Abstract
Background
Atrial fibrillation (AF) frequently complicates chronic kidney disease (CKD). AF treatment is challenging and requires complete pulmonary vein isolation (PVI). Recently, renal sympathetic denervation (RSD) has been reported to reduce AF recurrence when performed alongside PVI.
Methods
A prospective therapeutic study of patients with controlled hypertension and paroxysmal AF was undertaken. Renal function was evaluated using estimated glomerular filtration rate. Outcomes for patients with normal renal function who underwent PVI (n = 101) were compared with those for CKD patients who underwent either PVI alone (n = 96) or PVI + RSD (n = 39). The primary endpoint was recurrence of AF recorded by 24-h Holter monitoring.
Results
During the 22.4 ± 12.1 months following intervention, the incidence of AF recurrence was higher in CKD patients treated with PVI alone (61.5 %) than in CKD patients treated with PVI + RSD (38.5 %; HR 1.86, 95 % CI 1.14–3.03, P = 0.0251) or patients without CKD subjected to PVI (35.6 %; hazard ratio (HR) 2.27, 95 % confidence interval (CI) 1.51–3.42, P < 0.0001). In particular, the addition of RSD to PVI significantly reduced AF recurrence in CKD stage 4, but not stage 2 or 3, patients. Ambulatory blood pressure and mean heart rate were not different between groups or time points. No complications of either procedure were observed.
Conclusions
PVI + RSD is a safe treatment that is superior to PVI alone for treatment of paroxysmal AF in CKD patients.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
1 Introduction
Atrial fibrillation (AF) affects approximately 2 % of the population worldwide, and this percentage is projected to increase in the next 50 years [1, 2]. AF frequently complicates chronic kidney disease (CKD) and is associated with adverse outcomes. Progression to end-stage renal disease (ESRD) is a major complication of CKD, and the presence of AF is associated with a higher risk of developing ESRD in patients with CKD [3]. Furthermore, CKD is often associated with hypertension and high atrial pressure, both of which predispose to AF [3, 4]. Therefore, the development of AF in patients with CKD could simply reflect mechanical stress in the atrium.
Pokushalov and colleagues [5] recently reported that renal sympathetic denervation (RSD) reduces recurrence of AF when combined with pulmonary vein isolation (PVI). A strategy of percutaneous catheter-based delivery of radiofrequency (RF) energy was recently established to interrupt the sympathetic innervation of the kidneys. We propose that RSD can reduce the incidence of AF recurrence in patients with CKD by modulating the sympathetic hyperactivity present in this disease. The goal of this prospective study was to compare the impact of PVI in patients with controlled hypertension and paroxysmal AF with and without CKD, to PVI combined with RSD in patients with controlled hypertension, paroxysmal AF, and mild to moderate CKD.
2 Materials and methods
This paper reports a prospective, longitudinal study of 236 patients with controlled hypertension and either normal renal function or CKD, all of whom had a history of symptomatic paroxysmal AF (PAF). The study was undertaken in accordance with the Declaration of Helsinki and approved by the ethics committee of our institution. All patients gave their informed consent before inclusion.
2.1 Study subjects
This study was conducted at the Hospital e Clínica São Gonçalo, Rio de Janeiro, Brazil. Patients were recruited between January 2012 and January 2015 from the Arrhythmias and Artificial Cardiac Pacing Service of the same hospital. Enrolled patients met the following criteria: (i) mean 24-h systolic ambulatory blood pressure measurement (ABPM) of ≥100 and <130 mmHg; (ii) essential hypertension for >1 year; (iii) a physically normal heart with an ejection fraction of >50 % as measured by echocardiography (Simpson’s method); (iv) PAF (defined as AF episodes lasting <7 days with spontaneous termination) registered on ECG or 24-h Holter monitoring; (v) aged 18 to 80 years; (vi) estimated glomerular filtration rate (eGFR) of >15 mL/min/1.73 m2, calculated using the Chronic Kidney Disease Epidemiology Collaboration (CKD-EPI) equation [6] (patients with eGFR >60 mL/min/1.73 m2 were also required to have microalbuminuria); and (vii) the capacity to read, comprehend, and sign the informed consent form and attend the study.
