Abstract
Li2ZrO3-modified LiNi0.5Mn0.5O2 materials with improved electrochemical performance were directly synthesized by a simple mechanical milling route with ZrO2, Li2CO3 and Ni0.5Mn0.5(OH)2 precursors and a high temperature calcination in air atmosphere. The influences of ZrO2 contents on the microstructures and electrochemical properties of LiNi0.5Mn0.5O2 electrode materials were investigated through X-Ray diffraction, scanning electron microscope, energy dispersive spectroscopy and electrochemical tests. The results showed that ZrO2 can be completely converted into Li2ZrO3 in the form of a coating layer covering the surface of LiNi0.5Mn0.5O2 after a heat treatment process. Li2ZrO3 coating can be formed and dispersed homogenously on the surface of 1 mol% Li2ZrO3-modified LiNi0.5Mn0.5O2 materials. The electrochemical tests confirmed 1 mol% Li2ZrO3-modified LiNi0.5Mn0.5O2 materials exhibited the best discharge capacity of 158.3 mAh g−1 after 100 cycles between 2.75 and 4.35 V at 0.2 C, with an excellent capacity retention of 97.2% and higher discharge capacity at −20 °C than that of the pristine LiNi0.5Mn0.5O2. The enhanced cycling stability and low temperature performance may be attributed to the remarkable synergistic effects of Li2ZrO3 protective layer and its homogeneous distribution on LiNi0.5Mn0.5O2 surface with low Li/Ni cation mixing, high electric conductivity and good structure stability.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
1 Introduction
Rechargeable lithium ion batteries (LIBs) have been extensively used as efficient energy storage for mobile electronics, electrical vehicles (EVs) and some large-scale energy storage equipments due to their high power and energy densities, excellent cycle performance and long calendar life [1,2,3]. Recently, layered LiNixMn1-xO2 (0.5 ≤ x < 1) with a hexagonal α-NaFeO2 structure has been paid more attention to investigate as an alternative positive electrode material to commercial LiCoO2 [4,5,6]. Among LiNixMn1-xO2 materials, LiNi0.5Mn0.5O2 is especially attractive owing to its higher specific capacity, lower cost and excellent thermal stability, but it still has rapid capacity fading, poor cycling life at low temperature [7,8,9]. In LiNi0.5Mn0.5O2 formulation, the manganese ions are tetravalent while the nickel ions are generally divalent. For one thing, the electrochemically inactive Mn4+ may circumvent the Jahn-Teller distortions and play a crucial role in stabilizing the layer structures. But Mn addition can increase the Li/Ni cation disorder due to their similar ionic radius (0.69 Å(Ni2+) vs 0.76 Å(Li+)). A partial mutual substitution between Ni2+ and Li+ may impede the Li+ diffusion and lead to loss of Li+ electrochemical activity during cycling which induces a high initial capacity loss and low cycle life. For another, the electrochemically active Ni2+ can be oxidized successively to the Ni4+ upon charging, which can cause the partial collapse of the crystal layer (LiO6), hinder Li+ diffusion during discharging and result in capacity fading. As a result, LiNi0.5Mn0.5O2 usually delivers about 150 mAh/g capacity which is almost half of its theoretical value of ~280 mAh/g [10, 11].
Surface modification on cathode materials has been considered as a facile and effective strategy to improve the electrochemical properties of electrode materials [12, 13]. A proper coating layer can effectively reduce the electrolyte decomposition and side reactions and suppress the dissolution of transition metals, and further to improve the stability of electrode materials during the charge/discharge processes. For instance, coating on the surface of cathode material with a small amount of inert metal oxides, such as ZnO [14], Al2O3 [15], TiO2 [16], ZrO2 [17,18,19], can significantly improve the electrodes’ electrochemical performance by avoiding undesirable side reactions during cycling. Most of all, Al2O3 and ZrO2 coatings can efficiently hinder the erosion of cathode materials from the electrolyte, and have been applied to enhance the electrochemical performance of LiCoO2, LiNi0.5Mn0.5O2, Li(Ni1/3Co1/3Mn1/3)O2 and Li(Ni0.5Co0.2Mn0.3)O2 [20,21,22,23]. Nevertheless, these coating materials are usually poor electronic and ionic conductors, which might lead to a reduced reversible capacity and poor rate performance. Compared to these metal oxides, some fast Li-ion conductor such as LiAlO2, Li3VO4 and Li2TiO3 [24,25,26], have been focused and proven to enhance the electrochemical performance of cathode materials because they not only have good conductivity but also provide the tunnel for Li+ transportation during charge and discharge processes.
