Abstract
The dependence of structure and piezoelectric response d33 on individual excess A-site K or Na in K0.5Na0.5NbO3 based lead-free piezoceramics is reported. The coexistence of orthorhombic, tetragonal, and monoclinic phases at room temperature is observed in the investigated 0.94K0.5+xNa0.5NbO3–0.06LiNbO3 and 0.94K0.5Na0.5+yNbO3–0.06LiNbO3 ceramics (x, y = 0–0.025). The weight ratio of three phases, orthorhombic-tetragonal phase transition temperature, and d33 value are highly sensitive to A-site K or Na nonstoichiometry. A-site Na nonstiochiometry plays a dominant role in enhancing the electrical properties of KNN by significantly reducing the weight ratio percentage of monoclinic phase and shifting the orthorhombic-tetragonal phase transition temperature to lower temperatures.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
1 Introduction
Innovations in actuators, sensors, and ultrasonic transducers have been the driving force for the development of piezoelectric ceramics [1]. Due to their high piezoelectric response, large scale production capability and the tailoring of their properties through composition, lead zirconate-titanate ceramics (PZT) are the most widely used piezoelectrics [2, 3]. However, the lead-based PZT piezoelectrics contain more than 60 wt% toxic lead, leading to a various environmental problems [4]. One widely investigated alternative is based on potassium sodium niobate solid solutions [5, 6]. For example, K0.5Na0.5NbO3 (KNN) based piezoelectrics with relatively high piezoelectric constant and phase transition temperature are attracting more attention and emerging as a promising lead free piezoelectrics candidate [7–11]. Unlike the lead based PZT piezoelectrics, enhanced properties of KNN based lead-free ceramics are usually due to the shift of the orthorhombic-tetragonal phase transition temperature (OT-PTT) that is compositional dependence to near room temperature [12]. Guo et.al reported significantly enhanced piezoelectric properties for KNN based ceramics with the composition near the OT-PTT region by doping LiNbO3 [13].
However, Na and K evaporate unavoidably during the elevated temperature sintering. This can result in the formation of secondary phases and the nonstoichiometry of KNN [14, 15]. Additionally, KNN based piezoelectrics could be easily off stoichiometric from the beginning unless properly handled since alkali metal carbonates, such as Na2CO3 or K2CO3, are hygroscopic. While the influence of volatilization on the dielectric properties has been widely studied in Pb-based perovskite systems [16], investigations also indicate that volatile elements have distinct effect on the microstructure and properties of lead-free piezoelectric [17].
In particular, the difference in evaporation rate between A-site K and Na and induced point defects by nonstoichiometry make the analysis of relationship between structure and properties of KNN ceramics more complex. Several reports confirmed that it is of significant importance to adjust K/Na ratio and sintering temperature and A-site alkali excess amount to obtain enhanced dielectric and piezoelectric properties [18–20]. Zhang et.al predicted [21] that a large change in the structure over a small compositional change for K0.3Na0.7NbO3 could potentially result in enhanced physical properties based on the study of crystallographic structures of KNN based ceramics. Our recent study showed that A-site (K + Na) nonstoichiometry plays a key role in enhancing piezoelectric response by shifting the OT-PTT to lower temperatures [22]. However, it is not clear what the role of individual A-site K or Na nonstoichiometry plays in shifting the OT-PTT and affecting the electrical properties of KNN based ceramics.
In the present study, the dependence of phase structure and electrical properties on individual excess A-site K or Na in 0.94K0.5+xNa0.5NbO3–0.06LiNbO3 (KxNLN) with x = 0 ~ 0.025 and 0.94K0.5Na0.5+yNbO3–0.06LiNbO3 (KNyLN) with y = 0 ~ 0.025 was investigated. The coexistence of orthorhombic, tetragonal, and monoclinic phases was observed at room temperature.
