Abstract
Purpose
Late cure after a previously failed ablation of ventricular arrhythmias (VAs) is a relatively common phenomenon. The present study sought to delineate the incidence and electrophysiological characteristics of late cure in idiopathic VA patients.
Methods
Totally, 45 idiopathic VA cases (mean age 44 ± 18 years, 27 males) either failed acutely or recurred within 12 h were enrolled in this study. Based on intensive clinical observations in the acute period, 19 (42%) patients demonstrated late cure in the first week after the procedure.
Results
The late cure patients had significantly better acute and cumulative ablation effects during the procedure than did those without a late cure. Additionally, they had a prediction that originated from the right ventricular outflow tract, aortic-mitral continuum, and left summit area relative to other sites (13/18 vs 6/27, p < 0.01). In a median follow-up of 24 [14, 46] months, 7/19 (37%) patients had their VAs recurred. The late cure group had significantly more patients cured at long-term follow-up than those without (12/19 vs 0/26, p < 0.01). A cutoff value of the “time to eliminate VAs” > 7.0 s was able to predict a long-term recurrence of the VAs with 62.5% sensitivity and 85.7% specificity.
Conclusions
The late cure of VAs occurs in more than one third of patients who have a seemingly unsuccessful ablation session, which is clustered in the first week after the procedure. However, long-term recurrence of VAs occurred in 37% of the late cure patients, emphasizing the importance of long-term follow-up.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
1 Introduction
Ventricular arrhythmias (VAs) can be eliminated by radiofrequency catheter ablation, which has an acute success rate greater than 90% in the setting of idiopathic VAs [1,2,3,4,5,6,7]. It can be encountered in the clinical practice that VAs spontaneously collapse after a seemingly unsuccessful ablation session, which is termed late cure. The late cure phenomenon of arrhythmias post-ablation has been elucidated by several reports, which have only addressed the accessory pathway and atria-ventricular node reentry [8,9,10,11,12,13,14]. To date, there is a paucity of data addressing this issue in VAs. This present study sought to elucidate the electrophysiological characteristics, temporal course, and long-term outcome of the late cure of VAs in patients without structural heart disease.
2 Methods
2.1 Patient characteristics
From August 2010 to July 2016, a consecutive series of 46 patients who underwent catheter ablation for VAs without structural heart disease was enrolled in this study. These patients were drawn from a pool of 1065 non-structural VAs cases referred to The First Affiliated Hospital of Nanjing Medical University. Patients were included when they exhibited one of the following criteria: (1) VAs were not successfully eliminated in the index procedure (31 cases); or (2) VAs were successfully ablated in the procedure but recurred within 12 h after the procedure (15 cases). All patients provided written informed consent before the procedure, and the study protocol was approved by the Institutional Review Board (The First Affiliated Hospital of Nanjing Medical University).
2.2 Electrophysiological study
All procedures were performed under conscious sedation and local anesthesia. All antiarrhythmic medications were ceased for at least 5 half-lives before the procedure. If needed, a 6F quadripolar and a decapolar catheter were advanced into the high right atrium or right ventricle and coronary sinus, respectively. In the ventricular tachycardia ablation procedure, a standard programmed stimulation with and without isoproterenol was applied to induce tachycardia. For a premature ventricular complex ablation session, only isoproterenol challenge was attempted.
2.3 Mapping and ablation
Three-dimensional mapping was performed to identify the earliest activation site of VAs using the CARTO (Biosense-Webster Inc., Diamond Bar, CA, USA) or EnSite NavX/Velocity (Endocardial Solutions, Inc., Minneapolis, MN, USA) systems. Radiofrequency energy was applied to the earliest activation sites using an irrigated ablation catheter (Navistar Thermocool, Biosense Webster, Diamond Bar, CA, USA or IBI Coolflex, St. Jude Medical, St. Paul, MN, USA). Generally, the maximum ablation power was adjusted from 20 to 50 w at the discretion of the ablation. The temperature limit was set to 43 °C, and the irrigation rate was 17–30 ml/min.