Among the Li-ion conductors, Li2ZrO3, a layered structure similar to Li2TiO3, possesses three-dimensional tunnels for Li-ion diffusion and has excellent intrinsic structural stability and chemical inert [27,28,29]. Li2ZrO3-modified cathode materials, such as Li(Ni1/3Co1/3Mn1/3)O2 and Li(Ni0.5Co0.2Mn0.3)O2, can be prepared via a wet chemical process and followed by a high temperature solid-state reaction [29,30,31]. In order to find out a facile synthesis method to prepare Li2ZrO3-modified LiNi0.5Mn0.5O2 materials, a simple, green synthetic route without using bulk liquid solvents should be adopted. In this work, Li2ZrO3-modified LiNi0.5Mn0.5O2 materials were successfully synthesized by directly using a simple mechanical milling route for the mixtures of Li2CO3, ZrO2 and Ni0.5Mn0.5(OH)2 precursors and following a high temperature heat-treatment. And the influence of Li2ZrO3 contents on the internal structures, morphologies, and electrochemical performance of LiNi0.5Mn0.5O2 was carefully investigated. The results demonstrated that Li2ZrO3-modified LiNi0.5Mn0.5O2 materials showed the enhanced electrode’s cyclability and higher reversible capacity at low temperature (−20 °C).
2 Experimental
All reaction reagents were analytic grade and used as received. The corresponding spherical Ni0.5Mn0.5(OH)2 precursors were prepared based on our similar synthetic methods for Ni-rich layer-structured cathode materials [32, 33]. Stoichiometric amounts of NiSO4·6H2O and MnSO4·H2O were dissolved in deionized water to form 2.0 mol L−1 mixed salt solutions. Appropriate amounts of NH3·H2O solution were added into 4.0 mol L−1 NaOH solution to form mixed alkaline solutions. A certain amount of alkaline solution was first pumped into a stirred stainless steel tank reactor under N2 atmosphere and the pH of the solutions was carefully controlled as 11. Then both saline solutions and alkaline solutions were slowly pumped into and react at 55 °C for 26 h. After the reaction completed, the Ni0.5Mn0.5(OH)2 precursors were obtained by filtering, washing, and drying at 100 °C. The process of Li2ZrO3-modified LiNi0.5Mn0.5O2 materials prepared from a mechanical milling route was presented as follows: first, stoichiometric amounts of Ni0.5Mn0.5(OH)2 precursors and ZrO2 in a mole ratio of 99:1 were put into an agate jar. Then 8 mol% excess amount of Li2CO3 and an appropriate amount of absolute ethanol was also added in the agate jar. Second, the mixtures of Ni0.5Mn0.5(OH)2 precursors, ZrO2 and Li2CO3 were mechanically milled at 230 r min−1 for 5 h. Finally, the 1 mol% Li2ZrO3-modified LiNi0.5Mn0.5O2 materials can be synthesized by calcining the mechanical milling mixtures at 550 °C for 6 h, subsequently at 950 °C for 16 h in air atmosphere. Similarly, the mole ratio of Ni0.5Mn0.5(OH)2 precursors and ZrO2 was changed into 97:3 or 95:5, the corresponding 3 mol% or 5 mol% Li2ZrO3-modified LiNi0.5Mn0.5O2 materials can be also prepared through the same process. To better identify the products, the nominal content of 1, 3 and 5 mol% Li2ZrO3 (LZO)-modified LiNi0.5Mn0.5O2 (LNMO) were labeled as 1-LZO@LNMO, 3-LZO@LNMO and 5-LZO@LNMO, respectively.