2 Experimental
All ceramics were prepared by a conventional solid-state reaction method. Raw materials were sodium carbonate (Na2CO3, 99.8 %), potassium carbonate (K2CO3, 99 %), lithium carbonate (Li2CO3, 99.99 %), and niobium oxide (Nb2O5, 99.5 %). Since the changes in composition in the investigated samples were so small that several precautions were taken to make a series of non-stoichiometric samples. We dried all the powders in a vacuum dry oven to avoid possible water absorption by powders. Immediately, we used large batches of powders (60 g) and weighed it out using an analytic balance with a high precision (0.0001 g) to meet such tolerances and convinced that the actual compositions consistently followed the nominal compositions. Raw powders were wet-milled by mixing a certain amount of raw materials and ZrO2 milling balls for 24 h. After calcination at 750 °C for 3 h, the powders were ball milled and then pressed into disk samples, followed by sintering at 1080 °C for 3 h in air. The samples were poled in 130 °C silicon oil by applying a 30 kV/cm DC electric field for 30 min.
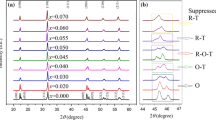
X-ray diffraction characterization was performed using Cu Kα radiation (λ = 1.54178 Å) in θ-2θ mode (D/max 2500PC, Rigaku, Japan). Scans were taken in a 2θ range from 20 to 80o with a step size of 0.02o and accumulation time 40 min. Due to the heavy overlapping of the peaks of these two phases (see the inset of Fig. 1(a)), the XRD patterns of KxNLN ceramics between 2θ = 44o and 47o with a step size of 0.01o were further recorded with a Panalytical Xpert Pro MPD diffractometer using only CuKα1 radiation. Rietveld refinement analysis using TOPAS software was carried out to further identify the unknown phase. All the samples used in XRD are as-synthesized powders by solid state reaction or crushed and ground powders.
The Raman spectra were measured by Renishaw Micro-Raman Spectroscopy System (Renishaw InVia Reflex). Using 514.5 nm Ar+ blue laser as exciting source, the Raman Spectroscopy scanned the region on the surface of pellets with ~2–3 μm in diameter on the range of wave number from 1000 cm−1 to 100 cm−1. The microstructure and morphology was characterized by a field emission scanning electron microscopy (FE-SEM, Hitachi S-4700). The temperature dependence of dielectric constant (ε r ) and loss tangent (tanδ) was measured by LCR meter (E4980A, Aglient). The piezoelectric constant d 33 was measured using a quasi-static d 33 meter (Model ZJ-6 A, Institute of Acoustics Academic Sinica).
3 Results and discussion
The XRD patterns for KxNLN (x = 0 ~ 0.025) and KNyLN (y = 0 ~ 0.025) ceramics are shown in Fig. 1(a) and (b), respectively. Two common phases in KNN based piezoelectrics, the orthorhombic and tetragonal, can be easily identified in all the investigated samples. Several very weak peaks can be occasionally observed between 2θ = 25o and 30o only in KNyLN (y = 0.005), which is usually associated to the existence of secondary phases with tungsten bronze structure due to the volatilization of alkaline elements [23]. However, the perovskite structures were formed in all the other investigated compositions. Due to the heavy overlapping of the peaks of these two phases, the XRD patterns of KxNLN ceramics between 2θ = 44o and 47o were further recorded with a Panalytical Xpert Pro MPD diffractometer using only CuKα1 radiation, as shown in the inset of Fig. 1(a). It is clearly seen from the inset that there are four peaks centered at ~44.78, ~45.25, ~45.90, and ~46.00o in all the samples, respectively. Especially, the peak at 2θ = 45.25o is very broad and obviously overlaps the peak of an unknown third phase. Given the appearance of the broad peak in all ceramics, the contribution of impurity phase to broad peaks can be ruled out.