2.4 Definitions
Two indicators were used to evaluate the acute effect of ablation at the sites of origin and the cumulative effect of the ablation during the session. The acute effect of burning was classified into one of two types: one was defined as PVCs that had been completely eliminated or a VT that was terminated during the application of radiofrequency energy, which was named an “adequate response”; however, if the PVCs were not eliminated by ablation, the acute effect was defined as a “poor response.” The cumulative ablation effect at the end of the procedure was classified into one of three types: “Elimination” was defined as no or rare VAs observed or induced. As a mandatory rule based on the inclusion criteria, the VAs of these patients recurred within 12 h after ablation. “Reduction of VAs” was defined by a marked reduction in PVC frequency (more than 80% reduction of VAs burdens) or the VT get difficult to induce. “No effect” means no obvious decrease in PVC frequency could be observed at the end of the procedure or no alterations in the induction of VT.
“Time to eliminate VAs” represented the shortest time required for ablation to eliminate VAs or terminate VT during the session.
During the follow-up, a decrease of ≥ 90% of the initial PVCs burden or no recurrent VT for sustained VT patients was defined as no recurrence, which reflected the maintenance of the late cure effect over a long-term period. However, patients who exhibited a < 90% reduction of their PVC burden or suffered from VT recurrence were classified as not maintaining the effect of the late cure.
2.5 Clinical observation and follow-up
After the procedure, patients were sent back to the cardiovascular ward and monitored for at least 12 h. All anti-arrhythmia drugs were not used regularly. Clinical evaluation to determine the acute recurrence of VAs was performed based on monitoring status. Prior to discharge, a regular 12-lead electrocardiogram was performed every day and when patients reported palpitations. The late cure in the acute period was defined as the abrupt disappearance of the VAs, as evaluated by regular surface ECG and pulse checks by physicians at least three times per day. Regularly, patients were discharged 3 to 5 days after the procedure or earlier if their VAs had resolved. Additionally, patients were asked to perform pulse checks after discharge.
After discharge, patients were asked to undergo 24-h Holter monitoring at 1, 3, 6, and 12 months, with a 6-month interval post-procedure thereafter to reassess any recurrence of symptoms.
2.6 Statistical analysis
Continuous variables were expressed as the means ± standard deviations (normally distributed variables) or medians [25th, 75th percentiles]. The normal distribution was assessed with the Shapiro-Wilk test. Comparisons between groups were performed with an unpaired Student’s t test or non-parametric test. Categorical variables were expressed as numbers and percentages. The chi-square test or Fisher’s exact test was employed to compare the categorical data. To identify the predictors of recurrence, all variables were entered into the model if a significant association was observed in the univariate analysis or if there was some clinical relevance of the variable with the recurrence. We used the likelihood ratio (LR+), defined as sensitivity/(1 − specificity), to evaluate the optimal cutoff value for predicting a left-sided arrhythmia in our sample. All tests were two-sided, and a p value < 0.05 was considered statistically significant. Statistical analyses were performed using SAS 9.2.
3 Results
3.1 Patient characteristics
A case of papillary muscle VT was excluded from the study due to amiodarone administration because of intolerable recurrent arrhythmia episodes (thus, it was unable to evaluate the presence of late cure). Consequently, 45 patients (mean age of 44 ± 18 years, 27 males) were included in the analysis. Before the procedure, these patients had taken 0.6 ± 0.7 types of medications, including β-blockers, amiodarone, propafenone, and mexiletine, with no benefit, and four of them had undergone a failed ablation procedure in other centers. One patient demonstrated sustained VT, and the others were premature ventricular complex and non-sustained VT cases, with a mean burden of VAs was 29 ± 12% of the total beats (confirmed by 24-h Holter). Clinical history, physical examination, and echocardiogram revealed no signs of structural heart disease, except one had reduced left ventricular ejection fraction, which normalized 1 year after the successful elimination of the VAs. There were 8, 3, and 3 patients suffered from primary hypertension, diabetes, and coronary artery disease, respectively, which were all well controlled by drugs.
