Abstract
Purpose
Cavotricuspid isthmus (CTI) ablation for typical atrial flutter (AFL) has become the preferred treatment for this arrhythmia. The aims of this study were to assess the impact of respiratory gating (RG) on electroanatomical mapping of CTI and to assess the efficiency of CTI ablation guided by the Carto3® system equipped with the new respiration gating software.
Methods
Forty-four consecutive patients (mean age, 60 ± 13 years; 25 male) undergoing cavotricuspid ablation for symptomatic common AFL were randomly assigned to CARTO™ mapping with or without enabling RG module (Group A, RG OFF, Group B, RG ON).
Results
A significant reduction in mean RA volume, CTI central length and CS ostium maximum diameter has been observed in the RG maps. The mean total procedural, fluoroscopy and radiofrequency (RF) time were 102.9 ± 35.3, 10.6 ± 3.3, 22.9 ± 14.2 min in group A and 75.3 ± 21.7, 3.6 ± 4.5, 10.4 ± 5.7 min in group B, respectively (p < 0.05).
Conclusions
Electroanatomical mapping systems’ accuracy may be strongly influenced by respiration movements. The current study showed that automatic respiratory gated acquisition resulted in a better visualization of CTI, and this determines a relevant reduction in fluoroscopy and RF times.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
1 Introduction
Cavotricuspid isthmus (CTI) ablation has become the preferred treatment for typical atrial flutter (AFL). The ablation target is constituted by the creation of a linear lesion between the tricuspid annulus and the inferior vena cava until a bidirectional conduction block is achieved. Usually typical flutter ablation is performed with conventional approach under fluoroscopy guidance, but sometimes this task can be challenging and may require repeated radiofrequency (RF) applications with prolonged X-ray exposure [1–3].
An underappreciated silent complication of lengthy electrophysiology procedures is radiation exposures [4]. Electroanatomical mapping (EAM) systems have been introduced into clinical electrophysiology over a decade ago in order to reduce radiation exposure and allow precise electroanatomical reconstruction with a better understanding of arrhythmia mechanisms. The CARTO 3™ electroanatomical mapping system (Biosense Webster, Diamond Bar, CA, USA) provides a new technology for three-dimensional fast anatomical mapping (FAM) that could be used to guide ablation. FAM enables continuous volume data acquisition via a sensor-based mapping catheter roving the cardiac chambers. During volume acquisition, mapping catheter movements may be affected by cardiac cycle and by respiration. Catheter motion is set to stable by averaging the catheter location over a 1-s period reducing the effect of cardiac cycle. The residual catheter motion is due to respiration.
Significant respiratory positional changes in the left atrium and pulmonary veins (LA–PV) during inspiration have been reported [5, 6], but the impact on right atrium (RA) and CTI is still unknown. CTI lies on the diaphragm, and its movement can significantly affect anatomical mapping and ablation. Therefore, volumetric anatomical reconstruction would be affected by respiration. Moreover, volume acquisition during deep inspiration could deform anatomical reconstruction. The analysis and visualization of respiratory pattern of the patient during all the procedure combined with the possibility to navigate in an anatomical reconstruction as close as possible to expirium phase would improve catheter tracking during CTI reconstruction and AFL ablation.
The aims of this study were to assess the impact of respiratory gating (RG) on EAM of the right atrium and CTI and to assess the efficiency of EAM and ablation procedure guided by the CARTO 3™ system equipped with the new RG software (AccuResp™).
2 Methods
2.1 Study design
Forty-four consecutive patients (mean age, 60 ± 13 years; 25 male; 35 with structural heart disease) undergoing cavotricuspid ablation for symptomatic common AFL were randomly assigned to CARTO 3™ mapping with or without enabling Accuresp™ module (Group A, RG OFF, 22 patients; Group B, RG ON, 22 patients). The demographics of the patients are summarized in Table 1.
In order to evaluate the impact of using the automatic RG anatomical reconstruction of cavotricuspid isthmus and the real-time monitoring of respiration pattern, procedural, fluoroscopy and radiofrequency time were considered. All antiarrhythmic drugs, except amiodarone, were discontinued five half-lives before the ablation procedure. The study protocol was approved by our Institutional Review Board established by the Ministry of Health and performed after written informed consent from the patient.
