Abstract
Purpose
Arrhythmia recurrence following pulmonary vein isolation (PVI) occurs predominantly due to the reconnection of previously isolated pulmonary veins (PVs). The prognostic implications of detection and treatment of acute PV reconnection are not well understood. We aim to examine the prognostic significance of acute PV reconnection on arrhythmia recurrence at 1 year following PVI.
Methods
This prospective study included 44 patients (22 men, 60 ± 7 years) who underwent index PVI procedure for treatment of atrial fibrillation (AF). Acute PV reconnection and/or dormant PV conduction were assessed sequentially in response to a 30-min waiting period, intravenous isoproterenol infusion and/or adenosine. All cases of acute PV reconnection and/or dormant conduction were successfully targeted with additional ablation.
Results
Freedom from AF at 1 year was 75 % (83.3 % in paroxysmal and 65 % in persistent AF, p = ns). Acute PV reconnection and/or dormant conduction were evident in 16 of 44 patients (36.3 %). AF recurrence was documented in eight of 16 patients with, but only in three of 28 patients without acute reconnection (p = 0.009). Three patients underwent a redo procedure, all from the group of patients with acute PV reconnection. In a multivariate model, acute PV reconnection was a strong independent predictor of arrhythmia recurrence (hazards ratio [HR], 6.36; 95 % confidence interval [CI], 1.12–31.6).
Conclusion
Identification of acute PV reconnection, even when successfully targeted, is a strong predictor of arrhythmia recurrence following PVI.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
1 Introduction
Pulmonary vein isolation (PVI) is an effective therapy for patients with symptomatic atrial fibrillation (AF) and is the cornerstone of ablation. However, arrhythmia recurrence is common and is accompanied by the inconvenient realization that durable PVI is difficult to achieve [1]. Pulmonary vein (PV) reconnection is a common feature in patients with recurrent AF after ablation and re-isolation can often facilitate arrhythmia freedom [2–4]. In addition, smaller studies indicate that patients without arrhythmia recurrence have fewer reconnected veins, suggesting chronic PV reconnection as the source of failed AF ablation [4].
PV reconnections are usually the result of small gaps in the ablation lesion set. This has been recently supported by histological data that correlated partial thickness radiofrequency ablation lesions with chronic and persistent PV conduction [5]. Although real-time assessment of lesion formation is lacking, data suggests that waiting period after PVI, isoproterenol and/or adenosine can unmask incompletely isolated PVs to reveal dormant reconnection or re-conduction that can be targeted for additional ablation [6, 7].
The premise of these methods to improve long-term PV isolation and clinical outcome resulted in several studies with varying results. While earlier reports showed improved long-term outcome following the use of adenosine to detect and treat dormant conduction when compared to historical cohorts [8–10], recent small prospective studies showed no difference [11] or a similar prognosis [12] in long-term freedom from AF between patients with paroxysmal AF with and without adenosine-reconnected PVs.
The purpose of this study was to prospectively examine the prognostic value of treated acute PV reconnection induced by adenosine, isoproterenol, and/or a waiting period on 1-year outcome in patients with AF. The primary end point of the study was freedom from AF and/or organized atrial tachyarrhythmias at 1 year after a single ablation procedure.
2 Methods
2.1 Study population
This prospective study consisted of 44 consecutive patients with drug-refractory, symptomatic, paroxysmal or persistent AF who underwent index PVI between August 2011 and February 2012, and were followed for 12 months after the ablation procedure. AF was defined as paroxysmal or persistent according to the 2012 HRS/EHRA/ECAS Expert Consensus Statement on Catheter and Surgical Ablation of AF [13]. Patients with persistent AF were only included if arrhythmia duration was less than 1 year, thus excluding long-term persistent AF patients. The study protocol was approved by the institutional review board of Beth Israel Deaconess Medical Center.
2.2 Mapping and ablation protocol
All anti-arrhythmic drugs (AADs) were discontinued for ≥5 half-lives (amiodarone was discontinued for ≥1 week) before the ablation procedure. All patients were anticoagulated for ≥1 month. Transesophageal echocardiography was performed to exclude atrial thrombi in all patients with persistent AF and in patients with ineffective anticoagulation during the 4 weeks preceding the ablation. The surface electrocardiogram and bipolar electrograms, filtered at 30–500 Hz, were continuously monitored and recorded on a computer-based digital recording system (Cardiolab system; Prucka Engineering, Houston, TX, USA).
