Abstract
Purpose
Modulation of the intrinsic cardiac autonomic nervous system (ICANS) has been described to occur during radiofrequency pulmonary vein (PV) isolation for paroxysmal atrial fibrillation (AF) and has been controversially proposed to increase clinical success. Energy source used for PV isolation might influence ICANS modulation. The effect of balloon-delivered cryoenergy on the ICANS is unknown. We conducted a study investigating acute periprocedural effect on ICAN as well as changes in heart rate variability (HRV) for standard deviation of normal-to-normal intervals (SDNN) and triangular index (TI) as surrogates for ICANS modulation after cryoballoon PV isolation.
Methods
Fourteen consecutive patients without structural heart disease underwent cryoballoon PV isolation for paroxysmal atrial fibrillation. Acute changes in heart rate requiring pacing during the procedure were recorded. HRV was tested by Holter ECG for SDNN and TI before ablation and after 1 week, 1 month, and 3 months following ablation.
Results
Fifty-five out of 56 PV were isolated (98%) with short-term 6-month freedom from paroxysmal AF of 64% by one single procedure. Five patients (36%) showed significant bradycardia during balloon thawing requiring temporary pacing. HRV decreased significantly immediately after PV isolation for both SDNN and TI until 1 month, gradually normalizing toward 3 months follow-up. HRV modulation was not different between patients with or without AF recurrences.
Conclusions
Cryoballoon PV isolation significantly modulates the ICANS, but only temporarily for up to 3 months, measured by HRV changes after ablation.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
1 Introduction
Catheter ablation has evolved as effective treatment option in symptomatic paroxysmal atrial fibrillation and is currently being investigated for first-line therapy in selected cases. After identifying focal activity within the pulmonary veins [1], pulmonary vein (PV) isolation has become the cornerstone in interventional treatment of paroxysmal atrial fibrillation [2].
The mechanism responsible for the cure of atrial fibrillation (AF) after PV isolation is still unresolved. It has been speculated that the inadvertent ablation of the adjacent intrinsic cardiac autonomic nervous system (ICANS) may play a crucial role for successful ablation of AF. The ICANS consists of an interconnected neural network composed of four ganglionated plexi (GP) at the posterior aspect of the left atrium, whereas free nerve endings aggregate subendothelially on the anterior surface of the pulmonary veins. The four ganglionated plexi are located epicardially in fat pads at the right superior pulmonary vein (anterior right GP), at the junction of the inferior vena cava and both atria (inferior right GP), at the root of the left superior vein (superior left GP), and at the root of the left inferior pulmonary vein (inferior left GP). Due to the proximity of GPs at the PV ostia, it is still unclear whether the disruption of electrical conduction of PVs or solely the destruction of the GPs is the clue for the cure of AF ablation.
Ablation of GPs is sufficient to significantly decrease the AF inducibility in dogs at both the ipsilateral and contralateral pulmonary veins [3]. Intact conducting PVs were not necessary whereas intact GPs were necessary for the maintenance of cholinergic AF in another dog model [4].
The results in human atrial fibrillation are controversial due to different patient populations, procedural approaches, and endpoint definition. Studies investigating ablation of the ganglia only without additional PV isolation were associated with an unfavorable outcome concerning AF recurrence or AF inducibility as compared to circumferential pulmonary vein isolation [5–8].
Parameters calculated from Holter ECG recordings such as heart rate variability (HRV) serve as surrogates for ICANS modulation [9]. It has been shown that circumferential PV isolation of left and right PVs by RF ablation significantly influences cardiac autonomic innervation with a significant reduction of HRV during short-term observations of 5-min ECG samples compared to HRV before PV isolation [10]. Longer observations showed significantly decreased HRV for a limited period of approximately 3–6 months duration after circumferential radiofrequency PV isolation [8]. Improvement of clinical success was proposed for patients, who demonstrated significant HRV reduction during follow-up, undergoing circumferential PV isolation by RF ablation [8].