2.2 CARTO 3™ system and AccuResp™ module
The EAM was performed before the ablation procedure by means of the new CARTO 3™ system (Biosense Webster, Inc., Diamond Bar, CA, USA). The CARTO 3™ system combines the magnetic technology with the advanced catheter location (ACL) technology allowing the visualization of all catheters connected to the system. The ACL is a hybrid technology that combines magnetic with additional current-based data [7, 8].
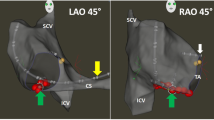
AccuResp™ module performs a respiration gating based on the end expiration (repeatable position) thus ensuring a respiration gating indifferent to changes in respiratory patterns throughout the procedure. During the training phase, AccuResp™ learns the inter-patch current measurements (respiration indications) and sensor-based catheter movements. The inter-patch currents passing through the lungs describe changes in impedance due to pulmonary air volume. The movements of a sensor-based catheter, placed in a stable location touching a chamber wall in an area with large respiratory swing, describe heart motion during respiration. During the training phase, the algorithm correlates these measurements and detects the end expiration and, throughout the procedure, it detects the inter-patch currents that are associated with end expiration. At the end of the training phase, the system displays the respiratory training real-time graph which plots the respiratory amplitude over time, from the end of expiration to the end of inspiration. A user-defined threshold defined on the real-time graph determines gate (orange data) vs out-of-gate acquisition (yellow data). Using a respiratory threshold as close as possible to the end of expirium phase provides for significant gating and reduces the effect of respiration on the anatomical reconstruction. Out-of-gate acquisition is automatically detected and excluded from the respiratory gated anatomical reconstruction (Fig. 1), thus slightly prolonging anatomical mapping time.
CARTO 3™ left anterior oblique (LAO) view of the right atrium (RA) showing differences between fast anatomical mapping (FAM) without (a) and with (b) respiratory gating (RG). During FAM with RG, anatomical data were acquired in closest proximity to completion of the expirium phase (orange volumes) by setting a specific threshold on the real-time graph (RTG). Over threshold volumes (yellow volumes) were detected by using RG (a) and are clearly visible on the isthmus, lateral wall and coronary sinus (blue arrows). FAM without RG (a) was affected by respiratory motion and would require manual editing. Accuresp™ gave accurate anatomical reconstruction by automatically excluding yellow volumes (b)
2.3 Electroanatomical mapping
All the procedures were performed while the patients were in a fasted state under mild sedation. Under local anaesthesia, all the diagnostic catheters were inserted into the RA. In all cases, a 7F decapolar catheter (EzSteer CS™, Biosense Webster, Inc., Diamond Bar, CA, USA) was placed into the coronary sinus (CS) via the right femoral vein.
In all patients, FAM of the whole chamber was performed via a sensor-based catheter (Navistar DS 8 mm, Biosense Webster, Inc., Diamond Bar, CA, USA) by setting the global reconstruction resolution to 14. FAM of the cavotricuspid isthmus region was performed by roving the catheter from CS ostium to the lateral aspect of tricuspid annulus and from the most inferior site of tricuspid annulus to the mouth of the inferior vena cava.
In group B, before starting the EAM, respiration gating was enabled. The training phase was completed by placing the mapping catheter at CS ostium, and a low respiratory threshold (10 % of the respiratory cycle) has been set to reconstruct the isthmus region to automatically collect anatomical data as close as possible to end expirium phase. The FAM of the RA was completed by using by setting a higher respiratory threshold (40 % of the respiratory cycle) in order to fasten anatomical map reconstruction of RA. For each patient, both respiratory gated (RG) and raw (no exclusion of respiratory data) map have been saved. In this case, any manual and empirical editing have been used in order to delete volume acquired during deep breath.
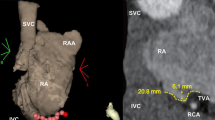
Specific analysis has been completed by measuring: the volume of the chamber (V), the central CTI length [9] the maximum diameter of CS ostium (Fig. 2). Real-time graph (RTG) was displayed during all the procedure in order to test visual correlation between catheter movements and respiratory pattern.