The crystallographic structure of the samples was characterized by X-ray diffraction with a step of 0.02° in the 2θ range between 10° and 80° on a PANalytical X’Pert Pro with Cu-Kα radiation. The morphological features were observed by scanning electron microscopy (TESCAN FE-SEM (MIRA3-XM) with an energy dispersive X-ray spectroscopy (EDS)). The average diameters (D50) of all samples were tested by laser particle size analyzer (BT-9300 ST, Dandong Bettersize Instruments Ltd. China). Specific surface area was determined by gas adsorption/desorption on TriStar II 3020 (Micromeritics Inc. USA).
For the fabrication of cathode sheets, the slurry formed by mixing the active material, acetylene black and polyvinylidene fluoride (PVDF) with a weight ratio of 90: 4:6 was coated on aluminum foil. After dried at 120 °C for 12 h in vacuum, the cathode disks were pressed and finished with ca. 8 mg cm−1 active material for a 2032 coin-type cell. The cells were assembled in an argon-filled glove box using Li metal as the reference electrode and 1 mol L−1 solution of LiPF6 dissolved in ethylene carbonate (EC)/dimethyl carbonate (DMC)/ ethyl methyl carbonate (EMC) (1:1:1, v/v/v) as the electrolyte. The cycle performance was carried out at 0.2 C between 2.75 and 4.35 V on a NEWARE cell test instrument. Electrochemical impedance spectroscopic (EIS) measurements were tested in a CHI660E electrochemical station with the frequency range of 0.01–100,000 Hz, applying an AC signal of 5 mV. All of the above electrochemical tests were operated at 25 °C or − 20 °C.
3 Results and discussion
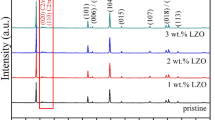
The crystal structures of the pristine and Li2ZrO3-modified LiNi0.5Mn0.5O2 materials different amounts of ZrO2 are investigated by XRD shown in Fig. 1. Diffraction patterns of all samples can be indexed to the hexagonal α-NaFeO2 structure with the space group of R-3 m. The clear peak splitting of doublets (006)/(102) and (108)/(110) are observed in these patterns, implying their high crystalline and layered characteristic [34, 35]. Although the XRD patterns of pristine and 1-LZO@LNMO samples show no significant difference, Li2ZrO3 phase is clearly observed in the diffraction patterns of 3 and 5% ZrO2 modified LiNi0.5Mn0.5O2 in the 2θ range of 42–43° and 60–63° shown in Figs. 1 and 2. For the local enlarged XRD patterns as shown in Fig. 2, these small diffraction peaks can be identified as Li2ZrO3 (JSPDC card No. 75-2157), suggesting that Li2ZrO3 could be formed on the surface of LiNi0.5Mn0.5O2 with increasing ZrO2 content.
To further investigate the influence of ZrO2 content on the crystal structures of LiNi0.5Mn0.5O2 materials, the lattice parameters calculated from XRD results are summarized in Table 1. There are not regular trend on the change of lattice parameters a and c with increasing ZrO2 content, and the c/a value has the similar irregular changing trend. A shrinkage of the cell volumes of LiNi0.5Mn0.5O2 from 103.12 Å3(x = 0) to 101.78 Å3(x = 0.05) with increasing ZrO2 content could be mainly ascribed to the change of monoclinic phase of Li2ZrO3, but not caused by Zr4+ doping into the host lattice due to the ion radius of Zr4+ (0.79 Å) larger than that of Ni2+ (0.69 Å) and Mn4+ (0.53 Å), which would result in the enlargement of the cell volumes of LiNi0.5Mn0.5O2. According to the reports before [36, 37], the intensity ratio of I(003)/I(104) has been regarded as an important index of the cation mixing, meaning that Ni2+ ions occupy the Li(3b) site in the layer-structured crystal lattice. The smaller the I(003)/I(104) value, the higher the disordering [38, 39]. Generally, the layered cathode materials with a high ratio of I(003)/I(104) > 1.2 showed a better electrochemical properties. As shown in Table 1, all samples have a high ratio of I(003)/I(104), and the ratio first increase then decreases with ZrO2 content increment. The value of I(003)/I(104) of 1-LZO@LNMO and 3-LZO@LNMO was over 1.7, suggesting that no undesirable cation mixing took place and a good electrochemical performance would be expected.
