Abstract
Purpose
Catheter cryoablation (CRYO) may eliminate inadvertent atrioventricular block (AVB) in the treatment of atrioventricular nodal reentrant tachycardia (AVNRT). However, higher recurrence was observed with CRYO delivered by 4 mm or 6 mm-tip catheter. This study was performed to investigate whether a comparably low treatment failure and recurrence rate as in radiofrequency (RF) ablation is achievable by CRYO with an 8-mm-tip catheter.
Methods
This is a retrospective case–control study including 40 patients with AVNRT treated with CRYO (n = 20) using an 8 mm-tip catheter or RF ablation (n = 20) from March 2009 to March 2011. Treatment failure was defined as the composite of acute procedural failure including inadvertent permanent AVB and documented recurrence.
Results
Acute procedural success of 90% (18/20) and 95% (19/20) were achieved in CRYO and RF ablation group, respectively (p = 0.998), with no permanent AVB in either group. With Kaplan–Meier analysis, there was no significant difference between the treatment groups in terms of recurrence rate (5.6% [1/18] vs. 0%; log-rank test p = 0.304) and treatment failure (15% [3/20] vs. 5% [1/20]; log-rank test p = 0.301). Shorter fluoroscopy time (15 ± 8.6 vs. 25.2 ± 12.1 min; p = 0.005) and more energy applications (median 4 [2–15] vs. 2 [1–8]; p = 0.005) were observed in the CRYO group compared with RF ablation group.
Conclusions
Compared to RF ablation, CRYO with an 8-mm-tip catheter for treating AVNRT achieves a comparable acute procedural success, comparably low recurrence rate and composite endpoint of treatment failure. Shorter fluoroscopy time and more energy applications were observed in the CRYO group.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
1 Introduction
Catheter ablation using radiofrequency (RF) is a well-established treatment for atrioventricular nodal re-entrant tachycardia (AVNRT) [1–3]. Although RF has been shown to be safe and effective in the treatment of AVNRT, there is a small, yet genuine risk of atrioventricular block (AVB) varying from 0.4% to 3% during or after the procedure [1, 2, 4]. Catheter cryoablation (CRYO) has been tested in treating AVNRT in previous studies and no AVB has occurred [4–7]. Other advantages in using CRYO over RF ablation in the treatment of AVNRT include less fluoroscopic exposure, less patient pain perception and reduced operator stress [4, 7, 8]. Although the acute procedural success achieved by CRYO was comparable to RF ablation, the recurrence rate has been shown to be consistently higher in the CRYO group in which either a 4 mm or 6 mm-tip cryocatheter was used [4, 5, 7]. Different strategies namely, large catheter size, longer duration of cryo-application, double freezing cycles, and linear lesion cryoablation have been proposed to reduce the recurrence rate [9, 10]. Use of an 8 mm-tip cryocatheter in the treatment of AVNRT was attempted in two small studies in adult and paediatric patients respectively with satisfactory results in terms of safety, acute procedural success, and recurrence [11, 12]. However, the performance of CRYO using this large size cryocatheter compared with RF ablation has not been studied.
2 Methods
This is a retrospective case–control study. The study protocol conforms to the ethical guidelines of the 1975 Declaration of Helsinki and has been approved by the Ethics Committee of the investigation center. Twenty consecutive patients with the diagnosis of AVNRT confirmed at cardiac electrophysiology study (EPS) and treated with CRYO with an 8-mm-tip cryocatheter (Freezor Max, Medtronic, Minneapolis, MN, USA) at the investigation center from March 2009 to March 2011 were included in the study. These patients were compared with another 20 consecutive patients who underwent RF catheter ablation for AVNRT within the same period. Informed consent was obtained from each patient before the procedure. Patients with age younger than 18 or older than 80, who had congenital heart disease or other structural heart diseases or who underwent catheter ablation for recurrent AVNRT were excluded. The selection of energy source for ablation was influenced both by the preference of the operator and the informed decision of the patient.