In patient with sinus rhythm, mapping and ablation were performed during CS stimulation with a cycle length of 600 ms. In patients with AFL, ablation and mapping were started until sinus rhythm was obtained. Then, the procedure was completed during coronary sinus stimulation. No patient was in atrial fibrillation at the time of procedure.
RF was applied with a power limit of 70 W in temperature-controlled modality (max 60 °C) and pulse duration of 60–90 s. Complete bidirectional isthmus block was verified with EAM remapping and double potential mapping during pacing from both sides of the lesion line. Validation was repeated after 20 min.
2.4 Statistical analysis
All variables are presented as a mean ± standard deviation. A paired t test or chi-square test was used to compare differences between the groups. A P value <0.05 was considered to be statistically significant.
3 Results
We analyzed 44 consecutive patients who underwent EAM for AFL catheter ablation procedures, from May 2011 to May 2012. Group A (22 patients, mean age 62 ± 13 years, 14 males, 16 with structural heart disease) underwent the procedure guided by the CARTO 3™ system without enabling Accuresp™ module, while group B (22 patients, mean age 63 ± 14 years, 9 males, 20 with structural heart disease) underwent the procedure by using the same EAM system after enabling respiration gating software.
In group A, map was generated within 2.1 ± 1.7 versus 4.8 ± 2.1 min with RG (P < 0.05). A mean of 52.0 ± 12.2 endocardial points were sampled.
No significant difference between group A and raw maps of group B was observed (Table 2). In group B, RG maps showed a significant reduction in central isthmus length (Δ = 4.95 ± 3.58 mm, Fig. 3), RA volume (Δ = 26.47 ± 19.85 mm3) and CS ostium maximum diameter (Δ = 4.58 ± 2.89 mm, Table 2).
The mean total procedural time, the mean duration of RF time and the mean total fluoroscopy time were significantly lower in group B when compared with group A, as shown in Table 3 (P = 0.026, P = 0.011 and P = 0,0001, respectively; P = 0.026). The reasons for using fluoroscopy should be referred to mobilization of catheters during FAM, placement of the reference catheter into the CS, ablation of CTI close to the mouth of inferior vena cava.
CTI ablation was achieved in all patients. No particular complications occurred during the procedure in any patient.
Different breathing patterns were observed among patients, with a varying component of thoracic expansion and diaphragmatic movement during inspiration. Although patients were instructed to breathe normally and regularly, some patients showed a trend towards shallow breathing pattern or even fell asleep. In other cases, patients had to be reminded to breathe regularly because they had a phasic pattern with clusters of deep inspirations followed by apnoea (Fig. 4). RTG was displayed during all the procedure and showed a good visual correlation with catheter movements.
4 Discussion
4.1 Main findings
The main findings of this study are: (a) the impact of respiration movements on CTI and CS anatomical reconstruction is significant; (b) RG acquisition of FAM allowed a precise and reliable reconstruction of RA, CTI and CS and avoided empiric editing of EAM; and (c) RG reduced procedural, fluoroscopy and RF time.
4.2 Respiration and electroanatomical mapping
The use of EAM for CTI ablation was introduced by Kottkamp et al. [10] in order to reduce fluoroscopy time. The benefits of EAM systems include the possibility to obtain a precise anatomical reconstruction, reduce fluoroscopy, identify arrhythmic mechanism and verify ablation efficacy.
Moreover, some investigators have reported that ablation of supraventricular arrhythmias guided by EAM systems can be carried out without fluoroscopy guidance, even if procedural time may be prolonged [11]. Respiration may cause important movements of cardiac chambers, and these can result in significant catheter displacements during the acquisition of the EAM. Although the introduction of fast anatomical mapping (FAM) in CARTO 3™ system fastened mapping of cardiac chambers, movements of the heart due to respiration could potentially introduce an error especially in non-ventilated patients.