Balloon-delivered cryoablation on the other hand has recently been introduced as effective alternative ablation energy for PV isolation [11–14]. The present prospective single-center study investigates whether cryoballoon ablation of pulmonary veins, similar to radiofrequency ablation of pulmonary veins, is associated with a modulation of the ICANS as assessed by acute bradycardia during ablation as well as changes of HRV. Since phrenic nerve injury evoked by cryoenergy or radiofrequency is different concerning reversibility (100% reversibility after cryoenergy ablation vs. only 60–70% reversibility after radiofrequency ablation) [15], it is of importance to know whether cryoenergy might affect the ICANS as extensive and sustained as radiofrequency energy does and whether the success of cryoablation relies on ICAN modulation.
2 Methods
Fourteen consecutive patients without structural heart disease, scheduled for pulmonary vein isolation for symptomatic paroxysmal atrial fibrillation, were eligible for the study. All patients gave written informed consent for use of data. A Holter ECG recorded on the day before interventional treatment for analysis of heart rate variability during sinus rhythm was acquired in all cases. Standard deviation of normal-to-normal intervals (SDNN) and triangular index (TI) were calculated from Holter data.
Holter analysis was performed using the pathfinder software (Pathfinder Digital V8.257, Reynolds Medical Ltd., Edinburgh, UK). Artifacts, premature complexes, and short atrial runs were manually edited and excluded from calculation. Patients presenting in persistent atrial fibrillation before ablation were excluded.
Pulmonary vein isolation was performed as described previously [12]. The entire ablation procedure was performed during mild sedation with midazolam and morphine as needed in 13 patients while one had asked for general anesthesia for additional general anxiety disorder. Briefly, following transesophageal echocardiography for exclusion of intra-atrial thrombi shortly before the procedure during oral anticoagulation pause, venous access was achieved for a hexapolar coronary sinus (CS) catheter (Bard, Lowell, MA,USA) and a quadropolar catheter (Bard, Lowell, MA, USA) in the superior vena cava (SVC) via two left femoral vein punctures. The right femoral vein was used to access the left atrium via transseptal puncture using standard fluoroscopy-guided technique with SR-0 sheath (Fast Cath SR-0, St. Jude Medical, Minnetonka, MN, USA) and transseptal needle (BRK-1, St. Jude Medical, Minnetonka, MN, USA) before changing to the steerable transseptal sheath (Flexcath sheath, Cryocath, Montreal, Canada) over the wire with a stable wire position inside the left superior PV. Heparin was administered after positioning of the steerable sheath in the left atrium. PV angiography was performed using the steerable sheath for identification of individual PV anatomy, in particular the left atrium to PV junction region and on the other hand for the localization of different PV branches for strategic planning of wire positioning during later balloon stabilization during ablation. A large 28-mm-diameter cryoballoon (Arctic Front, Cryocath, Montreal, Canada) was used for all cases in order to allow proximal isolation of all PVs, making complete PV isolation the prespecified endpoint of the procedure. Using the cryoballoon stabilized over the wire inside the PV to atrium junction region, one PV after another was treated with each freeze lasting 300 s. Optimum PV balloon occlusion was proven by contrast media injection through the distal tip of the inflated balloon. Ablation was started when no or only minimal leakage was shown. Pacing of the right phrenic nerve within the SVC was performed in order to show intact nerve conduction during ablation of the right superior PV. No endocardial pacing maneuvers within the left atrium were performed in order to identify ICANS. Temporal CS left atrial pacing was performed for a few seconds until recovery, when a symptomatic decrease in sinus node rate below 40 beats per minute or pauses during or after PV cryoablation occurred. Following ablation of all PVs, a lasso-shaped duodecapolar adjustable mapping catheter (Inquiry Optima, Irvine Biomedical, Irvine, CA, USA) was used to confirm successful proximal isolation of all PVs as previously described. Balloon ablation was repeated in nonisolated veins until they were successfully treated. The activated clotting time was kept at 300–350 s during the procedure and oral anticoagulation was reinitiated the following day. Previous antiarrhythmic medication was kept unchanged during and after ablation until the 3-month follow-up. After this period, class I and class III antiarrhythmic drugs were terminated in patients free of paroxysmal AF.