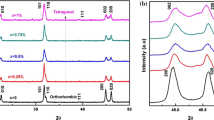
Rietveld refinement analysis was carried out to identify the unknown phase in the XRD patterns. As shown in Fig. 2, good reliable factors are achieved for the Rietveld refinement of the XRD patterns when the unknown structure is assigned to monoclinic phase. The reliable factors of Rexp, Rp and Rwp of KNLN are derived to be 3 ~ 4, 4.8 ~ 6.8 and 7.1 ~ 8.7 %, respectively. Rexp is an indicator of original measured XRD data and the limit of Rwp reliable factor, respectively. In contrast, Rp indicates the reliability fitting the XRD patterns. Rwp is the weighted average of Rp reliable factor. Gof factor denotes Rwp/Rexp. Usually, these derived results are considered to be reliable if the value of Gof and Rwp is below 2 and 10 %, respectively. We would like to mention that given the weak intensity of diffraction background peaks, the reliability of data listed in Table 2 can be very high.
It is well known that the Rietveld refinement analysis calculates the relative intensities of the XRD diffraction peaks based on the structural model of phases by adjusting the scale factor S to match the measured diffraction peaks’ intensity. By refining the preferred orientation and atomic positions of M (monoclinic) phase, the existence of M phases can be correctly determined. As an example, Fig. 3(a) and (b) show the Rietveld refinement analysis results of KNyLN (y = 0.015) ceramics for the three-phase and the two-phase refinement, respectively. In addition to O (orthorhombic) and T (tetragonal) phases, the existence of M is clearly observed in the Fig. 3(a). Especially, the Rwp factor will be over 15 % if M phase is not considered in the Rietveld refinement. Although TOPAS can not output the data of diffraction peaks if the phases have been refined, all the phases can be indicated by graph as shown in the Fig. 3. It is observed in Fig. 3 that these figures are the results of the screenshot superposition. It is clear that the results of two-phase refinement yield much larger error value than that of three-phase refinement. In a combination with the reliable factors from the Rietveld refinement, it is suggested that the orthorhombic, tetragonal and monoclinic phases coexist in KxNLN and KNyLN ceramics with a wide range of A-site K or Na nonstoichiometry at room temperature (Table 1).
Klein et al. [24] predicted that there could be three phase coexistence region of tetragonal (T), Orthorhombic (O) and an unknown “P″ phases in KNN ceramics. A similar third peak, which is featured by humps on the left side of the 020 peak and claimed to be a low temperature (< 0 °C) monoclinic phase, was reported in KNN based ceramics [25]. It should be mentioned that the structure of KNN is complex since it holds an orthorhombic structure. Especially, the perovskite type ABO3 sub-cell possesses monoclinic symmetry [26]. The uncertainties exist as to the extent of orthorhombic and monoclinic phase fields arising from different octahedral tilt systems [27]. The weight ratio percentages (WRPs) of the three phases in KxNLN and KNyLN ceramics derived from the Rietveld refinement are summarized in Table 2 and shown in Fig. 4. It is noticeable that that the WRPs of these phases, especially monoclinic phase, is highly sensitive to a small deviation of A-site Na or K stoichiometry.
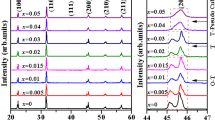
While XRD usually gives average structural information, Raman spectroscopy is further used to determine the structure change of these samples. Figure 4(a) and (b) show the Raman spectra of KxNLN and KNyLN (x, y = 0, 0.005, 0.01, 0.015, 0.02, and 0.025) between 100 and 1000 cm−1 at room temperature, respectively. Two strong peaks at ~625 cm−1 (ν5) and ~265 cm−1 (ν1) represent a double-degenerate symmetric O-Nb-O stretching vibration and a triply degenerate symmetric O-Nb-O bending vibration, respectively [24, 28]. Usually there are distinct shoulders in the Raman spectra on the low-frequency sides of the Raman mode at ~625 cm−1 in both orthorhombic and tetragonal phase, but the intensity of the tetragonal phase is weaker [29]. Furthermore, there is a broad weak peak in the 400–500 cm−1 regions, indicating the orthorhombic phase. As shown in Fig. 5, this characteristic shoulder in all Raman spectra is obviously visible. However, we observe a loss in Raman intensity in the 400–500 cm−1 regions. Its intensity becomes so weak that it is almost not observed for KxNLN with x = 0.025. In contrast, the intensity in the 400–500 cm−1 regions is still visible for KNxLN. The peaks around 625 cm−1 and 256 cm−1 obviously shift downward for overcompensated KNNLN by Na or K. This behavior is referred to a decrease in force constant caused by the change of length between Nb+5 and its coordinated oxygen in [BO6] by pushing the B-site cation shifting away from the center of [BO6]. This also implies that overcompensation of K or Na results in the formation of Nb+5 vacancies with negative charge. This can cause locally structural disorder and large lattice distortion, in order to maintain the charge balance [30, 31].