2.1 Cardiac electrophysiology study
After obtaining informed consent, patients underwent EPS in the fasting state. Antiarrhythmic drugs were discontinued for at least five half-life periods before study. A standard EPS was performed with four catheters positioned to the right atrium, His bundle, coronary sinus, and right ventricular apex, respectively. Incremental atrial and ventricular pacing and extrastimulus testing from the right atrium and right ventricle were performed. If sustained tachycardia could not be induced, isoprenalin could be infused to facilitate tachycardia induction. Dual AV nodal physiology was identified by a sudden AH or HA jump of at least 50 ms in response to programmed atrial or ventricular extrastimulation. AVNRT was diagnosed on the basis of standard criteria [13].
2.2 Radiofrequency catheter ablation
For both RF ablation and CRYO, a combination of the electrogram and anatomical approaches was used to identify appropriate target sites for ablation of slow pathway [14].
RF ablation was performed with the use of a 4-mm-tip catheter (Conductr, Medtronic, Minneapolis, MN, USA or Livewire, St Jude Medical, Minneapolis, MN, USA). At the target site, RF current was delivered through a generator with a preset temperature of 65°C and power limit of up to 40 W. If no junctional ectopic occurred within the first 20 s of RF current delivery, the energy application would be stopped. If one or more junctional ectopic occurred, energy application would be delivered for 60 s. To enhance safety, continuous fluoroscopy was encouraged to monitor catheter position during RF delivery. RF current delivery would be stopped immediately if ventriculoatrial block during junctional ectopic or PR interval prolongation with conducted sinus beats occurred. Programmed stimulation would be performed at baseline and during isoprenalin infusion after each RF application. If AVNRT or more than one AV nodal echo beat was inducible, the ablation catheter would be repositioned and RF energy applied at a new target site. EPS would be repeated 30 min at baseline and during isoprenalin infusion after last RF application to check for the effectiveness of ablation.
2.3 Catheter cryoablation
Catheter CRYO of the slow pathway was performed with the CRYO system (Universal console, Medtronic, Minneapolis, MN, USA). A 9 Fr 8-mm-tip CRYO catheter (Freezor Max, Medtronic, Minneapolis, MN, USA) was used. In contrast with the 4-mm or 6-mm-tip ablation catheter, the function of “cryomapping” by lowering the catheter tip temperature to −30°C is not available with the 8-mm-tip catheter. After positioning of the catheter at a target site, CRYO would be started by lowering the catheter tip temperature to −75 to −80°C. Each cycle of CRYO would last for 4 min. CRYO would be stopped immediately once AH prolongation or higher degree of AVB was observed. Testing of effectiveness of the ablation by programmed electrical stimulation would be performed if AH prolongation or higher degree of AVB did not occur after the first minute of ablation. If AVNRT or more than one AV nodal echo beat was still inducible, CRYO was stopped and ablation at a new target site was attempted. Continuous fluoroscopy was discouraged after the achievement of −70°C at the catheter tip because of the phenomenon of cryoadhesion. Programmed electrical stimulation was performed after the first successful CRYO to test the effectiveness of ablation. If AVNRT was noninducible or there was only one AV nodal echo beat inducible, a “security freeze” at the same or nearby site for 4 min was performed. If AVNRT or more than one AV nodal echo beat was inducible, CRYO at another site would be attempted. EPS would be repeated 30 min at baseline and during isoprenalin infusion after last CRYO to check for the effectiveness of ablation.
2.4 Follow-up
The patients were discharged the day after procedure with no antiarrhythmic drugs. Follow-up visits were arranged at 1, 3, and 9 months, and at the discretion of the attending cardiologists, the patients would then be followed up yearly or only when they experienced recurrence of symptoms. Patients would undergo assessment of symptoms, resting ECG during follow-up. Twenty-four-hour Holter monitoring or event recording would be performed in case of symptom recurrence. Patients who defaulted clinic follow-up would be contacted by telephone for recurrence of symptoms and appropriate investigations would be arranged accordingly.
2.5 Study endpoints
The primary endpoint in this study was treatment failure denoted by a composite of acute procedural failure (including permanent first-degree, second-degree, or third-degree AVB) and documented recurrence of AVNRT. Secondary endpoints included documented recurrence of AVNRT, procedure duration, fluoroscopy time, and number of energy applications. Any non-stop energy application of more than 2 min for CRYO and 30 s for RF was counted as one energy application.