A phased array intra-cardiac ultrasound catheter (AcuNav, Diamond Bar, CA, USA) was placed in the right atrium. Two diagnostic decapolar catheters were positioned in the coronary sinus and the anterolateral right atrium. Unfractionated heparin was administered before the transseptal punctures to maintain an activated clotting time of 300–400 s for the duration of the procedure. The first transseptal puncture was performed via a long steerable sheath (Agilis; St. Jude Medical, Minneapolis, MN, USA) and the second via a fixed-curve long sheath (SL1; St. Jude Medical). The mapping/ablation catheter (Thermocool; Biosense Webster, Diamond Bar, CA, USA) was introduced into the left atrium (LA) through the steerable sheath while the circular mapping catheter (Lasso; Biosense Webster) was advanced into the LA over the fixed curve sheath. A wide antral approach was used to perform PVI at a distance of 2–3 cm from the pulmonary vein–left atrial junction (Fig. 1). Ablation of the left anterior PV was performed on the ridge between the vein and the appendage. The energy delivery settings were as follows: power, ≤40 W (≤20 W over the posterior left atrium); and temperature, ≤42 °C. Radiofrequency application was delivered for a maximum of 40 s to achieve an impedance decrease of 5–10 Ω at the ablation site; over the posterior LA, lesion duration was restricted to 20 s. Successful PVI was defined by the loss of PV potentials (entrance block) and failure to capture the LA during pacing from all bipoles of the Lasso catheter when positioned at the PV ostium (output, 10 mA; pulse width, 2 ms; exit block). Ablation in the PV carina was performed only when all the following criteria were met: (1) presence of LA–PV conduction despite completely encircling ablation lesions; (2) lack of near-field electrograms on the ablation line; and/or (3) presence of near-field electrograms in the PV carina. All procedures were performed under general anesthesia.
Pulmonary vein isolation ablation strategy. Antero–posterior (left) and posterior–anterior (right) views of the left atrium. PVI was performed by isolating all pulmonary veins in a wide antral approach. Visitag (Carto 3, Biosense Webster) was used to annotate ablation lesions based on isochromatic scale corresponding to decrease in impedance (red signifies an impedance drop >5 %)
2.3 Detection of dormant conduction or re-conduction
Entrance and exit block for each PV was confirmed after a ≥30-min waiting period from the time of the initial isolation. If persistent entrance and exit blocks were documented, intravenous isoproterenol, adenosine, or both were administered as follows: isoproterenol was the first agent of choice and was used in 36 patients (82 %). Adenosine was administered to patients who could not tolerate the hemodynamic effects of isoproterenol (39 %, 17/44). In addition, patients who tolerated isoproterenol, but did not have acute PV reconnection were subsequently treated with adenosine (21 %, 9/44).
2.3.1 Isoproterenol challenge
Isoproterenol infusion was started at 3 μg/min with increments every 3 min to 6, 12, and 20 μg/min for an increase in heart rate of ≥50 %. In the majority of cases, concomitant infusion of phenylephrine hydrochloride was required due to hypotension.
2.3.2 Adenosine challenge
Adenosine administration in a dose producing temporary atrioventricular conduction block during coronary sinus pacing (12–48 mg) was performed separately for each of the PV. In cases of common ipsilateral ostia, adenosine was given once per ipsilateral set of PVs. The effect of adenosine on LA–PV conduction was documented (Fig. 2). If adenosine induced persistent LA–PV conduction, the presence of conduction from the PV to the LA (exit) was also examined.
Acute pulmonary vein reconnection. Each panel contains three surface electrocardiographic leads, ten bipolar electrograms of a circular mapping catheter positioned in the left superior pulmonary vein (PV), two right atrial (RA) electrograms and two coronary sinus (CS) electrograms. a Acute PV reconnection in response to adenosine. Adenosine produces transient atrioventricular block (red asterisk) and acute LA–PV reconnection (arrow). b Acute LA–PV reconnection in response to isoproterenol infusion (arrow)
In cases of persistent recovery of LA–PV conduction, further radiofrequency ablation was delivered until the vein was re-isolated. In cases of transient reconnection, ablation was performed anatomically, based on the earliest activation transiently observed using a circular mapping catheter. In all cases of reconnection, the same pharmacological challenge was repeated following a waiting period of at least 15 min, until all PVs remained isolated for ≥15 min.