Postinterventional follow-up included 12 lead ECG, Holter recordings with arrhythmia analysis, and HRV measurements as well as clinical assessment. Follow-up was performed within the first week after ablation before hospital discharge and during scheduled outpatient visits after 1 month and after 3 months.
SPSS statistics software (SPSS 15.0 for Windows, SPSS, Chicago, IL, USA) was used for statistical calculations. A two-sided paired Student’s t test was used for calculation of significance. A p < 0.5 was considered statistically significant.
3 Results
Fourteen consecutive symptomatic, predominantly male patients without structural heart disease receiving cryoballoon PV isolation for paroxysmal atrial fibrillation were included. Arterial hypertension was present in most cases but without enlarged left atrial diameters as measured by echocardiography as presented in Table 1.
Successful ablation of all PV was achieved in all but one case. A total number of 56 PVs were treated in 14 patients with successful isolation of 55 PVs (98%) leaving one PV with only significant conduction delay. Each patient received 13 ± 2.3 (range 10–17) cryoballoon ablations. Additional touch-up cryoablation (FreezorMax, Cryocath, Montreal, Canada) of a single PV was performed in three patients. The acute single balloon-only success rate of PV isolation was 92%. One right phrenic nerve palsy was observed during isolation of the right superior PV during continuous phrenic nerve pacing after approximately 120 s of ablation; therefore, ablation was immediately terminated with complete recovery of the nerve after 3 min. Five patients (36%) required temporary cardiac pacing for less than 30 s, each due to symptomatic sinus bradycardia and pauses, which occurred during cryoballoon thawing, particularly immediately after balloon thawing and deflation. Interestingly, four of them were later judged as free from paroxysmal AF. Complete normalization of sinus node rate was observed in all cases after pacing. Fluoroscopy duration was 34 ± 10 min (range 20–54 min).
Clinical success 6 months after initial PV isolation procedure, defined as freedom from symptomatic paroxysmal atrial fibrillation in clinical assessment, 12 lead ECG, and Holter ECG recordings was observed in nine patients (64%) after one single procedure cryoballoon PV isolation during 6 months follow-up. One patient underwent a second procedure showing reconduction in three out of four PVs and was free from paroxysmal AF following the second procedure. One patient underwent ablation of the right atrial cavotricuspid isthmus for documented atrial flutter and was free of paroxysmal AF thereafter. One patient received a pacemaker for symptomatic sick sinus syndrome with chronotropic incompetence during sinus rhythm and recurrent syncope following spontaneous AF termination, while a diagnostic invasive study showed persistent isolation of all PVs 5 months after the initial procedure.
Following cryoballoon PV isolation, a significant change in the cardiac autonomic tone indicated by HRV could be detected in a time-dependent way. Immediately after the procedure, a significant drop of SDNN was observed with a mean drop by 31 ± 28.6 ms to a significantly depressed mean SDNN of 84 ± 34.9 ms, while baseline SDNN values were normal in all cases before cryoballoon PV isolation (115 ± 13 ms). The ablation-induced change persisted during the first months after PV isolation and showed normalization of SDNN after 3 months. HRV analysis was performed until the 3-month follow-up. Individual development of HRV is displayed in Fig. 1(a); mean SDNN is shown in Fig. 2(a). SDNN returned to baseline levels 3 months after ablation.