Some authors have described relative (but not absolute) changes in LA–PV anatomy [5, 12]. They found splaying of the PVs during inspiration and surface-to-surface distances of 2–4 mm for different PVs and the LA between inspiration and expiration after rigid image registration. Relative changes in LA–PV geometry were most pronounced in the distal PVs and in the LA body near the mitral valve. Recently, Beinart et al. [5] demonstrated that manual acquisition of endocardial points limited to end expiratory phase resulted in more accurate definition of the PV ostia and may minimize the potential mistake attributed to respiratory cycle. Unfortunately, they focused only on a population of ventilated patients, and they found only a reduction of fluoroscopy time.
In our series, we evaluated the impact of respiration on RA-CS morphology during the acquisition of real-time EAM. We found a significant reduction of RA volumes, with a major impact on CTI length and CS ostium dimension. As expected, these errors for RA body and CS were most pronounced in the superior–inferior axis, based on the predominant displacement axis of the heart during respiration, while the CTI mapping was affected also in the anterior–posterior axis. No apparent geometrical changes in the geometry of the RA were noted, whilst FAM acquired with respiratory gating led a more reliable definition of CTI length and CS dimension.
4.3 Role of respiratory gated electroanatomical mapping and cavotricuspid isthmus ablation
CTI ablation is one of the most common procedures in EP laboratories. The CTI is limited posteriorly by the Eustachian ridge and anteriorly by the annular insertion of the septal leaflet of the tricuspid valve. Creation of a complete bidirectional conduction block across the CTI is the accepted target for long-term success in typical AFL [13, 14]. Limiting anatomical factors (variability of isthmus length and shape, presence of pouch-like recesses, a prominent Eustachian ridge, different muscular thickness, RCA cooling effect) may affect CTI ablation length [15, 16]. In a previous study, Kirchhof et al. [17] identified that difficult CTI ablation was associated with an angulation of the CTI close to 90°, the movement of CTI and its diastolic length. Fluoroscopy time required varies depending on the operators’ experience and technique used. In fluoroscopy-guided procedures of CTI ablation, mean fluoroscopy varies between 8 and 30 min [11, 18–20].
Recently, Ilg et al. [21] did not exclude a potential helpful role of 3D catheter navigation ablation conducted with large-tip catheters. In our study, the introduction of a respiratory gating during mapping (FAM and EAM) reduced both procedural and fluoroscopy time, but the most important was the reduction in RF time. Despite a slight increase in mapping time, CTI block was achieved in a shorter time, and this result reflected in a shorter procedural time.
In 5–10 % of cases, CTI ablation may be challenging, and CTI block may be achieved only after prolonged and repeated procedures with long RF times. Repeated RF applications may produce progressive oedematous swelling and create a barrier making progressively more difficult to obtain CTI block. As CTI movement and length may be limiting factors, EAM with respiration gating provided accurate maps and precise measurement of CTI. Direct visualization of RTG could facilitate catheter tracking and recognize catheter displacement during deep inspiration. Automatic respiration gating may allow to create a conduction block on CTI avoiding to repeat ineffective RF pulses.
Repeated RF ablations may increase the risks of rare complications such as right coronary artery (RCA) occlusion or AV nodal impairment. [22] Due to the proximity of RCA and the CTI, thermal damage (focal spasm, intravascular coagulation, vessel wall oedema) should always be considered as potentially harmful during ablation [23, 24.
5 Conclusions
EAM system accuracy may be strongly influenced by respiration movements. In the current study, automatic respiratory gated acquisition showed a significant reduction of RA volumes, with a major impact on CTI length and CS ostium dimension. The best visualization of CTI and RA resulted in a relevant reduction in fluoroscopy and RF times. In conclusion, RG-FAM could maximize both the efficiency and safety of CTI ablation: (1) by providing a correct CTI anatomical reconstruction, (2) by avoiding inadvertent redundant RF applications and (3) by providing a direct correlation between catheter movements and breathings during RF delivery.