2.4 Follow-up
Following the ablation, patients resumed their pre-procedure medical regimen including AADs. Long-term follow-up consisted of clinic visits at 1, 3, 6 and 12 months and at least two 14-day sessions of trans-telephonic rhythm monitoring (with auto- and patient-trigger capabilities) at 3, 6 or 12 months. Additional trans-telephonic monitoring was performed based on symptoms. At each outpatient visit, patients were queried for symptoms and a 12-lead electrocardiogram was obtained. In the absence of any documented arrhythmias, AADs were discontinued between 1 and 3 months after the initial procedure. Patients with documented recurrence of atrial tachyarrhythmias were treated with AADs or offered repeat ablation procedures. In patients undergoing a repeat ablation, the same monitoring approach was used. The follow-up duration of the study was 1 year.
2.5 Statistical analysis
Baseline clinical variables were compared between groups using two-sided Student's t-test (continuous variables) and Fisher's exact test (categorical variables). Event-free survival was estimated by the Kaplan–Meier survival function. Pairwise comparisons of survival rates were made using a log-rank test. The impact of the variables associated with the risk of AF recurrence on the 1-year AF-free survival end point (LA size, the presence of hypertension, persistent AF, and obstructive sleep apnea [OSA]) was assessed in a univariate model and the variables demonstrating significant association were entered in a multivariate model. Bootstrapping analysis was used for parameter estimation. A p value <0.05 was considered statistically significant. Analyses were conducted using IBM SPSS Statistics 20.0 (SPSS, Inc., Chicago, IL, USA).
3 Results
3.1 Baseline patient characteristics
A total of 44 consecutive patients were enrolled in this study. The mean patient age was 60 ± 6.9 years with 50 % of participants being men. Fifty-five percent (24/44) of patients had paroxysmal AF. The baseline clinical characteristics of this cohort are summarized in Table 1.
3.2 Acute pulmonary vein reconnection
Successful PVI was achieved in all patients. Acute PV reconnection and/or transient dormant conduction were evident in 16 of 44 patients (36.4 %). The rate of reconnection was equal in the paroxysmal and persistent AT cohort (8/16 each). A total of 26 focal reconnections were identified in these 16 patients. The anatomical distribution of acute PV reconnections (based on site of successful re-isolation or earliest activation on the circular mapping in cases of transient conduction) is displayed in Fig. 3. The frequency of acute reconnections was similar between the left and right PVs (14 and 12, respectively, p = ns). The most common areas of acute reconnections, in order of frequency, were as follows: the left PV anterior ridge (34.6 %), the septal side of the right PV (23 %), the bilateral posterior carina (19.2 %), and the inferior aspect of the lower PVs (15.3 %).
Anatomical distribution of focal pulmonary vein reconnections. LPV left pulmonary vein, LSPV left superior pulmonary vein, LIPV left inferior pulmonary vein, RPV right pulmonary vein, RSPV right superior pulmonary vein, RIPV right inferior pulmonary vein. Each dot represents a single focal reconnection
Acute PV reconnection was detected by waiting period alone in five patients (31.2 %; mean waiting time 37 ± 4 min), in response to isoproterenol in four patients (25 %), in response to adenosine in six patients (37.5 %), and in response to the concomitant administration of isoproterenol and adenosine in one patient (6.25 %). Waiting period and isoproterenol infusion both resulted in persistent bidirectional reconnection between the LA and the PVs, while adenosine resulted in transient LA–PV conduction in four patients and persistent conduction in two patients. In these two patients, conduction from the PV to the LA was also documented. Re-isolation of the PVs was achieved in all patients.
3.3 Arrhythmia-free survival
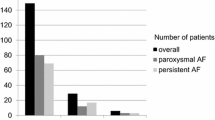
Thirty-three of the 44 patients (75 %) were free of AF and/or other atrial tachyarrhythmia at 1 year. Arrhythmia-free survival was 83.3 % in patients with paroxysmal AF compared to 65 % with persistent AF (p = ns). Thirty-five patients (80 %) were free of atrial arrhythmia between 3 and 12 months after ablation and without the need for AADs. Nine patients (20 %) remained on AADs at 1 year after PVI: in six of these patients, AAD was administered due to early recurrence of arrhythmia; however, three patients with persistent AF and no arrhythmia recurrence elected to continue therapy with AAD for at least 1 year. The recurrent atrial tachyarrhythmia was AF in all patients.