2.6 Statistical analysis
Descriptive statistics are presented for all data. For normally distributed data, they are expressed as mean and standard deviation and comparison is performed by Student's t test. For non-normally distributed data, they are expressed as median with maximum and minimum values stated, and the Mann–Whitney U test is used to assess statistical significance. For categorical data, absolute and relative frequencies are determined, and the exact 95% confidence interval is calculated. Fisher exact test or Chi-square test is performed to assess statistical significance. For statistical assessment of acute procedural failure and recurrence rate, the log-rank test and Kaplan–Meier survival analysis were performed. P value of less than 0.05 for the above statistical tests will be considered statistically significant.
3 Results
3.1 Patient characteristics
Twenty patients undergoing CRYO for AVNRT with an 8-mm-tip catheter were compared with 20 patients undergoing RF ablation for the same arrhythmia within the same period (Table 1). The baseline characteristics including age, sex, and comorbid cardiovascular diseases were comparable between the groups. Patients in the RF ablation group had significantly longer follow-up than patients in the CRYO group (Table 2).
3.2 Procedural characteristics
Three (15%) patients in the CRYO group and 1 (5%) patient in the RF group had inducible atypical AVNRT (p = 0.990). Acute procedural success rate of 90% and 95% was achieved by CRYO with 8-mm-tip catheter and RF ablation, respectively, with no significant statistical difference (p = 0.998). There were two procedural failures in the CRYO group. One of them was a 55-year-old man with typical AVNRT and no comorbid cardiovascular conditions. Cryo-applications were delivered from posterior to anterior parts of the triangle of Koch (TOK) without effect. He subsequently received RF ablation at the middle part of the TOK with power up to 50 W to achieve procedural success. The other patient was a 36-year-old woman who had typical AVNRT and no comorbid cardiovascular disease. Again, cryo-applications were delivered from posterior to anterior parts of the TOK without effect. Successful RF ablation was performed with energy application at the anterior part of the TOK. One patient who had failed RF ablation was a 23-year-old woman with typical AVNRT and no comorbid cardiovascular disease. RF ablation was attempted at the posterior and middle part of the TOK. Ablation was not performed to more anterior sites because of risk of inadvertent AVB. Cryo-application at the middle part of the TOK resulted in procedural success (Table 2).
There was no incidence of permanent AVB in either the CRYO group or RF group. There were three patients who had transient AVB during CRYO. One of them developed transient PR interval prolongation and another patient had two to one AVB. Both patients had subsidence of AVB within 45 s. The third patient developed transient atrioventricular dissociation during cryo-application at the posterior part of TOK and the AVB subsided within 20 min. Notably, all transient AVB occurred within the first minute of CRYO.
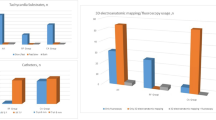
There was no significant difference in the procedure time between CRYO and RF group (135.8 ± 38.9 vs. 116.3 ± 28.7 min; p = 0.08). Fluoroscopy time in the CRYO group is significantly shorter than that of RF group (15.0 ± 8.6 vs. 25.2 ± 12.1 min; p = 0.005). The median number of energy applications is significantly higher in the CRYO group than RF group (4 vs. 2; p = 0.005).
3.3 Follow-up
The median follow-up duration in the RF group is significantly longer than that of the CRYO group (8.4 vs. 10.4 months; p = 0.038). One out of 18 patients (5.6%) who had acute procedural success had documented AVNRT recurrence in the CRYO group. No patient in the RF group experienced recurrence of arrhythmia. No statistical significance was observed between the groups (log-rank test, p = 0.304).
In terms of the primary composite endpoint which includes acute procedural failure and documented recurrence of AVNRT, there is no significant difference between the CRYO and RF groups (15% vs. 5%; log-rank test, p = 0.301) with Kaplan–Meier analysis (Fig. 1).
4 Discussion
4.1 Cryoablation with an 8-mm-tip catheter
The higher recurrence rate consistently demonstrated in the treatment of AVNRT by CRYO compared with RF ablation may be due to the biophysical properties of cryolesion [15, 16]. The ablation lesion created by CRYO using 4-mm-tip catheter has been shown to be smaller than that created by RF [15, 16]. On the other hand, the size of the cryolesion is further limited by the phenomenon of cryoadhesion which prevents catheter movement on cardiac tissues during cardiac and respiratory cycles [17]. This is in contrast with RF ablation lesion which tends to cover a larger surface area with catheter movement. Moreover, RF has the characteristic of late lesion maturation which manifests as late lesion progression by fibrosis and vascular damage [18, 19]. On the contrary, with rewarming in the border zone, cryolesion tend to contract over time [20].