3.2 Acute ablation outcome and late cure in the acute period
In these patients, 45 VAs were mapped and ablated. The VA origin was assumed to be at the earliest activation site based on three-dimensional mapping. These VA origins were classified into the following sites: 1. Ventricular papillary muscles (9 cases, including left and right ventricles); 2. Left ventricular summit area and aortic-mitral annulus continuum (5 cases); 3. Right ventricular outflow tract (13 cases, including below and above the pulmonary artery valve); 4. Para-His area (6 cases); 5. Epicardial sites and coronary vein system (5 cases); and 6. Ventricular septum (7 cases).
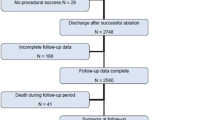
In terms of the acute response to ablation, 27 and 17 cases demonstrated an “adequate response” and “poor response,” respectively; in the remaining one patient, the acute response to ablation could not be evaluated because of the inadvertent disappearance of PVCs before ablation. At the end of the procedure, “elimination,” “reduction in burdens,” and “no effect” were obtained in 14, 15, and 16 patients, respectively. All “elimination” patients demonstrated acute recurrence in 12 h. Among the 31 cases without successful ablation, 7 cases were supposed to be due to unable to locate the catheter tip at the origin of VAs (most from papillary muscle), 8 cases were due to energy delivery restraints afraid of His or coronary vessels damage, 7 cases were supposed to be originated from intramural sites, 1 was due to inaccessible site (distal part of the great cardiac vein), and the other were due to unknown causes. A total of 19 patients (42%) demonstrated late cure, which occurred exclusively in the first week after the procedure (Fig. 1). A comparison of the clinical and electrophysiological characteristics of the enrolled patients based on the presence of late cure is summarized in Table 1. The acute response of VAs to the ablation demonstrated a significant difference between groups with and without late cure (adequate vs poor: 18/1 vs 9/15, p = 0.03). No significant difference was found in other clinical and electrophysiological variables, including the max power output and prematurity on the ablation site. Supplementary Table 1 provides detailed information as per anatomic sites of origin regarding patients with and without late cure. The VAs originating from sites 2 and 3 had a significantly higher incidence of late cure relative to those from other sites (13/18 vs 6/27, p < 0.01). The incidence of late cure, as per the classification of the cumulative ablation effect at the end of the procedure, is provided in Supplementary Table 2. The group of “elimination” patients had a significantly higher incidence of late cure than did those in the “no effect” group (13/14 vs 6/31, p < 0.01).
The number of patients with VAs occurrence after a seemingly unsuccessful ablation session is shown in the Fig. 1. It is clearly seen that all of the “late cures” occurred in the first week after the ablation procedure. However, from 1 to 12 months after the ablation, 7 cases had their VAs recurred, suggesting the effect of late cure warrants long-term follow-up
3.3 Long-term follow-up results and comparison of clinical and electrophysiological characteristics based on the maintenance of the late cure effect
After the failed procedure, four patients (all of those in the group without late cure) underwent re-ablation after one (3 patients) and three (1 patient) months; in 3 of these patients, the VAs were successfully ablated. These four patients were classified as failed with a follow-up period of 1 and 3 months. At a median of 24 [18, 41] months follow-up, 7 of the 19 late cure patients had their VAs recurred (Fig. 1). The late cure group had a significantly better long-term outcome than did patients without late cure (12/19 vs 0/26, p < 0.01, Table 1).