Acute PV reconnection was strongly associated with worse arrhythmia-free survival at 1-year (8/16 patients, 50 %) compared to patients with no acute reconnection (25/28 patients, 89.3 %), hazards ratio (HR) = 8.3 (95 % confidence interval [CI], 1.78–39.2), p = 0.009 (Table 2, Fig. 4).
The effect of acute pulmonary vein reconnection on AF recurrence. Eighty-nine percent of patients without acute reconnection were free of AF at 1 year after the index PVI. In contrast, 50 % of patients with acute reconnection had recurrence of AF. The AF-free survival rate was significantly different between both groups (KM p = 0.002; AF atrial fibrillation, PVI pulmonary vein isolation)
3.4 Clinical variables associated with AF recurrence
Acute PV reconnection and OSA were the only variables associated with AF recurrence in univariate analysis. In the multivariate model, the effect of OSA was no longer significant and acute PV reconnection remained the only independent predictor of arrhythmia recurrence (Table 2).
During the 1-year follow-up period of the study, three patients with symptomatic recurrence of arrhythmia that was not well controlled with AADs underwent a repeat ablation procedure. In all three patients, acute PV reconnection was observed during the initial procedure. Chronic PV reconnection was found in all three patients (1.66 focal reconnection sites per patient). Interestingly, chronic reconnections occurred at a distance (>3 cm) from the previously documented acute reconnection sites. At the redo procedure, only re-isolation of the connected PVs was performed. These patients remained arrhythmia-free for the rest of the follow-up period (mean period 6 ± 3 months).
4 Discussion
The main findings of this study are: (1) acute PV reconnections are common and their detection can be enhanced by a waiting period, isoproterenol and/or adenosine; (2) identification of acute PV reconnection, even when successfully targeted, is a strong predictor of arrhythmia recurrence following PVI; (3) in this cohort, acute PV reconnection was the only independent predictor of arrhythmia recurrence in a multivariate analysis, even when compared with the type of AT before ablation.
The findings of our study are consistent with the recent report by Miyazaki and colleagues [12], and are extended also to patients with persistent AF. In their study, 109 patients with paroxysmal AF underwent PVI followed by pharmacological challenge with isoproterenol in conjunction with adenosine. Acute PV reconnections were documented in about one third of patients and then targeted with additional ablation until elimination of adenosine-dependent conduction. Recurrence of AF was significantly higher in subjects with acute PV reconnections compared to subjects without acute PV reconnection (49 % vs. 27 %, respectively; HR = 1.37).
Gula and colleagues published equally disappointing results using acute provocation with adenosine. In 76 consecutive patients, adenosine was administered after PVI and revealed transient reconnection in 35 % of subjects. In contrast to the study by Miyazaki et al., these patients did not receive additional ablation in an attempt to test the predictive power of adenosine for arrhythmia recurrence. There was in fact no difference in long-term freedom from AF between patients with and without adenosine-responsive veins, limiting the predictive utility of adenosine in long-term arrhythmia control [11]. Although the results of our study confirm the limited utility of acute PV reconnection ablation for long-term arrhythmia control, we found that acute PV reconnection is a strong predictor of arrhythmia recurrence. These different results may be explained by the following: (1) different patient population, as our study was not limited to subjects with paroxysmal AF and included an equal amount of subjects with persistent AF; (2) in the study by Gula et al. [11], patients who exhibited persistent reconnection in response to adenosine did not receive additional ablation until re-isolation; (3) perhaps, the most important difference between the studies was the method to detect reconnections. In our study, detection of reconnection was not limited to adenosine, and was detected by either a waiting period or isoproterenol in 62.5 % of all subjects.