Patients with any of the following were excluded: (i) pregnancy; (ii) valvular disease with significant adverse sequelae; (iii) unstable angina, myocardial infarction, transient ischemic attack, or stroke within the preceding 6 months; (iv) renovascular abnormalities; (v) psychiatric disease; (vi) allergy to ionic contrast medium; (vii) the inability to be monitored clinically after the procedure; (viii) a known addiction to alcohol or drugs that affects the intellect; (ix) a serious health condition that, in the investigator’s opinion, may have adversely affected the safety and/or efficacy of the participant or the study (e.g., abdominal aortic aneurysm, clinically significant peripheral vascular disease, diseases that might have caused bleeding due to thrombocytopenia, hemophilia, or significant anemia); (x) congestive heart failure (symptoms of functional class II to IV heart failure on the New York Heart Association scale); (xi) a previous AF ablation procedure; and (xii) treatment with amiodarone.
The subjects were divided into three groups according to their CKD status and the procedures to be performed: patients with normal renal function undergoing PVI (n = 101), CKD patients undergoing PVI (n = 96), and CKD patients undergoing PVI + RSD (n = 39). All of them were followed for at least 6 months after the blanking period of the procedures to assess maintenance of sinus rhythm and monitor variations in blood pressure, renal function, and echocardiographic parameters.
The primary endpoint of this study was a recurrence of AF of 30-s duration recorded by 24-h Holter monitoring. The blanking period (the first 3 months after ablation) was excluded from the analysis [7], and the 24-h Holter monitoring was evaluated at baseline and quarterly after the performance of the nominated procedures. The secondary endpoints were an evaluation of 24-h ABPM, eGFR, albuminuria, and echocardiographic parameters at baseline and 6 months after the procedures. Additionally, in the subjects that underwent RSD, safety was evaluated by a renal arterial duplex scan at baseline and 6 months after this procedure.
2.2 Transthoracic echocardiography
Transthoracic echocardiography was performed at baseline and 6 months after RSD using a Vivid I ultrasound system (General Electric, Frankfurt, Germany) equipped with a multifrequency transducer and tissue Doppler imaging software, according to the guidelines of the American Society of Echocardiography [8]. Data were analyzed and interpreted by one experienced echocardiographer who was blinded to treatment status and imaging sequence. The left ventricular (LV) mass was calculated from the LV linear dimensions using the Devereux formula [8, 9] and normalized to body surface area (BSA) [8, 10]. LV hypertrophy was considered to be present when the LV mass exceeded 115 g/m2 for men and 95 g/m2 for women [8]. The left atrial (LA) volume was measured using a disk sum algorithm similar to that used to measure LV volume [11, 12] and normalized to BSA. Although the LA size depends on sex, variation due to sex is generally recognized when it is normalized to the BSA [13]. Furthermore, whereas several methods of normalizing this measurement have been proposed [14, 15], normalizing to BSA produces the most reliable data. Normalizing to BSA compensates for sexual dimorphism in LA size, so only the normalized value is reported. The recommended highest value for LA size is 34 ml/m2 [15–18].
2.3 24-h ABPM
ABPM was performed for 24 h before each procedure with a clinically validated device (CardioMapa; Cardios, São Paulo, Brazil). The device was set to measure every 15 min during the day (06:00 to 22:00) and every 30 min during sleep (22:00 to 06:00). Patients were instructed to continue their regular activities during the recording and go to bed no later than 23:00. The waking period typically included 08:00 to 22:00 and the sleep period 24:00 to 06:00 [19]. All individuals were trained to record in a diary their sleeping and waking hours, meals, intake of medications, and any symptoms or events that could influence blood pressure during this period. Measurements were transferred to a computer for analysis. Monitoring was repeated as necessary until ≥70 % of the measured values obtained during both daytime and nighttime were satisfactory [20].
2.4 24-h Holter monitoring
Patients underwent 24-h Holter monitoring (Galix Biomedical Instrumentation, Florida, USA) at baseline and quarterly during the follow-up period to the nominated procedure. A three-channel recorder was used to record the electrocardiographic traces and to identify the minimum, mean, and maximum heart rate and the rhythm.
2.5 Pulmonary vein isolation
The AF ablation procedure has been described in detail previously [21]. All patients underwent complete PVI using a three-dimensional mapping system (EnSite Velocity; St. Jude Medical) without additional ablation lesion sets or lines. Patients still in AF at the end of the procedure were converted to sinus rhythm by cardioversion.