The detailed clinical and electrophysiological characteristics based on the maintenance of the late cure effect are provided in Table 2. All but one patient in the late cure group demonstrated an adequate response to the ablation in the session. The “time to eliminate VAs” in patients did not recur was significantly shorter than that in the patients who recurred (6.4 ± 3.4 vs 9.9 ± 3.1 s, p = 0.002; the data was not obtained in three patients, one for data missing, two were due to PVC disappeared due to trauma before burning; all these four patients were identified as “adequate response”). A cutoff value of the “time to eliminate VAs” > 7.0 s was able to predict a long-term recurrence of the VAs with 62.5% sensitivity and 85.7% specificity (Fig. 2). There were no significant differences in terms of clinical and other electrophysiological variables.
The “time to eliminate VAs” differed significantly between patients who did and did not recur in the long-term period, although there is an overlap. A cutoff value of the “time to eliminate VAs” > 7.0 s was able to predict a long-term recurrence of the VAs with 62.5% sensitivity and 85.7% specificity. The data could not be obtained in three patients; one for data missing; two were due to PVC disappeared before ablation
4 Discussion
4.1 Major findings
The study has several major findings: 1. In patients who are refractory to ablation or exhibit acute recurrence within 12 h, late cure in the acute period was observed in 42% of patients, and they were all in the first week after the procedure. 2. The late cure effect in the acute period does not guarantee a long-term cure, with 37% patients had their VAs recurred. Approximately 27% of the patients manifested a long-term cure after a seemingly unsuccessful ablation. The shortest time to eliminate the VAs in the ablation session predicted whether the patient would recur in long-term period. 3. After a failed ablation, those patients who manifested a late cure in the acute period had a significantly better outcome compared with those who did not.
4.2 Late cure of arrhythmias after ablation
The late cure of arrhythmias after a seemingly unsuccessful ablation session has been sporadically reported in several articles, most of which focused on supraventricular tachycardia. In the three-dimensional mapping era, the spectrum of arrhythmias referring to ablation has been dramatically widened. This makes the late cure of VAs relatively common in clinical practice, particularly for idiopathic focal VAs without structural heart disease.
Edema and inflammation may be one of the possible mechanisms of late cure after ablation. Histological studies showed that lesions produced by ablation are well-demarcated areas of coagulative necrosis surrounded by an inflammatory cell infiltrate and hemorrhage [12, 15, 16]. Late cure may be the result of lesion extension due to the inflammatory response induced peripheral zone fibrosis. Another possibility is that ablation creates an inflammatory milieu mimicking the focal ectopy. That is, the ablation was actually successful, but ventricular myocardium adjacent was now irritated after ablation, causing ectopy. As the inflammation resolves over time, VAs became less prevalent. In this study, the fact that all of the late cure cases were observed in the first week after the procedure could be a reflection of this pathological explanation [8,9,10, 13]. Another possible cause of the late effect may be microcirculatory damage to the surrounding tissue [11]. However, it is reasonable to find that in more than half of the late cure patients, the VAs recurred during the follow-up period in this study.
Few studies have explored late cure in terms of its clinical and electrophysiological characteristics. This may be partly due to its rareness in the clinical practice of supraventricular tachycardia ablation. The development of idiopathic focal VA ablation using three-dimensional mapping systems provided a unique milieu to observe the late cure effect. One of the most obvious differences between three-dimensional mapping-navigated VA ablation and conventional supraventricular tachycardia ablation lies in that the former allows repeating ablation attempts at the assumed site of origin and its surrounding areas, without any catheter movement, but the latter could not provide this accuracy. To evaluate the acute response of the most efficacious ablation and the cumulative effect of extensive ablation at sites of origin and its surrounding areas, two variables, the “acute effect of ablation” and “cumulative ablation effect”, were employed in the analysis, respectively. Although the exact ablation time could not be obtained in this study, we think it is reasonable that the cumulative effect of repeating ablation may partially account for the higher incidence in the present study than that in a previous study [8].