In this cohort of patients, acute PV reconnection was a stronger predictor for clinical recurrence when compared to the type of arrhythmia or left atrial dimension. This may be explained by the somewhat different patient population and ablation technique compared with our earlier cohort [14]. The current group of patients included subjects with either paroxysmal or early persistent AF according to the 2012 HRS/EHRA/ECAS Expert Consensus Statement on Catheter and Surgical Ablation of AF [13]. However, the group of persistent AF included patients with AF episode duration greater than 7 days requiring cardioversion, while patients with long-term AF (AF > 1 year) were excluded. In addition, a wider circumferential ablation approach was used, resulting in greater substrate ablation. Lastly, acute PV reconnection was associated with early clinical recurrence, and thus may have attenuated the longer-term effects of other variables.
Several studies examined the differential efficacy of waiting period, isoproterenol or adenosine on dormant PV conduction. Datino et al. [15] compared the differential efficacy of adenosine and isoproterenol in 25 subjects undergoing PVI. They found that adenosine was superior to isoproterenol in revealing dormant conduction (10 % vs. 87 %, p < 0.001). In addition, they examined the tissue response to adenosine and isoproterenol in coronary-perfused canine preparations. While radiofrequency lesions led to partial depolarization of the resting membrane potential causing inexcitability, the reversal effect of hyperpolarization was more profound with adenosine than isoproterenol (9.1 ± 0.6 vs. 3.8 ± 0.6 mV; p < 0.001).
Unlike pharmacological provocation with adenosine or isoproterenol, PVs that persistently reconnect during a waiting period appear to be critically relevant for long-term AF recurrence. In a recent study, 90 subjects with AF who underwent PVI were randomized into three groups of waiting period: 0, 30, and 60 min. By re-isolating PVs that reconnected over a 30- or 60-min waiting period, freedom from AF at 6.7 months improved from 61 % to 85 % [16]. In another report, by combining adenosine and 90 min waiting period after PVI, the 1-year freedom from AF was a remarkable 92 % [17].
While controversy still exists regarding the optimal method to detect and potentially treat acute PV reconnections, there is cumulative evidence to suggest that acute PV reconnections, especially in response to a 30- to 60-min waiting period, are a strong marker of arrhythmia recurrence following PVI.
The importance of acute PV reconnection as the mechanism of long-term arrhythmia recurrence is also reflected by the usual early recurrence of arrhythmia and the uniform presence of PV reconnection during the redo procedure, possibly implicating tissue edema and lack of lesion transmurality. This has been supported by histological data that correlated partial thickness radiofrequency ablation lesions with persistent PV conduction [5].
The high recurrence rate in patients with acute PV reconnection, even when targeted, can be explained by the following: first, inability to precisely map and ablate the focal area of reconnection due to multiple ineffective lesions, resulting in edema and tissue swelling, limiting adequate recording and effective ablation. Second, while a waiting period or pharmacological provocation can unmask conduction by hyperpolarizing partially depolarized tissue with modest edema, other areas with more significant edema, and hence lower resting membrane potential, may take longer to detect. This may explain the anatomic discrepancy between areas of acute and chronic PV reconnections.
Until a technology allowing real-time lesion assessment is available, physicians need to maximize efforts to deliver effective energy in a uniform and linearly continuous fashion, in an attempt to minimize gap formation and potential conduction recovery. Recent data showed that the use of contemporary tools including image registration, steerable sheaths and high frequency jet ventilation reduced the incidence of acute PV reconnection and resulted in improved 1-year freedom from AF after ablation [18]. In this regard, contact force sensing catheter technology may improve effective energy application and thereby reduce gaps resulting in vein reconnections. One prospective study found that PVI guided by contact sensing resulted in significantly lower incidence of acute PV reconnections when compared to a matched group of patients in whom contact force was not used to guide ablation [19]. Similarly, Reddy and colleagues recently published findings detailing the close relationship between contact force and clinical outcomes in the TOCCATA study, showing a higher rate of AF recurrence following PVI at 12 months in those with low catheter tip-to-tissue contact force. In this cohort, patients with average contact force >20 g had lower probability of recurrence (20 %) compared to those with an average contact force of <10 g, where all experienced recurrence of AF [20]. Moreover, differential contact force was found in specific areas around the PVs that prove more challenging to ablate, raising the possibility for operators to modify the approach by adjusting other parameters for ablation, such as RF power and/or duration of RF application to create a more durable lesion. The advent of this and other technologies, including balloon-based energy delivery as well as curvilinear and mesh catheters may also reduce the frequency of ablation gaps, however randomized studies comparing radiofrequency and cryoballoon ablation in regard to acute vein reconnections are currently lacking.