The SEM images of the pristine, 1-LZO@LNMO, 3-LZO@LNMO and 5-LZO@LNMO materials are given in Fig. 3. The initial LiNi0.5Mn0.5O2 secondary particles have a clear spherical diameters almost all from ca. 9 to 12 μm with an average value of 11 μm, which is well consistent with the results tested by laser particle size analyzer in Fig. 4. The secondary particles of initial LiNi0.5Mn0.5O2 are mainly composited of the primary particles with the particle size of ca. 0.5–2 μm seen in its enlarged image shown in the inset of Fig. 3a. After a certain amount of ZrO2 powders are added, the modified LiNi0.5Mn0.5O2 powders show a relatively irregular incompact aggregates with different sizes. Figure 4 shows the average diameters and BET surface areas of different content of Li2ZrO3-modified LiNi0.5Mn0.5O2 samples. Among all positive electrode materials, 1-LZO@LNMO shows the largest average particles size of 12.26 μm and the smallest BET surface area of 1.57 cm2 g−1. With the increment of ZrO2 content, the small amount of Li2ZrO3 aggregates can be easily found on LiNi0.5Mn0.5O2 surface, which can be further confirmed by energy dispersive spectrum analysis.
To further examine the controllability on the morphologies of Li2ZrO3-modified LiNi0.5Mn0.5O2 materials, element mapping results of the 1-LZO@LNMO sample were displayed in Fig. S1 to understand the location and identity of atoms. As observed, four elements of O, Mn, Ni and Zr can be clearly found on the element mapping, indicating a certain amount of Li2ZrO3 has been deposited on the surface of LiNi0.5Mn0.5O2. To gain more information of Zr distribution on the surface of LiNi0.5Mn0.5O2, the corresponding element mapping of Zr in 1-LZO@LNMO, 3-LZO@LNMO and 5-LZO@LNMO samples was also compared in Fig. S2. From the Fig. S2a, Zr element can be found and dispersed sparsely on the surface for 1-LZO@LNMO. With the ZrO2 amount increment to 3 mol% and 5 mol%, the coating layer becomes thicker and denser. However, few small aggregates of Zr could be formed on LiNi0.5Mn0.5O2 surface with 3 mol% Zr in Fig. S2b, more small aggregates have been grew into bigger irregular aggregates on LiNi0.5Mn0.5O2 surface with 5 mol% Zr shown in the inset of Fig. 3d and Fig. S2c. The differences amounts of Zr contents and distribution conditions (with or without aggregates) of Li2ZrO3 on the surface of LiNi0.5Mn0.5O2 materials might have different effects on their electrochemical performance.
To further investigate the influences of ZrO2 contents on electrochemical performance for LiNi0.5Mn0.5O2 materials, coin-type half cells are cycled between 2.75 and 4.35 V at a current density of 0.2 C (30 mA g−1) at 25 °C. Figure 5 shows the charge and discharge profiles of the different materials after 1st, 2nd, 50th and 100th cycle, respectively. These results show the charge and discharge curves of all cathode materials are quite smooth, the discharge average voltage plateau at ca. 3.7 V does not change noticeably with Zr content increment. As shown in Table 2, the initial discharge capacity of LiNi0.5Mn0.5O2 is 164.9 mAh g−1, which was higher than 158.3, 154.0 and 132.4 mAh g−1 of 1-LZO@LNMO, 3-LZO@LNMO and 1-LZO@LNMO. The initial capacity of LiNi0.5Mn0.5O2 is obviously reduced by adding ZrO2, due to the electrochemically inactive Li2ZrO3 materials.