2.6 Renal sympathetic denervation
The RSD procedure has been described in detail previously [22]. The patients remained hospitalized in the ward for 24 h after the procedure.
2.7 Statistical analysis
The results are expressed as mean ± standard deviation for normally distributed data and as median and interquartile range otherwise. All statistical tests were two-sided. Comparisons between two paired values were performed with the paired t test in cases of a Gaussian distribution and using the Wilcoxon test otherwise. Comparisons between more than two paired values were made by repeated-measures analysis of variance or by Kruskal–Wallis analysis of variance and complemented by post hoc testing as appropriate. Categorical variables were compared using Fisher’s exact test. A P value <0.05 was considered significant. Correlations between two variables were performed using Pearson’s chi-square test in case of a Gaussian distribution and with the Spearman correlation test otherwise. Kaplan–Meier analysis was performed to determine the probability of success, estimated as the percentage of patients free of AF. Differences in arrhythmia-free survival time were assessed with the log-rank test. All statistical analyses were performed using GraphPad Prism v.7.0 (GraphPad Software, La Jolla, CA, USA).
3 Results
3.1 Baseline characteristics of patients
The general features of the groups of patients are listed in Table 1. The mean follow-up period for the 236 patients was 22.4 ± 12.1 months.
3.2 Safety evaluation of RSD and PVI
No patient developed procedural complications related to RSD. Real-time renal artery imaging was performed to evaluate structural changes that developed following the RSD. Six months after the procedure, all patients in the RSD group underwent a Doppler scan of their renal arteries, but no evidence of stenosis or flow limitation was detected. However, regarding PVI, there were ten cases of cardiac tamponade (4.3 %), six of them being self-limiting and four were drained quickly, continuing the procedure even in heparin use. No deaths occurred.
3.3 Effects of CKD and procedure on blood pressure and heart rate
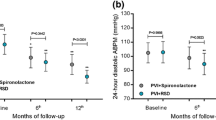
No significant changes were observed in the mean 24-h ABPM or the mean heart rate during the 24-h Holter monitoring between baseline and 6 months postprocedure within each treatment group. In addition, there were no significant differences between the three groups at each time point, as shown in Table 2.
3.4 Effects of CKD and procedure on renal function
The effects of PVI alone or PVI + RSD on the creatinine concentration, eGFR, and albumin/creatinine ratio during the first 6 months of follow-up are shown in Table 3.
3.5 Effects of CKD and procedure on echocardiographic parameters
Baseline values of the normalized LA volume, interventricular septal thickness, LV posterior wall thickness, LV ejection fraction (LVEF), end-diastolic LV internal dimensions (LVEDD), end-systolic LV internal dimensions, and LV mass index are displayed in Table 4. Changes in these parameters 6 months after PVI or PVI + RSD vs. the respective baseline values, as well as comparisons between groups at the same time points, are shown in Table 5.
3.6 Monitoring of AF
During a mean follow-up period of 22.4 ± 12.1 months, the incidence of AF recurrence was higher in CKD patients who underwent PVI (61.46 %) than in those without CKD subjected to PVI (35.64 %) (hazard ratio (HR) 2.272, 95 % confidence interval (CI) 1.512–3.415, P < 0.0001 by log-rank test), as well as in those subjects with CKD who underwent PVI + RSD (38.46 %) (HR 1.861, 95 % CI 1.142–3.031, P = 0.0251 by log-rank test). There was no difference in risk of AF recurring in patients without CKD subjected to PVI and patients with CKD who underwent PVI + RSD (HR 0.6841, 95 % CI 0.331–1.268, P = 0.1427 by log-rank test. P = 0.0002 by log-rank test for all comparisons; Fig. 1.
There was no significant effect of PVI vs. PVI + RSD on the subsequent incidence of recurrence of AF in stage 2 and 3 CKD patients (P = 0.1689 and P = 0.2914, respectively). However, CKD stage 4 patients who underwent only PVI (64.41 %) demonstrated a higher rate of AF recurrence than those subjected to PVI + RSD (33.33 %, P = 0.0409; Fig. 2).
4 Discussion
In the present study, we demonstrate that there is a greater likelihood of recurrence of AF in CKD patients who undergo PVI than in those without CKD and also versus those CKD patients who undergo PVI + RSD ∼2 years after each procedure. Furthermore, CKD stage 4 patients who undergo PVI alone demonstrate a higher incidence of AF recurrence than those who undergo PVI + RSD.