(a) Standard deviation of normal-to-normal intervals measured by Holter ECG before ablation and after ablation within the first week, after 1 month, and after 3 months follow-up. Data presented as mean values ± standard deviation. *p = 0.01; **p = 0.09. (b) Triangular index measured by Holter ECG before ablation and after ablation within the first week, after 1 month, and after 3 months follow-up. Data presented as mean values ± standard deviation. *p = 0.01; **p = 0.02
Similar changes were detected measuring the triangular index as shown individually for each patient in Fig. 1(b). With normal baseline values before cryoballoon PV isolation (31 ± 2.8), a significant mean drop by 8 ± 10.5 was observed during the first week, again with normalization of this parameter 3 months after the initial procedure as shown in Fig. 2(b). Changes of SDNN and TI from baseline values are shown in Fig. 3. The ablation-induced decrease in HRV was observed in 12 out of 14 patients (86%). The two patients without significant changes of HRV shown in Fig. 1 were judged as free from paroxysmal AF during follow-up. Symptomatic bradycardia suggesting vagal reaction during ablation and thawing was present in one of the two patients without HRV decrease during follow-up. The three largest reductions of HRV occurred in patients without reflex bradycardia during the procedure.
Patients without AF recurrences (nine patients) and patients with AF recurrence (five patients) after a single procedure did not show differences of left atrial diameter (38.9 ± 4.7 vs. 35.4 ± 7.8 mm, p = 0.31). The calculated changes from baseline levels in SDNN and TI as shown in Fig. 3 did not differ between both subgroups neither within the first week, at 1 month nor at 3 months after ablation.
4 Discussion
Our study demonstrates significant changes in heart rate variability, a surrogate for ICANS modulation, following cryoballoon PV isolation for paroxysmal atrial fibrillation. However, modulation of the ICANS did not sustain for more than 3 months after ablation, and modulation of the ICANS was not associated with freedom of AF recurrence. The population was a typical cohort for patients with paroxysmal AF without structural heart disease with normal left ventricular function, no significant valvular disease, and normally sized left atria, predominantly with a history of hypertension.
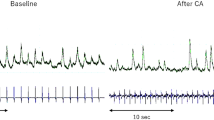
Acute procedural success of 98%, defined as PV isolation, and clinical success (64% at 3 and 6 months), defined as freedom from atrial fibrillation, are consistent with previously published reports on larger numbers of patients treated by cryoballoon PV isolation [11–14]. Temporary sinus bradycardia or even pauses have not been described systematically for cryoballoon ablation of paroxysmal atrial fibrillation. We attribute this observation to cardiac autonomic plexus modulation, which was observed frequently in 36% of the procedures. Bradycardia observed during balloon-based cryoablation was only observed during the period of balloon (and tissue) thawing and balloon deflation following thawing at the end of ablation. Cell damage from cryoablation occurs during cell freezing on the one hand and cell thawing followed by reactive hyperemia and edema on the other hand [16]. We hypothesize that bradycardia during the balloon and tissue thawing period may be induced by balloon-induced stretch of left atrial tissue, hyperemia within cryoablation border zones, or possibly by direct damage to cardiac nerve tissue during thawing. This observation may be similar to reflex bradycardia during mapping of plexi by rapid endocardial pacing. Reflex bradycardia was not associated with a better clinical outcome or greater change of HRV during follow-up.
Our study population consisted of nondilated left atria only. Only large 28-mm balloons were used, which have been shown to create PV isolation in the PV to left atrium junction region [17]. We hypothesize that balloon-based cryoablation not within the PV but at the PV to left atrium junction region would affect cardiac ganglia. Hence, cryoballoon PV isolation is associated with a significant decrease of HRV in a time-dependent manner. The greatest decrease of SDNN and TI can be detected shortly after PV isolation. Gradual recovery of HRV is observed with normalization of HRV levels after 3 months. This finding confirms previous data from circumferential radiofrequency PV isolation. RF ablation of GPs as indicated by depressed HRV was associated with a better clinical outcome [8]. However, superiority of GP ablation was shown by excluding early AF recurrences. This classification for early and late recurrences has not been used in our study. Most of the other studies comparing circumferential PV isolation with GP ablation only could not find superior results with GP ablation [5–7]. Thus, cryoenergy appears to behave quite similar to radiofrequency energy concerning ICANS modulation.