In addition, the effect of ZrO2 contents on the capacity retention for different materials was also investigated by using cycling performance. As shown in Fig. 6, the pristine LiNi0.5Mn0.5O2 shows a rapid capacity fading from 164.9 to 131.1 mAh g−1 during 100 cycles, but the 1-LZO@LNMO showed an excellent capacity retention of 153.8 mAh g−1 after 100 cycles. The capacity retention at end of the 100th cycle (Table 2) is 81.9% (Zr = 0%), 97.2% (Zr = 1%), 94.6% (Zr = 3%) and 91.4% (Zr = 5%), respectively, indicating the electrode cycling performance could be greatly improved with Zr addition. For a typical example, a nominal content of 1 mol% Zr difference between pristine LiNi0.5Mn0.5O2 and 1-LZO@LNMO results in a 15.3% retention increase, while a nominal 3 mol% Zr content difference between pristine LiNi0.5Mn0.5O2 and 3-LZO@LNMO with a 12.7% retention raise. Namely, the appropriate amount of LZO modification for LiNi0.5Mn0.5O2 is crucial to the cycle life of cathode materials. As the nominal content of Zr was 1 mol%, the overlap of the charge/discharge curves for 50th and 100th cycle became obvious, illustrating that the materials contained appropriate amount of Li2ZrO3 possessed better cycle reversibility. The superiority of the 1-LZO@LNMO in high capacity retention may be attributed to the remarkable synergistic effects of Li2ZrO3 incorporation and its homogeneous distribution on LiNi0.5Mn0.5O2 surface [27,28,29]. The Li2ZrO3 coating on the electrode surface not only can improve the electrical conductivity, but also as a protective layer to decrease the direct contact between the electrolyte and electrode, thus suppress the dissolution of transition metal elements and electrolyte decomposition in repetitive lithium insertion and extraction reactions, which can achieve a higher capacity retention and better cycle life.
To further investigate rate capability of the different LZO-modified LiNi0.5Mn0.5O2, the influence of discharge rate on the capacity retention between 2.75 and 4.35 V was studied as shown in Fig. S3. The half cells were charged at 0.5C to ensure initial conditions for each charge, and discharge rates were ranged from 0.5C to 8C at 25 °C. With the increased current rate, all the capacities decreased regularly. Obviously, the rate capability of the modified LiNi0.5Mn0.5O2 was slight lower than that of the bare LiNi0.5Mn0.5O2, which suggests the rate capability was not improved by the Li2ZrO3 modification on LiNi0.5Mn0.5O2. The low rate capability of LZO-modified LiNi0.5Mn0.5O2 might be partly attributed to the obstruction of Li+ diffusion from the LZO coating to the interior of the secondary particles at a high charge-discharge current density [30, 40].
The Li-storage properties at low temperature of −20 °C for pristine LiNi0.5Mn0.5O2 and Li2ZrO3-modified LiNi0.5Mn0.5O2 materials different amounts of Zr are also compared in Fig. 7. The corresponding cells are cycled between 2.75 and 4.2 V at a current density of 0.2 C at −20 °C. Obviously, the discharge capacity of the pristine material decreased faster than that of 1-LZO@LNMO when ambient temperature is changed from 25 °C to −20 °C. The discharge capacity of the pristine material at −20 °C was 28.3 mAh g−1, about only 14.6% of that at 25 °C, while the 1-LZO@LNMO shows the best discharge capacity of 64.3 mAh g−1, about 50% of that at 25 °C. These results show the low temperature performance of LiNi0.5Mn0.5O2 can be greatly enhanced by Li2ZrO3 modification, which may be attributed to the excellent Li+ conductor of Li2ZrO3 coating on the electrode surface at low temperature.