All subjects in the study had been diagnosed with paroxysmal AF and controlled hypertension and were classified according to preexisting differences in renal function at baseline. The mean 24-h systolic and diastolic ABPMs in CKD patients did not change from baseline during the follow-up period to either procedure and there were no differences between the PVI + RSD and PVI groups at any time points, consistent with previous studies [23, 24]. No change from baseline was observed in average heart rate during 24-h Holter monitoring 6 months postprocedure, either within or between groups.
CKD and AF share risk factors and putative mechanisms, suggesting that common pathophysiologic processes may drive both pathologies. One possible common link between AF and CKD is activation of the renin–angiotensin–aldosterone system [25–27]. Evidence suggesting a role for the renin–angiotensin–aldosterone system in the pathogenesis of AF has been provided previously [25–27]. Angiotensin II can increase atrial pressure, promote atrial fibrosis, and modulate ion channels, all of which are involved in structural and electrical remodeling of the atria, resulting in AF [25]. In addition, polymorphisms in genes encoding components of this pathway have been linked to the development of AF [26].
The addition of RSD to PVI had a positive impact on AF recurrence. Once PVI was achieved, the dominant initiating source was eliminated. As the patients in our study were individuals with controlled hypertension, we believe that overactivity of both the sympathetic nervous system and the feedback loop of the renin–angiotensin–aldosterone system were reduced in the subjects without CKD. However, in CKD patients with substantial pathology in the atrial substrate, additional intervention might be required to maximize the antiarrhythmic response, given that the normalized LA volume was significantly higher in CKD than in non-CKD patients at baseline. We consider that atrial remodeling caused by CKD may contribute to the higher incidence of AF recurrence in patients with CKD than in those without. The PVI in patients without CKD resulted in a reduction in the posterior wall thickness (PWT) 6 months postprocedure and a reduction in both the IST and PWT in those with CKD. However, PVI + RSD resulted in an improvement in additional parameters in CKD patients, including normalized LA volume, LVEF, LVEDD, and LV mass index. Six months after PVI + RSD, almost all echocardiographic parameters in CKD patients were superior to those in patients who underwent PVI, as previously reported [28, 29].
We believe that during follow-up, AF recurrence was higher in CKD patients who underwent PVI than in those without CKD subjected to PVI or those with CKD who underwent PVI + RSD (38.46 %), because of the sympathetic hyperactivity inherent to CKD. As expected, there was no difference in AF recurrence between patients without CKD subjected to PVI and patients with CKD who underwent PVI + RSD, suggesting that RSD can suppress sympathetic overactivity and the associated arrhythmogenic foci. Moreover, subjects with stage 4 CKD who underwent PVI alone were more likely to redevelop AF than were those who underwent PVI + RSD, further reinforcing the hypothesis that RSD can suppress sympathetic hyperactivity and the resulting arrhythmogenic activity.
4.1 Study limitations
Although our data show an independent contribution of RSD to reduce AF recurrence in patients with controlled hypertension and CKD, our patient cohort was small, which could be seen as a limitation. To our knowledge, however, the present series is the first to address the efficacy of percutaneous renal artery denervation in patients with concurrent controlled hypertension, CKD, and AF.
The presence of AF hampers measurement of LV ejection fraction because of tachycardia and beat-to-beat (i.e., R-to-R) LV filling variability. Our measurements could have been less precise because we did not use a three-dimensional single-beat ultrasound system. In addition, the use of Doppler echocardiography to assess damage in the renal arteries might also be seen as less than ideal. However, early complications due to the RF applications were excluded by angiography performed at the end of the procedure. Any other method, such as magnetic resonance angiography, computed tomographic angiography, or further angiography of the renal arteries, could expose patients to additional undesirable toxic insults. Carbon dioxide angiography is not available at our institution.
More precise methods of eGFR assessment, such as cystatin C or iothalamate measurement, should be used in future studies to further evaluate our findings concerning the effects of RSD on the eGFR, especially considering that only one serum creatinine measurement was performed at each time point of the study. Neuromuscular sympathetic activity could also be measured, which would contribute greatly to the assessment of the degree of sympathetic blockade.