Increasing the lesion size of CRYO is a potential strategy to reduce recurrence rate after AVNRT treatment [21]. The size of the cryolesion is dependent on local blood flow, electrode orientation, electrode contact pressure, and electrode size [22]. By increasing the electrode size from 4 to 6 and to 8 mm, a progressive increase in lesion volume was observed with similar lesion depth [23]. The use of a 6-mm-tip cryocatheter in the treatment of AVNRT, however, still resulted in significantly higher recurrence rate compared with RF ablation [4, 7]. This observation leads to the subsequent attempt in the use of an 8-mm-tip cryocatheter in AVNRT ablation. Under favorable catheter orientation, contact pressure, and local blood flow, lesion created by an 8-mm-tip cryocatheter may be even larger than the lesion created by a 3.5-mm-tip saline-irrigated RF catheter [24]. Silver et al. [12] has shown that AVNRT ablation by using 8-mm-tip cryocatheter was safe and effective with a low recurrence rate. Whether CRYO using an 8-mm-tip catheter to create a larger lesion in the TOK reduces the recurrence rate comparable with RF ablation, and at the same time preserves the “AVB-free” property, is the focus of the current study.
4.2 Recurrence
The recurrence rate after AVNRT treatment by CRYO varies from 7–20% with the use of a 4-mm-tip cryocatheter [5, 24, 25]. Despite the use of a 6-mm-tip cryocatheter, the recurrence rate is still significantly higher than RF ablation and is around 9% in a retrospective case–control study [7] subsequently confirmed by a recently published large randomized controlled trial [4]. In the current study, 20 patients underwent CRYO with an 8-mm-tip catheter for the treatment of AVNRT, an acute procedural success of 90% was achieved and 1 (5.6%) patient had documented recurrence with a median follow-up of 8.4 months. Comparing with the data reported by Silver et al. [12], the acute procedural success of 91% was similar, while the recurrence rate of 2.8% was slightly better. In either study, the recurrence rate seems to be lower than that observed in studies in which 6-mm-tip cryocatheter was used. In our study, another 20 patients undergoing RF ablation for AVNRT in the same period were compared with the CRYO cohort. In the RF ablation group, the acute procedural success was 95% and no patient had recurrence of AVNRT with median follow-up of 10.4 months. There was no significant difference in the recurrence rate between the CRYO and RF groups (log-rank test, p = 0.304). The shorter median follow-up in the CRYO group compared to RF group (8.4 vs. 10.4; p = 0.038) may under-estimate the recurrence rate in the former. Non-inferiority in recurrence rate between CRYO with an 8-mm-tip catheter and RF ablation has to be confirmed by larger scale randomized control study.
4.3 Atrioventricular block
Since there is only 0.4–3% risk of inadvertent AVB in RF ablation for AVNRT[1–2.4], a randomized controlled study of large sample size is required to confirm the advantage of CRYO in this aspect. In a recent review, pooled data from nine studies comparing CRYO with RF ablation for AVNRT, 6 out of 917 patients (0.65%) suffered from inadvertent permanent AVB during RF ablation [26]. Although there was a 4–23% of transient inadvertent AVB in the CRYO group, no patients developed permanent AVB. The potential of an 8-mm-tip cryocatheter to produce a larger lesion leading to lower recurrence rate is desirable. However, whether the merit of “AVB-free” property is preserved without the function of “cryomapping” by lowering the catheter tip temperature to −30°C is still unknown. In the study performed by Silver et al. [12], 5 out of 77 (6.5%) patients undergoing AVNRT CRYO with an 8-mm-tip catheter developed transient AVB, while no patients had inadvertent permanent AVB. It has been shown in an animal study that there was a safety window of at least 10 s after development of AVB created by an 8-mm-tip cryocatheter, meaning that by stopping CRYO within 10 s of detection of AVB, permanency of this complication can be prevented [27]. In our study, development of AVB during CRYO was monitored throughout the whole ablation cycle especially during the first minute of application. CRYO would be immediately stopped once AVB was observed. Transient AVB was observed in three patients and all developed this complication within the first minute of cryo-application. AVB subsided within 45 s in two patients and within 20 min in one patient. Our findings are in consistency with the animal study [27] and the approach of stopping CRYO with an 8-mm-tip catheter prevents the development of permanent AVB even without the function of “cryomapping”. The potential ability of CRYO to eliminate the small but genuine risk of inadvertent AVB associated with RF ablation for AVNRT makes this technology especially appealing for young patients. The safety of this approach has to be tested further in future studies with larger sample size.