To further understand the improvement in the electrochemical properties by Li2ZrO3 modification, the electrochemical impedance spectroscopy (EIS) of different Zr-content materials was carried out after charging to 4.35 V in five cycles at 0.2 C. As shown in Fig. 8, the EIS curves can be considered to consist of one depressed semicircles at the high frequency region (enlarged in inset) and two depressed semicircles at the high to medium frequency region, followed by a straight line at the low frequency region, which are very similar to the previous results of transition metal Ni, Co, Mn-oxide positive electrode materials [41, 42]. All EIS spectra can be fitted using the equivalent circuit shown in inset of Fig. 8. For this equivalent circuit, Rs, RSEI, Rint and Rct represent the ohmic resistance between the working electrode and the reference electrode, the resistance for Li+ diffusion in the SEI layer, interphase electronic contact and the charge transfer resistance, respectively. CPESEI is constant phase element depicting the non-ideal capacitance of the SEI layer, CPEint is constant phase element depicting the non-ideal capacitance of the interphase electronic properties and CPEdl is constant phase element depicting the non-ideal capacitance of the double layer. The rising line in the low-frequency region is assigned to Warburg impedance (Zw), which is connected with the solid-state diffusion of Li+ into the positive electrode [42,43,44,45]. The Rs decreases from 6.8 Ω for the pristine LNMO to 6.0 Ω for 1-LZO@LNMO and further to 3.4 Ω for 5-LZO@LNMO, implying that the electronic conductivity can be improved with Zr addition. As Zr content increases, the value of Rct begins to decrease, then increases. The Rct value of 1-LZO@LNMO is 68.5 Ω g−1, lower than 88.4, 138.2 and 159.6 Ω g−1 of 3-LZO@LNMO, 5-LZO@LNMO and LNMO, respectively. The variation of the Rct value might be partly attributed to Li2ZrO3 aggregates of 3-LZO@LNMO and 5-LZO@LNMO obstructing the Li+ diffusion since the electrolytes cannot fully permeate into the primary inter-particle spaces, which might cause local polarization to increase the Rct value during the lithium insertion and extraction reactions. Because 1 mol% Li2ZrO3 coating on the surface of LiNi0.5Mn0.5O2 materials is very thin, it might not reduce the electronic conductivity of the whole electrode material, and also not cause the electrode to produce large polarization during the reaction process. 1-LZO@LNMO electrode possesses the smallest Rct among the four as-prepared electrode materials, confirming that the right amount of Li2ZrO3 on LiNi0.5Mn0.5O2 surface would be an efficient method to improve the electrochemical properties for the whole positive electrode materials.
4 Conclusions
Li2ZrO3-modified LiNi0.5Mn0.5O2 materials have been successfully synthesized through calcining the mixtures of ZrO2, Li2CO3 and Ni0.5Mn0.5(OH)2 precursors assisted by a simple mechanical milling route in air atmosphere. The results show that Li2ZrO3-modified materials still have a-NaFeO2 type layered structure with small amount of Li2ZrO3 phase in high ZrO2 contents. The discharge specific capacity and rate capability have not gained improvement, but the cycle stability and low temperature performance of the modified LiNi0.5Mn0.5O2 material have been improved by coating small amount of Li2ZrO3. The low initial discharge specific capacity and rate capability may be ascribed to the added mass of the inactive Li2ZrO3 and the obstruction of Li+ diffusion from the Li2ZrO3 coating to the interior of the secondary particles at a high charge-discharge current density. The improved cycle stability and low temperature performance of the modified LiNi0.5Mn0.5O2 material can be attributed to appropriate amounts of Zr contents and distribution on surface of LiNi0.5Mn0.5O2 with low Li/Ni cation mixing, high electric conductivity and good structure stability. This work will provide a simple and convenient, but promising method to construct Li2ZrO3-modified Ni-rich cathode materials to apply in LIBs with improved performance in the future.