Abbreviations
- CTI:
-
Cavotricuspid isthmus
- AFL:
-
Atrial flutter
- EAM:
-
Electroanatomical mapping/map
- FAM:
-
Fast anatomical mapping
- RA:
-
Right atrium
- LA–PV:
-
Left atrium–pulmonary vein
- RG:
-
Respiratory gating
- CS:
-
Coronary sinus
- RTG:
-
Real-time graph
- RF:
-
Radiofrequency
References
Poty, H., Saoudi, N., Nair, M., Anselme, F., & Letac, B. (1996). Radiofrequency catheter ablation of atrial flutter. Further insights into various types of isthmus block: application to ablation during sinus rhythm. Circulation, 94(12), 3204–3213.
Cauchemez, B., Haissaguerre, M., Fischer, B., Thomas, O., Clementy, J., & Coumel, P. (1996). Electrophysiological effects of catheter ablation on inferior vena cava-tricuspid annulus isthmus in common atrial flutter. Circulation, 93(2), 284–294.
Schwartzman, D., Callans, D. J., Gottlieb, C. D., Dillon, S. M., Movsowitz, C., & Marchlinski, F. E. (1996). Conduction block in the inferior vena cava-tricuspid valve isthmus: association with outcome of radiofrequency ablation of type I atrial flutter. Journal of the American College of Cardiology, 28(6), 1519–1531.
Houmsse, M., & Daoud, E. G. (2012). Radiation exposure: a silent complication of catheter ablation procedures. Heart Rhythm, 9(5), 715–716.
Beinart, R., Kabra, R., Heist, K. E., Blendea, D., Barret, C. D., Danik, S. B., et al. (2011). Respiratory compensation improves the accuracy of electroanatomical maping of the left atrium and pulmonary veins during atrial fibrillation. Journal of Interventional Cardiac Electrophysiology, 32(2), 105–110.
Noseworthy, P. A., Malchano, Z. J., Ahmed, J., Holmvang, G., Ruskin, J. N., & Reddy, V. Y. (2005). The impact of respiration on left atrial and pulmonary venous anatomy: implications for image-guided intervention. Heart Rhythm, 2(11), 1173–1178.
Stabile, G., Scaglione, M., del Greco, M., De Ponti, R., Bongiorni, M. G., Zoppo, F., et al. (2012). Reduced fluoroscopy exposure during ablation of atrial fibrillation using a novel electroanatomical navigation system: a multicentre experience. Europace, 14(1), 60–65.
Scaglione, M., Biasco, L., Caponi, D., Anselmino, M., Negro, A., Di Donna, P., et al. (2011). Visualization of multiple catheters with electroanatomical lapin reduces X-ray exposure during atrial fibrillation ablation. Europace, 13(7), 955–962.
Cabrera, J. A., Ho, S. Y., & Sanchez-Quintana, D. (2009). How anatomy can guide ablation of isthmic atrial flutter. Europace, 11(1), 4–6.
Kottkamp, H., Hugl, B., Krauss, B., Wetzel, U., Fleck, A., Schuler, G., et al. (2000). Electromagnetic versus fluoroscopic mapping of the inferior isthmus for ablation of typical atrial flutter. A prospective randomized study. Circulation, 102(17), 2082–2086.
Alvarez, M., Tercedor, L., Herrera, N., Munoz, L., Galdeano, R. S., Valverde, F., et al. (2011). Cavotricuspid isthmus ablation without the use of fluoroscopy as a first-line treatment. Journal of Cardiovascular Electrophysiology, 22(6), 656–662.
Ector, J., de Buck, S., Loeckx, D., Coudyzer, W., Maes, F., Dymarkowski, S., et al. (2008). Changes in left atrial anatomy due to respiration: impact on three-dimensional image integration during atrial fibrillation ablation. Journal of Cardiovascular Electrophysiology, 19(8), 828–834.
Feld, G. K., Flech, R. P., Chen, P. S., Boyce, K., Bahnson, T. D., Stein, J. B., et al. (1992). Radiofrequency catheter ablation for the treatment of human type 1 atrial flutter: identification of a critical zone in the reentrant circuit by endocardial mapping techniques. Circulation, 86(4), 1233–1240.
Cosio, F., Lopez Gil, M., Goicolea, A., Arribas, F., & Barroso, J. L. (1993). Radiofrequency ablation of the inferior vena cava-tricuspid valve isthmus in common atrial flutter. The American Journal of Cardiology, 71(8), 705–709.