5 Limitations
This study has several limitations to note. First, the study population and event rate is relatively small, resulting in wide CI margins. Second, we limited the waiting period to 30 min, which may account for the relatively small number of subjects with acute reconnection over time. In addition, although the order of PV isolation alternated between left and right ipsilateral PV, the overall waiting period for detection of reconnection was slightly longer for the left PVs. This time interval, however, was not statistically significant and the rate of PV reconnection was similar between the ipsilateral PVs. Moreover, it is possible that the higher incidence of isoproterenol to reveal acute PV reconnection relative to adenosine was related to the prolonged waiting period required to achieve an isoproterenol effect. Lastly, all acute reconnections were targeted with additional ablation, thus limiting the ability to discern the sole value of targeting acute reconnections.
6 Conclusion
Acute PV reconnections following PVI in patients with both paroxysmal and persistent AF is a strong predictor of arrhythmia recurrence even when successfully targeted. Technologies to optimize energy delivery and tissue transmurality are warranted.
Abbreviations
- AAD:
-
Anti-arrhythmic drug
- AF:
-
Atrial fibrillation
- BMI:
-
Body mass index
- LA:
-
Left atrium
- LVEF:
-
Left ventricular ejection fraction
- OSA:
-
Obstructive sleep apnea
- PV:
-
Pulmonary vein
- PVI:
-
Pulmonary vein isolation
References
Kowal, R. C. (2012). PVI's inconvenient truths: lights out for dormant reconnection? Journal of Cardiovascular Electrophysiology, 23(3), 261–263.
Cappato, R., Negroni, S., Pecora, D., Bentivegna, S., Lupo, P. P., Carolei, A., et al. (2003). Prospective assessment of late conduction recurrence across radiofrequency lesions producing electrical disconnection at the pulmonary vein ostium in patients with atrial fibrillation. Circulation, 108(13), 1599–1604.
Gerstenfeld, E. P., Callans, D. J., Dixit, S., Zado, E., & Marchlinski, F. E. (2003). Incidence and location of focal atrial fibrillation triggers in patients undergoing repeat pulmonary vein isolation: implications for ablation strategies. Journal of Cardiovascular Electrophysiology, 14(7), 685–690.
Ouyang, F., Antz, M., Ernst, S., Hachiya, H., Mavrakis, H., Deger, F. T., et al. (2005). Recovered pulmonary vein conduction as a dominant factor for recurrent atrial tachyarrhythmias after complete circular isolation of the pulmonary veins: lessons from double Lasso technique. Circulation, 111(2), 127–135.
Kowalski, M., Grimes, M. M., Perez, F. J., Kenigsberg, D. N., Koneru, J., Kasirajan, V., et al. (2012). Histopathologic characterization of chronic radiofrequency ablation lesions for pulmonary vein isolation. Journal of the American College of Cardiology, 59(10), 930–938.
Arentz, T., Macle, L., Kalusche, D., Hocini, M., Jais, P., Shah, D., et al. (2004). “Dormant” pulmonary vein conduction revealed by adenosine after ostial radiofrequency catheter ablation. Journal of Cardiovascular Electrophysiology, 15(9), 1041–1047.
Tritto, M., De Ponti, R., Salerno-Uriarte, J. A., Spadacini, G., Marazzi, R., Moretti, P., et al. (2004). Adenosine restores atrio-venous conduction after apparently successful ostial isolation of the pulmonary veins. European Heart Journal, 25(23), 2155–2163.
Hachiya, H., Hirao, K., Takahashi, A., Nagata, Y., Suzuki, K., Maeda, S., et al. (2007). Clinical implications of reconnection between the left atrium and isolated pulmonary veins provoked by adenosine triphosphate after extensive encircling pulmonary vein isolation. Journal of Cardiovascular Electrophysiology, 18(4), 392–398.
Matsuo, S., Yamane, T., Date, T., Inada, K., Kanzaki, Y., Tokuda, M., et al. (2007). Reduction of AF recurrence after pulmonary vein isolation by eliminating ATP-induced transient venous re-conduction. Journal of Cardiovascular Electrophysiology, 18(7), 704–708.