The recovery of HRV and triangular index in our study 3 months after ablation might be due to cardiac reinnervation. This has also been shown after heart transplantation [18, 19]. Since epicardial modulation of the ICANS is only temporary, we speculate that GP modulation is rather a bystander than essential part of the cure in cryoballoon PV isolation.
5 Conclusion
Cryoballoon PV isolation significantly modulates the ICANS, but only temporarily for up to 3 months, as assessed by HRV parameters SDNN and TI. HRV modulation did not indicate clinical cryoballoon ablation success. Thus, ICANS modulation may likely represent a bystander effect rather than necessary target for cryoballoon ablation of paroxysmal AF.
6 Limitations
Our study is limited by the fact that we included patients with paroxysmal atrial fibrillation and did not exclusively study patients with clinical characteristics of vagal atrial fibrillation such as bradycardia-associated and nocturnal atrial fibrillation episodes. Persistent ICANS modulation might be of utmost importance in this subgroup of AF patients.
This study describes temporary modulation of the ICANS in the majority of patients following cryoballoon PV isolation. However, follow-up using single Holter ECG recordings may be insufficient in proving freedom from atrial fibrillation. Considering its small sample size of 14 patients, our study is not powered to prove that ablation of ICANS is essential in achieving clinical long-term success. A larger study is needed to investigate the role of ICANS cryomodulation on long-term freedom from atrial fibrillation.
References
Haissaguerre, M., Jais, P., Shah, D. C., Takahashi, A., Hocini, M., Quiniou, G., et al. (1998). Spontaneous initiation of atrial fibrillation by ectopic beats originating in the pulmonary veins. The New England Journal of Medicine, 339(10), 659–666.
Fuster, V., Ryden, L. E., Cannom, D. S., Crijns, H. J., Curtis, A. B., Ellenbogen, K. A., et al. (2006). ACC/AHA/ESC 2006 guidelines for the management of patients with atrial fibrillation: a report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines and the European Society of Cardiology Committee for Practice Guidelines (Writing Committee to Revise the 2001 Guidelines for the Management of Patients With Atrial Fibrillation): developed in collaboration with the European Heart Rhythm Association and the Heart Rhythm Society. Circulation, 114(7), e257–e354.
Lu, Z., Scherlag, B. J., Lin, J., Yu, L., Guo, J. H., Niu, G., et al. (2009). Autonomic mechanism for initiation of rapid firing from atria and pulmonary veins: evidence by ablation of ganglionated plexi. Cardiovascular Research, 84(2), 245–252.
Lemola, K., Chartier, D., Yeh, Y. H., Dubuc, M., Cartier, R., Armour, A., et al. (2008). Pulmonary vein region ablation in experimental vagal atrial fibrillation: role of pulmonary veins versus autonomic ganglia. Circulation, 117(4), 470–477.
Danik, S., Neuzil, P., d’Avila, A., Malchano, Z. J., Kralovec, S., Ruskin, J. N., et al. (2008). Evaluation of catheter ablation of periatrial ganglionic plexi in patients with atrial fibrillation. The American Journal of Cardiology, 102(5), 578–583.
Katritsis, D., Giazitzoglou, E., Sougiannis, D., Goumas, N., Paxinos, G., & Camm, A. J. (2008). Anatomic approach for ganglionic plexi ablation in patients with paroxysmal atrial fibrillation. The American Journal of Cardiology, 102(3), 330–334.
Scanavacca, M., Pisani, C. F., Hachul, D., Lara, S., Hardy, C., Darrieux, F., et al. (2006). Selective atrial vagal denervation guided by evoked vagal reflex to treat patients with paroxysmal atrial fibrillation. Circulation, 114(9), 876–885.