In this study, we found that VAs in the outflow tract, left ventricular summit area, and aortic-mitral continuum are more prone to exhibit the late cure effect than those from other sites. The reason underlying this predilection is to be determined. We attribute this specificity to the fact that in our practice, more extensive ablation in these two areas has been performed than in other sites. However, the quantity of detailed ablation, especially in the vicinity of target sites, was not systematically evaluated, which may be a limitation of this study.
We also found that better response to the ablation, mirrored by shorter time to eliminate VAs, was the only parameter to predict the long-term maintenance of this effect. Actually, among all cases maintained the long-term effect, there were three demonstrated disappearance by mechanical trauma of catheter manipulation at vicinity of origin; this fact implied that the prediction of better ablation response is beyond what the cutoff value showed. It is reasonable to speculate that shorter time to eliminate VAs implicates more superficial location under the myocardium.
4.3 Clinical implications
The results of this study have some implications for clinical practice. First, because the VAs at a failed ablation session may have some chance of self-healing, it is appropriate to interrupt a long and difficult session to observe the occurrence of delayed effects, rather than continue to burn, which will lead to the possibility of high radiation exposure and complications. Additionally, after an unsuccessful ablation, at least 1 week of no re-ablation should be used to observe the late effect. Finally, the late cure of VAs in the acute period does not guarantee a permanent effect; thus, long-term follow-up is needed, particularly in those patients with long time to eliminate VAs.
4.4 Limitations
The evaluation of VA burden by which to determine the late cure and its long-term effect was a technical challenge [17]. Intensive clinical observation rather than continuous ECG recording was employed to evaluate the collapse or recurrence of VAs, which was the inherent limitation of this observational study. The natural spontaneous variation of VAs also weakens the precision of 24-h Holter recording at follow-up. To overcome this shortcoming, more strict criteria (< 90%) were employed as the definition of an effective ablation procedure [18].
Another limitation of this study was that the total ablation time and number of lesions could not be obtained. Additionally, the definition of acute and cumulative effect of ablation was arbitrarily defined by the operator with more or less subjectivity.
The observational results in this series represent the experience of a tertiary electrophysiological center, in which all cases received detailed mapping and ablations by an experienced ablationist. Also, contact force catheter was not available in this study, which was considered to help to monitor the ablation effect.
5 Conclusions
The late cure of VAs after ablation occurred in more than one third of patients who experienced a seemingly unsuccessful ablation session, which clusters in the first week after the ablation. Those patients who demonstrate a better acute and cumulative ablation effect are more prone to demonstrate late cure. More than one third of the late cure patients had their VAs recurred during follow-up, suggesting the importance of long-term follow-up.
References
Liao Z, Zhan X, Wu S, Xue Y, Fang X, Liao H, et al. Idiopathic ventricular arrhythmias originating from the pulmonary sinus cusp: prevalence, electrocardiographic/electrophysiological characteristics, and catheter ablation. J Am Coll Cardiol. 2015;66:2633–44.
Ouyang F, Mathew S, Wu S, Kamioka M, Metzner A, Xue Y, et al. Ventricular arrhythmias arising from the left ventricular outflow tract below the aortic sinus cusps: mapping and catheter ablation via transseptal approach and electrocardiographic characteristics. Circ Arrhythm Electrophysiol. 2014;7:445–55.
Crawford T, Mueller G, Good E, Jongnarangsin K, Chugh A, Pelosi F Jr, et al. Ventricular arrhythmias originating from papillary muscles in the right ventricle. Heart Rhythm Off J Heart Rhythm Soc. 2010;7:725–30.
Yamada T, Doppalapudi H, McElderry HT, et al. Idiopathic ventricular arrhythmias originating from the papillary muscles in the left ventricle: prevalence, electrocardiographic and electrophysiological characteristics, and results of the radiofrequency catheter ablation. J Cardiovasc Electrophysiol. 2010;21:62–9.
