Buszko J, Stankiewicz AM (2005) Maculinea arion in Poland: distribution, ecology and prospects of conservation. In: Settele J, Kühn E, Thomas JA (eds) Studies on the ecology and conservation of butterflies in Europe. Species ecology along a European gradient: Maculinea butterflies as a model, vol 2. Pensoft, Sofia, Moscow, pp 231–233
Stankiewicz A, Sielezniew M (2002) Host specificity of Maculinea teleius Bgstr. and M. nausithous Bgstr. (Lepidoptera: Lycaenidae) the new insight. Ann Zool 53:403–409
Stankiewicz AM, Sielezniew M, Švitra G (2005) Myrmica schencki rears Maculinea rebeli in Lithuania: new evidence for geographical variation of host-ant specificity of an endangered butterfly. Myrmecol Nachr 7:51–54
Tartally A, Varga Z (2008) Host ant use of Maculinea teleius in the Carpathian Basin (Lepidoptera: Lycaenidae). Acta Zool Acad Sci Hung 54:257–268
Tartally A, Nash DR, Lengyel S, Varga Z (2008) Patterns of host ant use by sympatric populations of Maculinea alcon and M. ‘rebeli’ in the Carpathian Basin. Insect Soc 55:370–381
Thomas JA (1995) The ecology and conservation of Maculinea arion and other European species of large blue butterfly. In: Pullin AS (ed) Ecology and conservation of butterflies. Chapman and Hall, London, pp 180–197
Thomas JA (2002) Larval niche selection and evening exposure enhance adoption of a predacious social parasite, Maculinea arion (large blue butterfly), by Myrmica. Oecologia 132:531–537
Thomas JA, Elmes GW (1998) Higher productivity at the cost of increased host-specificity when Maculinea butterfly larvae exploit ant colonies through trophallaxis rather than by predation. Ecol Entomol 23:457–464
Thomas JA, Settele J (2004) Butterfly mimics of ants. Nature 432:283–284
Thomas JA, Wardlaw JC (1990) The effect of queen ants on the survival of Maculinea arion larvae in Myrmica ant nest. Oecologia 85:87–91
Thomas JA, Elmes GW, Wardlaw JC, Woyciechowski M (1989) Host specificity among Maculinea butterflies in Myrmica ant nests. Oecologia 79:425–457
Thomas JA, Elmes GW, Schonrogge K, Simcox DJ, Settele J (2005) Primary hosts, secondary hosts and ‘non-hosts’: common confusion in the interpretation of host specificity in Maculinea butterflies and other social parasites of ants. In: Settele J, Kühn E, Thomas JA (eds) Studies on the ecology and conservation of butterflies in Europe. Species ecology along a European gradient:Maculinea butterflies as a model, vol 2. Pensoft, Sofia, Moscow, pp 99–104
Thomas JA, Simcox DJ, Clarke RT (2009) Successful conservation of a threatened Maculinea butterfly. Science 325:80–83
Van Swaay CAM, Warren MS (1999) Red data book of European butterflies (Rhopalocera). Nature and Environment, vol 99. Council of Europe, Strasbourg
Witek M, Sliwinska E, Skórka P, Nowicki P, Wantuch M, Vrabec V, Settele J, Woyciechowski M (2008) Host ant specificity of large blue butterflies Phengaris (Maculinea) (Lepidoptera: Lycaenidae) inhabiting humid grasslands in east-central Europe. Eur J Entomol 105:871–877
Acknowledgments
We are grateful to Paweł Borkowski, Cezary Bystrowski, Krzysztof Frąckiel, Adam Górnicki, Tadeusz Janik and Krzysztof Pałka for the precise location of study sites and/or for logistic help during field studies. Sarah Łuczaj made linguistic improvements on the draft of the manuscript. We also thank our two referees for their valuable comments on the manuscript. The Polish Minister of the Environment and the Director of the Biebrza National Park issued the relevant permissions for our studies. This work was supported by the Polish Ministry of Science and Higher Education (grant no 2 P04G 024 30).
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Sielezniew, M., Dziekańska, I. & Stankiewicz-Fiedurek, A.M. Multiple host-ant use by the predatory social parasite Phengaris (=Maculinea) arion (Lepidoptera, Lycaenidae). J Insect Conserv 14, 141–149 (2010). https://doi.org/10.1007/s10841-009-9235-0
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10841-009-9235-0