Pappone, C., Santinelli, V., Manguso, F., Vicedomini, G., Gugliotta, F., Augello, G., et al. (2004). Pulmonary vein denervation enhances long-term benefit after circumferential ablation for paroxysmal atrial fibrillation. Circulation, 109(3), 327–334.
Redfearn, D. P., Skanes, A. C., Gula, L. J., Griffith, M. J., Marshall, H. J., Stafford, P. J., et al. (2007). Noninvasive assessment of atrial substrate change after wide area circumferential ablation: a comparison with segmental pulmonary vein isolation. Annals of Noninvasive Electrocardiology, 12(4), 329–337.
Ketels, S., Houben, R., Van Beeumen, K., Tavernier, R., & Duytschaever, M. (2008). Incidence, timing, and characteristics of acute changes in heart rate during ongoing circumferential pulmonary vein isolation. Europace, 10(12), 1406–1414.
Neumann, T., Vogt, J., Schumacher, B., Dorszewski, A., Kuniss, M., Neuser, H., et al. (2008). Circumferential pulmonary vein isolation with the cryoballoon technique results from a prospective 3-center study. Journal of the American College of Cardiology, 52(4), 273–278.
Klein, G., Oswald, H., Gardiwal, A., Lusebrink, U., Lissel, C., Yu, H., et al. (2008). Efficacy of pulmonary vein isolation by cryoballoon ablation in patients with paroxysmal atrial fibrillation. Heart Rhythm, 5(6), 802–806.
Malmborg, H., Lonnerholm, S., & Blomstrom-Lundqvist, C. (2008). Acute and clinical effects of cryoballoon pulmonary vein isolation in patients with symptomatic paroxysmal and persistent atrial fibrillation. Europace, 10(11), 1277–1280.
Van Belle, Y., Janse, P., Rivero-Ayerza, M. J., Thornton, A. S., Jessurun, E. R., Theuns, D., et al. (2007). Pulmonary vein isolation using an occluding cryoballoon for circumferential ablation: feasibility, complications, and short-term outcome. European Heart Journal, 28(18), 2231–2237.
Klein, G., Gardiwal, A., & Oswald, H. (2008). Catheter-based cryoablation of atrial fibrillation: state of the art. Minerva Cardioangiologica, 56(6), 623–633.
Lustgarten, D. L., Keane, D., & Ruskin, J. (1999). Cryothermal ablation: mechanism of tissue injury and current experience in the treatment of tachyarrhythmias. Progress in Cardiovascular Diseases, 41(6), 481–498.
Van Belle, Y., Knops, P., Janse, P., Rivero-Ayerza, M., Jessurun, E., Szili-Torok, T., et al. (2009). Electro-anatomical mapping of the left atrium before and after cryothermal balloon isolation of the pulmonary veins. Journal of Interventional Cardiac Electrophysiology, 25(1), 59–65.
Singh, T. P., Gauvreau, K., Rhodes, J., & Blume, E. D. (2007). Longitudinal changes in heart rate recovery after maximal exercise in pediatric heart transplant recipients: evidence of autonomic re-innervation? The Journal of Heart and Lung Transplantation, 26(12), 1306–1312.
Yap, K. S., Gould, P., Kalff, V., Kaye, D. M., Esmore, D., & Kelly, M. J. (2006). Evaluation of sympathetic re-innervation in heterotopic cardiac transplants by iodine-123-metaiodobenzylguanidine (I-123-MIBG) imaging. The Journal of Heart and Lung Transplantation, 25(8), 977–980.
Conflict of interest statement
There are no conflicts of interests for any author of this manuscript.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Oswald, H., Klein, G., Koenig, T. et al. Cryoballoon pulmonary vein isolation temporarily modulates the intrinsic cardiac autonomic nervous system. J Interv Card Electrophysiol 29, 57–62 (2010). https://doi.org/10.1007/s10840-010-9491-7
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10840-010-9491-7