Gami, A. S., Edwards, W. D., Lachman, N., Friedman, P. A., Talreja, D., Munger, T. M., et al. (2010). Electrophysiological anatomy of typical atrial flutter: the posterior boundary and causes for difficulty with ablation. Journal of Cardiovascular Electrophysiology, 21(2), 144–149.
Tai, C. T., & Chen, S. A. (2009). Cavotricuspid isthmus: anatomy, electrophysiology, and long-term outcome of radiofrequency ablation. Pacing and Clinical Electrophysiology, 32(12), 1591–1595.
Kirchhof, P., Ozgun, M., Zellerhoff, S., Monning, G., Eckardt, L., Wasmer, K., et al. (2009). Diastolic isthmus length and vertical isthmus angulation identify patients with difficult catheter ablation of typical atrial flutter: a pre-procedural MRI study. Europace, 11(1), 42–47.
Hindrincks, G., Willems, S., Kaurtzner, J., de Chillou, C., Weidemann, M., Schepel, S., et al. (2009). Effect of electroanatomically guided versus conventional catheter ablation of typical atrial flutter on the fluoroscopy time and resource use: a prospective randomized multicenter study. Journal of Cardiovascular Electrophysiology, 20(7), 734–740.
Anselme, F., Savoure, A., Cribier, A., & Saoudi, N. (2001). Catheter ablation of typical atrial flutter. A randomized comparison of 2 methods for determining complete bidirectional block. Circulation, 103(10), 1434–1439.
Vollmann, D., Luthje, L., Seegers, J., Hasenfuss, G., & Zabel, M. (2009). Remote magnetic catheter navigation for cavotricuspid isthmus ablation in patients with common-type atrial flutter. Circulation: Arrhythmia and Electrophysiology, 2(6), 603–610.
Ilg, K. J., Kuhne, M., Crawford, T., Chugh, A., Jongnarangsin, K., Good, E., et al. (2011). Randomized comparison of cavotricuspid isthmus ablation for atrial flutter using an open irrigation-tip versus a large-tip radiofrequency ablation catheter. Journal of Cardiovascular Electrophysiology, 22(9), 1007–1012.
Belhassen, B., Glick, A., Rosso, R., Michowitz, Y., & Viskin, S. (2011). Atrioventricular block during radiofrequency catheter ablation of atrial flutter: incidence, mechanism, and clinical implications. Europace, 13(7), 1009–1014.
Sassone, B., Leone, O., Martinelli, G. N., & Di Pasquale, G. (2004). Acute myocardial infarction after radiofrequency catheter ablation of typical atrial flutter: histopathological findings and etiopathogenetic hypothesis. Italian Heart Journal, 5(5), 403–407.
Mykytsey, A., Kehoe, R., Bharai, S., Maheshwari, P., Halleran, S., Kousik, K., et al. (2010). Right coronary occlusion during RF ablation of typical atrial flutter. Journal of Cardiovascular Electrophysiology, 21(7), 818–821.
Conflict of interest
Ermenegildo de Ruvo received consulting honoraria from Biosense Webster, Inc.; Serena Dottori is an employee of Biosense Webster Inc. No financial or other relations that could lead to a conflict of interest is present for other authors.
Author information
Authors and Affiliations
Corresponding author
Additional information
Editorial Commentary
In a randomized study De Ruvo et al. evaluated the impact of respiratory gating on electroanatomical mapping of the cavo-tricuspid isthmus (CTI) region during catheter ablation of typical atrial flutter. The authors showed a reduction of CTI central length and right atrial volume. Furthermore a reduction of fluoroscopy and radiofrequency times was also reported. Whether such benefits translate in better long-term freedom from recurrent arrhythmia is unclear.
Rights and permissions
About this article
Cite this article
de Ruvo, E., Dottori, S., Sciarra, L. et al. Impact of respiration on electroanatomical mapping of the right atrium: implication for cavotricuspid isthmus ablation. J Interv Card Electrophysiol 36, 33–40 (2013). https://doi.org/10.1007/s10840-012-9745-7
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10840-012-9745-7