5 Conclusions
We have shown that PVI + RSD is a safe combination and appears to be superior to PVI alone in the treatment of paroxysmal AF in CKD patients, especially in the most advanced stages of CKD. Furthermore, RSD can also improve renal function and some cardiac parameters in these patients. Although encouraging, our data are preliminary and need to be validated in a large population over a longer follow-up period. However, PVI + RSD is a potentially valuable combination therapy for incorporation into future clinical practice.
References
Stewart S, Hart CL, Hole DJ, McMurray JJ. Population prevalence, incidence, and predictors of atrial fibrillation in the Renfrew/Paisley study. Heart. 2001;86:516–21.
Go AS, Hylek EM, Phillips KA, Chang Y, Henault LE, Selby JV, et al. Prevalence of diagnosed atrial fibrillation in adults: national implications for rhythm management and stroke prevention: the AnTicoagulation and Risk Factors in Atrial Fibrillation (ATRIA) study. JAMA. 2001;285:2370–5.
Bansal N, Xie D, Tao K, Chen J, Deo R, Horwitz E, Hsu CY, Kallem RK2, Keane MG, Lora CM, Raj D, Soliman EZ, Strauss L, Wolf M, Go AS; CRIC Study: Atrial Fibrillation and Risk of ESRD in Adults with CKD. Clin J Am Soc Nephrol 2016.
Benjamin EJ, Levy D, Vaziri SM, D’Agostino RB, Belanger AJ, Wolf PA. Independent risk factors for atrial fibrillation in a population-based cohort. The Framingham Heart study. JAMA. 1994;271:840–4.
Pokushalov E, Romanov A, Corbucci G, Artyomenko S, Baranova V, Turov A, et al. A randomized comparison of pulmonary vein isolation with versus without concomitant renal artery denervation in patients with refractory symptomatic atrial fibrillation and resistant hypertension. J Am Coll Cardiol. 2012;60:1163–70.
Levey AS, Stevens LA, Schmid CH, Zhang YL, Castro III AF, Feldman HI, et al. CKD-EPI (chronic kidney disease epidemiology collaboration): a new equation to estimate glomerular filtration rate. Ann Intern Med. 2009;150:604–12.
Joshi S, Choi AD, Kamath GS, Raiszadeh F, Marrero D, Badheka A, et al. Prevalence, predictors, and prognosis of atrial fibrillation early after pulmonary vein isolation: findings from 3 months of continuous automatic ECG loop recordings. J Cardiovasc Electrophysiol. 2009;20:1089–94.
Lang RM, Bierig M, Devereux RB, Flachskampf FA, Foster E, Pellikka PA, et al. Recommendations for chamber quantification: a report from the American Society of Echocardiography’s Guidelines and Standards Committee and the Chamber Quantification Writing Group. J Am Soc Echocardiogr. 2005;18:1440–63.
Devereux RB, Alonso DR, Lutas EM, Gottlieb GJ, Campo E, Sachs I, et al. Echocardiographic assessment of left ventricular hypertrophy: comparison to necropsy findings. Am J Cardiol. 1986;57:450–8.
Mosteller RD. Simplified calculation of body-surface area. N Engl J Med. 1987;317:1098.
Thomas L, Levett K, Boyd A, Leung DYC, Schiller NB, Ross DL. Compensatory changes in atrial volumes with normal aging: is atrial enlargement inevitable? J Am Coll Cardiol. 2002;40:1630–5.
Yamaguchi K, Tanabe K, Tani T, Yagi T, Fujii Y, Konda T, et al. Left atrial volume in normal Japanese adults. Circ J. 2006;70:285–8.
Kou S, Caballero L, Dulgheru R, Voilliot D, De Sousa C, Kacharava G, et al. Echocardiographic reference ranges for normal cardiac chamber size: results from the NORRE study. Eur Heart J Cardiovasc Imaging. 2014;15(6):680–90. doi:10.1093/ehjci/jet284.
Pritchett AM, Jacobsen SJ, Mahoney DW, Rodeheffer RJ, Bailey KR, Redfield MM. Left atrial volume as an index of left atrial size: a population-based study. J Am Coll Cardiol. 2003;41:1036–43.
Vasan RS, Levy D, Larson MG, Benjamin EJ. Interpretation of echocardiographic measurements: a call for standardization. Am Heart J. 2000;139:412–22.