References
P. Simon, Y. Gogotsi, B. Dunn, Science 343, 1210–1211 (2014)
Z.Q. Yan, W.L. Yao, L. Hu, D.D. Liu, C.D. Wang, C.S. Lee, Nanoscale 7, 5563–5577 (2015)
Y. Yang, S. Li, Q. Zhang, Y. Zhang, S. Xu, Ind. Eng. Chem. Res. 56, 175–182 (2017)
T.E. Quine, M.J. Duncan, A.R. Armstrong, A.D. Robertson, P.G. Bruce, J. Mater. Chem. 10, 2838–2841 (2007)
Y. Hinuma, Y.S. Meng, K. Kang, G. Ceder, Chem. Mater. 19, 1790–1800 (2007)
X.L. Meng, S.M. Dou, W.L. Wang, J. Power Sources 184, 489–493 (2008)
M. Yoncheva, R. Stoyanova, E. Zhecheva, R. Alcantara, J.L. Tirado, J. Alloys Compd. 475, 96–101 (2009)
K. Sakamoto, M. Hirayama, H. Konishi, N. Sonoyama, N. Dupre, D. Guyomard, K. Tamura, J. Mizuki, R. Kanno, Phys. Chem. Chem. Phys. 12, 3815–3823 (2010)
Y.M. Liu, B.L. Chen, F. Cao, X. Zhao, J. Yuan, J. Mater. Chem. 21, 10437–10441 (2011)
F. Li, G. Yang, G. Jia, X. Shangguan, Q. Zhuge, B. Bai, J. Appl. Electrochem. 47, 1189–1201 (2017)
Y. Ding, D. Mu, B. Wu, R. Wang, Z. Zhao, F. Wu, Appl. Energy 195, 586–599 (2017)
H. Kobayashi, H. Sakaebe, H. Kageyama, K. Tatsumi, Y. Arachi, J. Mater. Chem. 13, 590–595 (2003)
Z. Wang, E. Liu, L. Guo, C. Shi, C. He, J. Li, N. Zhao, Surf. Coat. Technol. 235, 570–576 (2013)
J.Z. Kong, C. Ren, G.A. Tai, X. Zhang, A.D. Li, D. Wu, H. Li, F. Zhou, J. Power Sources 266, 433–439 (2014)
J. Cho, J.K. Yong, B. Park, Chem. Mater. 32, 3788–3791 (2000)
W. Feng, W. Meng, Y. Su, C. Shi, B. Xu, J. Power Sources 191, 628–632 (2009)
H. Liu, G.X. Wang, D. Wexler, J.Z. Wang, H.K. Liu, Electrochem. Commun. 10, 165–169 (2008)
J.Q. Zhao, Y. Wang, Nano Energy 2, 882–889 (2013)
H. Han, F. Qiu, Z. Liu, X. Han, Ceram. Int. 41, 8779–8784 (2015)
E. Jung, Y.J. Park, J. Electroceram. 29, 23–28 (2012)
B.J. Hwang, S.K. Hu, C.H. Chen, C.Y. Chen, H.S. Sheu, J. Power Sources 174, 61–765 (2007)
S.K. Hu, G.H. Cheng, M.Y. Cheng, B.J. Hwang, R. Santhanam, J. Power Sources 188, 564–569 (2009)
J.Z. Kong, S.S. Wang, G.A. Tai, L. Zhu, L.G. Wang, H.F. Zhai, D. Wu, A.D. Li, H. Li, J. Alloys Compd. 657, 593–600 (2016)
L. Li, Z. Chen, Q. Zhang, M. Xu, X. Zhou, H. Zhu, K. Zhang, J. Mater. Chem. A 3, 894–904 (2015)
W. Wang, Z. Yin, Z. Wang, X. Li, H. Guo, D. Wang, J. Alloys Compd. 646, 454–460 (2015)
J. Wang, Y. Yu, B. Li, T. Fu, D. Xie, J. Cai, J. Zhao, Phys. Chem. Chem. Phys. 17, 32033–32043 (2015)
W. Wang, Z. Yin, J. Wang, Z. Wang, X. Li, H. Guo, J. Alloys Compd. 651, 737–743 (2015)
S.B. Lim, H. Lee, Y.J. Park, J. Electroceram. 37, 92–97 (2016)
C. Wang, L. Chen, H. Zhang, Y. Yang, F. Wang, F. Yin, G. Yang, Electrochim. Acta 119, 236–242 (2014)
D. Wang, X. Li, Z. Wang, H. Guo, Z. Huang, L. Kong, J. Ru, J. Alloys Compd. 647, 612–619 (2015)