4.4 Primary composite endpoint
Treatment failure of catheter ablation for AVNRT can be measured by the primary composite endpoint of acute procedural failure including permanent inadvertent AVB and recurrence. CRYO with a 4- or 6-mm-tip catheter has been consistently shown to be inferior to RF ablation in terms of this primary composite endpoint or treatment failure in randomized control studies [4, 5]. The acute procedural success was similar in both groups. However, the gain in AVB is negated by the increase in recurrence rate. In the current study, no significant difference was present in the primary composite endpoint between the CRYO and RF ablation groups (15% vs. 5%; p = 0.301). Again, the small sample size in our study makes our data speculative rather than confirmatory. With a larger sample size, significant difference in the primary composite endpoint favoring RF ablation group may be observed.
4.5 Procedural characteristics
Whether there is a difference in procedure time or fluoroscopy time between CRYO and RF ablation for AVNRT is not agreeable among the published studies. In some studies, the procedure time was longer in the CRYO group [4, 5]. In other studies, the procedure time was similar between different treatment groups [6, 7]. Fluoroscopy time was shown to be shorter in CRYO group in some studies [7, 8] but similar between the treatment groups in others [4–6]. In the current study, between CRYO group and RF ablation group, the procedure time (135.8 ± 38.9 vs. 116.3 ± 28.7 min; p = 0.08) was similar and the fluoroscopy time was significantly shorter in the former (15.0 ± 8.6 vs. 25.2 ± 12.1; p = 0.005). Longer procedure time in CRYO group in some studies may be related to learning curve of new technique. In our study, CRYO were performed by a single operator who had significant experience with CRYO in treating AVNRT and other arrhythmias like atrioventricular reentrant tachycardia, atrial flutter, and atrial fibrillation. This may explain the comparable procedural time in the CRYO and RF ablation group. The fluoroscopy time is significantly shorter in the CRYO group than in RF group. The reduction of fluoroscopy time in the CRYO group in this study may be related to the phenomenon of cryoadhesion which facilitated minimization of fluoroscopy screening during CRYO. In contrast, continuous fluoroscopy was required during RF ablation. The median number of energy applications was 2 (1–8) in the RF group and 4 (2–15) in the CRYO group, and the difference is statistically significant (p = 0.005). The number of energy applications in our study is similar to what was reported from the study of Deisenhofer et al. [4] in which a 6-mm-tip cryocatheter was used. The failure to reduce the number of energy applications in our study despite the use of a larger tip cryocatheter may reflect the less accurate mapping with the use of an 8-mm-tip catheter. On the other hand, we recorded less RF applications in our study compared with Deisenhofer's (2 vs. 4). In our study, one energy application is defined as any non-stop energy application of more than 2 min for CRYO and 30 s for RF. Exact definition for counting the number of energy application was not found in the previous studies. The discrepancy of results may be due to a difference in the relevant methodology.
5 Limitations
This is a retrospective case–control study with known limitations. The sample size of the study is small, and true difference between CRYO and RF ablation group may not be detected. The median follow-up duration is significantly shorter in the CRYO group and the recurrence rate in that group may be under-estimated. However, the absolute difference in follow-up was only 2 months, and all patients have completed the 6-month follow-up.
6 Conclusions
In this small retrospective case–control study, treatment of AVNRT by CRYO with an 8-mm-tip catheter was compared with RF ablation. No statistically significant difference was found in the acute procedural success, recurrence rate, and treatment failure (acute procedural failure including permanent AVB plus recurrence) between the two treatment groups. Permanent AVB was not observed in either group. Shorter fluoroscopy time and more energy applications were observed in the CRYO group. Future large randomized control study is warranted to confirm the non-inferiority of CRYO with 8-mm-tip catheter in treatment failure compared with RF ablation in treating patients with AVNRT. CRYO with 8-mm-tip catheter may become a more attractive treatment option than RF ablation in AVNRT especially for young patients with its potential of eliminating small but genuine risk of inadvertent AVB associated with RF ablation and comparable treatment failure.