Kumagai, K., Naito, S., Nakamura, K., Hayashi, T., Fukazawa, R., Sato, C., et al. (2010). ATP-induced dormant pulmonary veins originating from the carina region after circumferential pulmonary vein isolation of atrial fibrillation. Journal of Cardiovascular Electrophysiology, 21(5), 494–500.
Gula, L. J., Massel, D., Leong-Sit, P., Gray, C., Fox, D. J., Segal, O. R., et al. (2011). Does adenosine response predict clinical recurrence of atrial fibrillation after pulmonary vein isolation? Journal of Cardiovascular Electrophysiology, 22(9), 982–986.
Miyazaki, S., Kuwahara, T., Kobori, A., Takahashi, Y., Takei, A., Sato, A., et al. (2012). Impact of adenosine-provoked acute dormant pulmonary vein conduction on recurrence of atrial fibrillation. Journal of Cardiovascular Electrophysiology, 23(3), 256–260.
Calkins, H., Kuck, K.-H., Cappato, R., Brugada, J., Camm, A. J., Chen, S.-A., et al. (2012). 2012 HRS/EHRA/ECAS Expert Consensus Statement on Catheter and Surgical Ablation of Atrial Fibrillation: recommendations for patient selection, procedural techniques, patient management and follow-up, definitions, endpoints, and research trial design. Europace : European Pacing, Arrhythmias, and Cardiac Electrophysiology, 14(4), 528–606.
Fein, A. S., Shvilkin, A., Shah, D., Haffajee, C. I., Das, S., Kumar, K., et al. (2013). Treatment of obstructive sleep apnea reduces the risk of atrial fibrillation recurrence following catheter ablation. Journal of the American College of Cardiology.
Datino, T., Macle, L., Chartier, D., Comtois, P., Khairy, P., Guerra, P. G., et al. (2011). Differential effectiveness of pharmacological strategies to reveal dormant pulmonary vein conduction: a clinical–experimental correlation. Heart Rhythm : The Official Journal of the Heart Rhythm Society, 8(9), 1426–1433.
Wang, X.-H., Liu, X., Sun, Y.-M., Gu, J.-N., Shi, H.-F., Zhou, L., et al. (2007). Early identification and treatment of PV re-connections: role of observation time and impact on clinical results of atrial fibrillation ablation. Europace: European Pacing, Arrhythmias, and Cardiac Electrophysiology, 9(7), 481–486.
Yamane, T., Matsuo, S., Date, T., Lellouche, N., Hioki, M., Narui, R., et al. (2011). Repeated provocation of time- and ATP-induced early pulmonary vein reconnections after pulmonary vein isolation: eliminating paroxysmal atrial fibrillation in a single procedure. Circulation Arrhythmia and Electrophysiology, 4(5), 601–608.
Hutchinson, M. D., Garcia, F. C., Mandel, J. E., Elkassabany, N., Zado Pa-C, E. S., Riley, M. P., et al. (2012). Efforts to enhance catheter stability improve atrial fibrillation ablation outcome. Heart Rhythm, 10(3), 347–353.
Haldar, S., Jarman, J. W. E., Panikker, S., Jones, D. G., Salukhe, T., Gupta, D., et al. (2012). Contact force sensing technology identifies sites of inadequate contact and reduces acute pulmonary vein reconnection: a prospective case control study. International journal of cardiology.
Reddy, V. Y., Shah, D., Kautzner, J., Schmidt, B., Saoudi, N., Herrera, C., et al. (2012). The relationship between contact force and clinical outcome during radiofrequency catheter ablation of atrial fibrillation in the TOCCATA study. Heart Rhythm, 9(11), 1789–1795.
Conflict of interest
E. Anter received research grants from Biosense Webster and Rhythmia Medical. F.M. Contreras-Valdes, A. Shvilkin and C.M. Tschabrunn have no conflicts of interest. M.E. Josephson received research grants and speaking honoraria from Medtronic.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Anter, E., Contreras-Valdes, F.M., Shvilkin, A. et al. Acute pulmonary vein reconnection is a predictor of atrial fibrillation recurrence following pulmonary vein isolation. J Interv Card Electrophysiol 39, 225–232 (2014). https://doi.org/10.1007/s10840-013-9864-9
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10840-013-9864-9