Y. Xu, Y. Liu, Z. Lu, H. Wang, D. Sun, G. Yang, Appl. Surf. Sci. 361, 150–156 (2016)
S. Zhong, P. Chen, W. Yao, ECS Electrochem. Lett. 4, A45–A48 (2015)
S. Zhong, M. Lai, W. Yao, Z. Li, Electrochim. Acta 212, 343–351 (2016)
H.Q. Liu, Y.M. Hu, Y.B. Li, H.S. Gu, Mater. Chem. Phys. 138, 440–443 (2013)
H. Gwon, S.W. Kim, Y.U. Park, J. Hong, G. Ceder, S. Jeon, K. Kang, Inorg. Chem. 53, 8083–8087 (2014)
T. Ohzuku, A. Ueda, M. Nagayama, J. Electrochem. Soc. 140, 1862–1870 (1993)
A.M.A. Hashem, A.E. Abdel-Ghany, A.E. Eid, J. Trottier, K. Zaghib, J. Power Sources 196, 8632–8637 (2011)
X. Zhang, A. Mauger, L. Qi, H. Groult, L. Perrigaud, Electrochim. Acta 55, 6440–6449 (2010)
S. Jouanneau, K.W. Eberman, L.J. Krause, J.R. Dahn, J. Electrochem. Soc. 150, A1637–A1642 (2003)
S.N. Lim, W. Ahn, S.H. Yeon, S.B. Park, Electrochim. Acta 136, 1–9 (2014)
F. Nobili, S. Dsoke, M. Minicucci, F. Croce, R. Marassi, J. Phys. Chem. B 110, 11310–11313 (2006)
X.Y. Qiu, Q.C. Zhuang, Q.Q. Zhang, R. Cao, Y.H. Qiang, P.Z. Ying, S.G. Sun, J. Electroanal. Chem. 687, 35–44 (2012)
W. Yao, Y. Liu, Q. Dai, D. Li, Y. Yu, Q. Jing, J. Chin. Chem. Soc. 64, 539–546 (2017)
C.H. Liang, L.B. Liu, Z. Jia, C. Dai, Y. Xiong, Electrochim. Acta 186, 413–419 (2015)
W. Yao, Q. Dai, Y. Liu, Q. Zhang, S. Zhong, Z. Yan, ChemElectroChem 4, 1236–1242 (2017)
Acknowledgments
The authors are grateful for the financial support of this work by National Natural Science Fund of China (No.51372104), Jiangxi Province Science and Technology Plan Project (No.20141BBE50019), Foundation of Jiangxi Educational Committee (No. GJJ160601), Finance and Education Plan of Ganzhou City (No.197[2017]) and Doctoral scientific research foundation of Jiangxi University of Science and Technology (No. jxxjbs16025).
Author information
Authors and Affiliations
Corresponding author
Electronic supplementary material
Fig. S1
SEM image and the corresponding element mapping of O, Ni, Mn, Zr and EDS images of 1-LZO@LNMO material. (JPG 443 kb)
Fig. S2
SEM image and the corresponding element mapping of Zr for LZO-modified LiNi0.5Mn0.5O2 materials. (JPG 365 kb)
Fig. S3
Rate capability of LZO-modified LiNi0.5Mn0.5O2 materials from 0.2C to 5C between 2.75 and 4.35 V. (JPG 119 kb)
Rights and permissions
About this article
Cite this article
Yao, W., Zhang, H., Zhong, S. et al. Facile synthesis of Li2ZrO3-modified LiNi0.5Mn0.5O2 cathode material from a mechanical milling route for lithium-ion batteries. J Electroceram 43, 84–91 (2019). https://doi.org/10.1007/s10832-018-0158-6
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10832-018-0158-6