References
Calkins, H., Yong, P., Miller, J. M., Olshansky, B., Carlson, M., Saul, J. P., et al. (1999). Catheter ablation of accessory pathways, atrioventricular nodal reentrant tachycardia, and the atrioventricular junction: final results of a prospective, multicenter clinical trial. The Atakr Multicenter Investigators Group. Circulation, 99, 262–270.
Hindricks, G. (1993). The Multicenter European Radiofrequency Survey (MERFS): complications of radiofrequency catheter ablation of arrhythmias. The Multicenter European Radiofrequency Survey (MERFS) investigators of the Working Group on Arrhythmias of the European Society of Cardiology. European Heart Journal, 14, 1644–1653.
Morady, F. (2004). Catheter ablation of supraventricular arrhythmias: state-of-the-art. Journal of Cardiovascular Electrophysiology, 15, 124–139.
Deisenhofer, I., Zrenner, B., Yin, Y. H., Pitschner, H. F., Kuniss, M., Grobmann, G., et al. (2010). Cryoablation versus radiofrequency energy for the ablation of atrioventricular nodal reentrant tachycardia (the CYRANO study). Results from a large multicenter prospective randomized trial. Circulation, 122, 2239–2245.
Zrennar, B., Dong, J., Schreieck, J., Deisenhofer, I., Estner, H., Luani, B., et al. (2004). Transvenous cryoablation versus radiofrequency ablation of the slow pathway for the treatment of atrioventricular nodal reentrant tachycardia: a prospective randomized pilot study. European Heart Journal, 25, 2226–2231.
Kimman, G. P., Theuns, D. A. M. J., Szili-Torok, T., Scholten, M. F., Res, J. C., & Jordaens, L. T. (2004). CRAVT: a prospective, randomized study comparing transvenous cryothermal and radiofrequency ablation in atrioventricular nodal reentrant tachycardia. European Heart Journal, 25, 2232–2237.
Chan, N. Y., Mok, N. S., Lau, C. L., Lo, Y. K., Choy, C. C., Lau, S. T., et al. (2009). Treatment of atrioventricular nodal re-entrant tachycardia by cryoablation with a 6 mm-tip catheter vs radiofrequency ablation. Europace, 11(8), 1065–1070.
Chan, N. Y., Choy, C. C., Lau, C. L., Lo, Y. K., Chu, P. S., Yuen, H. C., et al. (2011). Cryoablation versus radiofrequency ablation for atrioventricular nodal re-entrant tachycardia: patient pain perception and operator stress. Pacing and Clinical Electrophysiology, 34(1), 2–7.
Schwagten, B., van Belle, Y., & Jordaens, L. (2010). Cryoablation: how to improve results in atrioventricular nodal reentrant tachycardia ablation. Europace, 12(11), 1522–1525.
Czosek, R. J., Anderson, J., Marino, B. S., Connor, C., & Knilans, T. K. (2010). Linear lesion cryoablation for the treatment of atrioventricular nodal re-entry tachycardia in paediatrics and adult patients. Pacing and Clinical Electrophysiology, 33, 1304–1311.
Dumont, F., Khairy, P., Guerra, P. G., Talajic, M., Roux, J. F., Thibault, B., et al. (2007). Initial experience with an 8 mm tip cryocatheter for slow pathway modification in AVNRT (abstract). Heart Rhythm, 4, S113.
Silver, E. S., Silva, J. N. A., Ceresnak, S. R., Chiesa, N. A., Rhee, E. K., Dubin, A. M., et al. (2010). Cryoablation with an 8 mm tip cryocatheter for pediatric AVNRT is safe and efficacious with a low incidence of recurrence. Pacing and Clinical Electrophysiology, 33(6), 681–686.
Jackman, W. M. (1995). Three forms of atrioventricular nodal (junctional) reentrant tachycardia: differential diagnosis, electrophysiological characteristics, and implications for anatomy of the reentrant circuit. In D. P. Zipes & J. Jalife (Eds.), Cardiac electrophysiology. From cell to bedside (pp. 620–637). Philadelphia (PA): WB Saunders.