Spencer KT, Mor-Avi V, Gorcsan J, DeMaria AN, Kimball TR, Monaghan MJ, et al. Effects of aging on left atrial reservoir, conduit, and booster pump function: a multi-institution acoustic quantification study. Heart. 2001;85:272–7.
Knutsen KM, Stugaard M, Michelsen S, Otterstad JE. M-mode echocardiographic findings in apparently healthy, non-athletic Norwegians aged 20–70 years. Influence of age, sex and body surface area. J Intern Med. 1989;225:111–5.
Wang Y, Gutman JM, Heilbron D, Wahr D, Schiller NB. Atrial volume in a normal adult population by two-dimensional echocardiography. Chest. 1984;86:595–601.
Mancia G, De Backer G, Dominiczak A, Cifkova R, Fagard R, Germano G, et al. 2007 Guidelines for the management of arterial hypertension: the task force for the management of arterial hypertension of the European Society of Hypertension (ESH) and of the European Society of Cardiology (ESC). J Hypertens. 2007;25:1105–87.
Stergiou GS, Kollias A, Destounis A, Tzamouranis D. Automated blood pressure measurement in atrial fibrillation: a systematic review and meta-analysis. J Hypertens. 2012;30:2074–82.
Pokushalov E, Romanov A, Corbucci G, Artyomenko S, Turov A, Shirokova N, et al. Ablation of paroxysmal and persistent atrial fibrillation: 1-year follow-up through continuous subcutaneous monitoring. J Cardiovasc Electrophysiol. 2011;22:369–75.
Kiuchi MG, E Silva GR, Paz LM, Chen S, Souto GL. Proof of concept study: renal sympathetic denervation for treatment of polymorphic premature ventricular complexes. J Interv Card Electrophysiol. 2016.
Azizi M, Sapoval M, Gosse P, Monge M, Bobrie G, Delsart P, et al. Optimum and stepped care standardised antihypertensive treatment with or without renal denervation for resistant hypertension (DENERHTN): a multicentre, open-label, randomised controlled trial. Lancet. 2015;385:1957–65.
De Jager RL, Sanders MF, Bots ML, Lobo MD, Ewen S, Beeftink MM, et al. Renal denervation in hypertensive patients not on blood pressure lowering drugs. Clin Res Cardiol. 2016.
Goette A, Staack T, Röcken C, et al. Increased expression of extracellular signal-regulated kinase and angiotensin-converting enzyme in human atria during atrial fibrillation. J Am Coll Cardiol. 2000;35:1669–77.
Tsai CT, Lai LP, Lin JL, et al. Renin-angiotensin system gene polymorphisms and atrial fibrillation. Circulation. 2004;109:1640–6.
Wachtell K, Lehto M, Gerdts E, et al. Angiotensin II receptor blockade reduces new-onset atrial fibrillation and subsequent stroke compared to atenolol: the Losartan Intervention For End Point reduction in hypertension (LIFE) study. J Am Coll Cardiol. 2005;45:712–9.
Brandt MC, Mahfoud F, Reda S, Schirmer SH, Erdmann E, Böhm M, et al. Renal sympathetic denervation reduces left ventricular hypertrophy and improves cardiac function in patients with resistant hypertension. J Am Coll Cardiol. 2012;59:901–9.
Kiuchi MG, Mion Jr D, Graciano ML, de Queiroz Carreira MA, Kiuchi T, Chen S, et al. Proof of concept study: improvement of echocardiographic parameters after renal sympathetic denervation in CKD refractory hypertensive patients. Int J Cardiol. 2016;207:6–12.
Acknowledgments
The authors are grateful to all participants included in this study. The authors also thank Pacemed for stimulating the development of this study and for providing technical support.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Ethics statement
The study was piloted in accordance with the Declaration of Helsinki and approved by the ethics committee of our institution. All patients gave their informed consent before inclusion.
Conflict of interest
The authors declare that they have no conflict of interest.
Funding
This study was funded by Pacemed (US$400,000), Rio de Janeiro, Brazil.
Rights and permissions
About this article
Cite this article
Kiuchi, M.G., Chen, S., e Silva, G.R. et al. The addition of renal sympathetic denervation to pulmonary vein isolation reduces recurrence of paroxysmal atrial fibrillation in chronic kidney disease patients. J Interv Card Electrophysiol 48, 215–222 (2017). https://doi.org/10.1007/s10840-016-0186-6
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10840-016-0186-6