Tada H, Ito S, Naito S, Kurosaki K, Kubota S, Sugiyasu A, et al. Idiopathic ventricular arrhythmia arising from the mitral annulus: a distinct subgroup of idiopathic ventricular arrhythmias. J Am Coll Cardiol. 2005;45:877–86.
Joshi S, Wilber DJ. Ablation of idiopathic right ventricular outflow tract tachycardia: current perspectives. J Cardiovasc Electrophysiol. 2005;16(Suppl 1):S52–8.
Aliot EM, Stevenson WG, Almendral-Garrote JM, et al. EHRA/HRS expert consensus on catheter ablation of ventricular arrhythmias: developed in a partnership with the European Heart Rhythm Association (EHRA), a registered branch of the European Society of Cardiology (ESC), and the Heart Rhythm Society (HRS); in collaboration with the American College of Cardiology (ACC) and the American Heart Association (AHA). Europace Eur Pacing Arrhythm Card Electrophysiol J Work Groups Card Pacing Arrhythm Card Cell Electrophysiol Eur Soc Cardiol. 2009;11:771–817.
Langberg JJ, Borganelli SM, Kalbfleisch SJ, Strickberger SA, Calkins H, Delayed MF. Effects of radiofrequency energy on accessory atrioventricular connections. Pacing Clin Electrophysiol. 1993;16:1001–5.
Wagshal AB, Pires LA, Mittleman RS, Cuello C, Bonavita GJ, Huang SK. Early recurrence of accessory pathways after radiofrequency catheter ablation does not preclude long-term cure. Am J Cardiol. 1993;72:843–6.
Chen X, Kottkamp H, Hindricks G, et al. Recurrence and late block of accessory pathway conduction following radiofrequency catheter ablation. J Cardiovasc Electrophysiol. 1994;5(8):650–8.
Nath S, Whayne JG, Kaul S, Goodman NC, Jayaweera AR, Haines DE. Effects of radiofrequency catheter ablation on regional myocardial blood flow. Possible mechanism for late electrophysiological outcome. Circulation. 1994;89:2667–72.
Fenelon G, Brugada P. Delayed effects of radiofrequency energy: mechanisms and clinical implications. Pacing Clin Electrophysiol. 1996;19:484–9.
Pelargonio G, Fogel RI, Knilans TK, Prystowsky EN. Late occurrence of heart block after radiofrequency catheter ablation of the septal region: clinical follow-up and outcome. J Cardiovasc Electrophysiol. 2001;12:56–60.
Sarubbi B, D'Alto M, Calvanese R, Russo MG, Calabro R. Late cure after radiofrequency catheter ablation in a pediatric patient. J Cardiovasc Med. 2006;7:356–61.
Wittkampf FH, Hauer RN, Robles de Medina EO. Control of radiofrequency lesion size by power regulation. Circulation. 1989;80:962–8.
Huang SK, Bharati S, Graham AR, Lev M, Marcus FI, Odell RC. Closed chest catheter desiccation of the atrioventricular junction using radiofrequency energy—a new method of catheter ablation. J Am Coll Cardiol. 1987;9:349–58.
Fontenla A, Castro L, Julia J, Arribas F. When is an outflow tract PCV completely ablated? J Cardiovasc Electrophysiol. 2015;26:814–5.
Baser K, Bas HD, Belardi D, et al. Predictors of outcome after catheter ablation of premature ventricular complexes. J Cardiovasc Electrophysiol. 2014;25:597–601.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Electronic supplementary material
ESM 1
(DOCX 16 kb)
Rights and permissions
About this article
Cite this article
Ju, W., Gu, K., Yang, B. et al. Late cure of focal ventricular arrhythmias post-catheter ablation: electrophysiological characteristics and long-term outcome. J Interv Card Electrophysiol 52, 31–37 (2018). https://doi.org/10.1007/s10840-018-0328-0
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10840-018-0328-0