Kalbfleisch, S. J., Strickberger, S. A., Williamson, B., Vorperian, V. R., Man, C., Hummel, J. D., et al. (1994). Randomized comparison of anatomic and electrogram mapping approaches to ablation of the slow pathway of atrioventricular node reentrant tachycardia. Journal of the American College of Cardiology, 23, 716–723.
Khairy, P., Chauvet, P., Lehmann, J., Lambert, J., Macle, L., Tanguay, J. F., et al. (2003). Lower incidence of thrombus formation with cryoenergy versus radiofrequency catheter ablation. Circulation, 107, 2045–2050.
Rodriguez, L. M., Leunissen, J., Hockstra, A., Korteling, B. J., Smeets, J. L., Timmermans, C., et al. (1998). Transvenous cold mapping and cryoablation of the AV node in dogs: observation of chronic lesions and comparison to those obtained using radiofrequency ablation. Journal of Cardiovascular Electrophysiology, 9, 1055–1061.
Arentz, T. (2005). Return to the ice age? Journal of Cardiovascular Electrophysiology, 16, 370–371.
Fenelon, G., & Brugada, P. (1996). Delayed effects of radiofrequency energy: mechanisms and clinical implications. Pacing and Clinical Electrophysiology, 19, 484–489.
Nath, S., Whayne, J. G., Kaul, S., Goodman, N. C., Jayaweera, A. R., & Haines, D. E. (1994). Effects of radiofrequency catheter ablation on regional myocardial blood flow. Possible mechanism for late electrophysiological outcome. Circulation, 89, 2667–2672.
Lustgarten, D. L., Keane, D., & Ruskin, J. (1999). Cryothermal ablation: mechanism of tissue injury and current experience in the treatment of tachyarrhythmias. Progress in Cardiovascular Diseases, 41, 481–498.
Schwagten, B., Van Belle, Y., & Jordaens, L. (2010). Cryoablation: how to improve results in atrioventricular nodal re-entrant tachycardia ablation? Europace, 12(11), 1522–1525.
Wood, M. A., Parvez, B., Ellenbogen, A. L., Shaffer, K. M., Goldberg, S. M., Gaspar, M. P., et al. (2007). Determinants of lesion sizes and tissue temperatures during catheter cryoablation. Pacing and Clinical Electrophysiology, 30, 644–659.
Khairy, P., Rivard, L., Guerra, P. G., Tanguay, J.-F., Roy, D., Mawad, W., et al. (2008). Morphomeric ablation lesion characteristics comparing 4, 6 and 8 mm electrode-tip cryocatheter. Journal of Cardiovascular Electrophysiology, 19(11), 1203–1207.
Collins, K. K., Dubin, A. M., Chiesa, N. A., Avasarala, K., & Van Hare, G. F. (2006). Cryoablation versus radiofrequency ablation for treatment of pediatric atrioventricular reentrant tachycardia: initial experience with 4-mm cryocatheter. Heart Rhythm, 3, 564–570.
Gupta, D., Al-Lamee, R. A., Earley, M. J., Kistler, P., Harris, S. J., Nathan, A. W., et al. (2006). Cryoablation compared with radiofrequency ablation for atrioventricular nodal re-entrant tachycardia: analysis of factors contributing to acute and follow-up outcome. Europace, 8, 1022–1026.
De Sisti, A., & Tonet, J. (2011). Cryoablation of atrioventricular nodal reentrant tachycardia: a clinical review. Pacing and Clinical Electrophysiology (in press).
Atienza, F., Almendral, J., Sanchez-Quintana, D., Zaballos, M., Murillo, M., Jimeno, C., et al. (2009). Cryoablation time-dependent dose–response effect at minimal temperatures (−80°C): an experimental study. Europace, 11, 1538–1545.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Chan, Ny., Mok, Ns., Choy, Cc. et al. Treatment of atrioventricular nodal re-entrant tachycardia by cryoablation with an 8-mm-tip catheter versus radiofrequency ablation. J Interv Card Electrophysiol 34, 295–301 (2012). https://doi.org/10.1007/s10840-012-9670-9
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10840-012-9670-9