Figure 6(a)–(f) show the SEM images of the microstructure of thermally etched KxNLN ceramics with 0.00 ≤ x ≤ 0.025 sintered at 1080 °C for 3 h. In addition to the existence of pores and small size irregular grains, all ceramics consist of square-like or near square-like grains. The morphology of grain in all samples does not change significantly with increasing excess K. However, the appearance of abnormal grains can be observed when excess K content increases. It is reported [32, 33] that the liquid phase and a small amount of secondary phase, which melts at ~1000 °C, can form in KNN based ceramics due to the evaporation of Na. The liquid phases enhance sintering processing by accelerating particle rearrangment and atomic diffusion [34]. We believe the abnormal grain growth results from the liquid phases as well.
In contrast, it is interesting to observe that the microstructure evolution of KNyLN is significantly different from that of KxNLN. Figure 7(a)–(f) show the SEM images of the microstructure of thermally etched KNyLN ceramics with 0.00 ≤ y ≤ 0.025 sintered at 1050 °C for 3 h. There exist two stages in the microstructure evolution with increasing excess Na. The microstructure displaying abnormal grains surrounded by small grains in KNyLN with y ≤ 0.005 is similar to that of KxNLN. It is expected since the small amount of excess Na is not enough to compensate the loss of Na. This can also promote the formation of Na-deficient liquid phase and subsequently abnormal grain growth. However, grain size of KNyLN with y ≥ 0.01 is much larger than that of KxNLN. The grain size of KNyLN with y = 0.025 reaches 8-10 μm. Another noticeable feature is that the grain size of KNyLN is relatively homogeneous as compared with that of KxNLN. The difference in the evolution of microstructure can be explained as follows. The addition of y ≥ 0.01 excess Na is enough to compensate the loss of Na and then limit the formation of Na deficiency liquid phase. Although B-site vacancies are not common in peroviskite ceramics [35], the overcompensation by either K or Na in KNLN can result in the presence of Nb+5 vacancies, which are considered to enhance mass transport during sintering. These observations leads us to conclude that the dominant mechanism of anormal grain growth in KxNLN with 0.00 ≤ x ≤ 0.025 and KNyLN with 0.00 ≤ y ≤ 0.005 is assisted by formation of liquid phase while coarsening of grains is assisted by presence of Nb+5 vacancies in KNyLN with 0.01 ≤ y ≤ 0.025.
Figure 8(a) and (b) show the temperature dependence of dielectric constant (εr) from room temperature to 160 °C on excess K and Na compensation, respectively. Two phase transition points are observed in all ceramics corresponding to the orthorhombic-tetragonal (OT-PTT) and cubic- tetragonal (Tc) phase transitions as shown as insets. Dielectric constant (εr), loss tangent, OT-PTT, T c (Curie temperature), and piezoelectric response d33 for KxNLN and KNyLN ceramics are summarized in Table 1. As expected, there is no regular rule in the change of εr since the OT-PTT near around room temperature can result in the comparison of dielectric constants being complicated. Additionally, the dielectric constant is closely related to the grain size [36]. However, higher εr values usually lie in a narrow range in KxNLN and KNyLN ceramics with x = 0.010–0.015 and y = 0.010–0.020.
The dependence of OT-PTT and d33 on excess K and Na is further plotted in Fig. 9. As shown, the OT-PTT shows a trend of a shift to lower temperatures for KNyLN ceramics with increasing excess Na until the actual stoichiometry is obtained. The lowest OT-PTT point appears between 40 °C and 52 °C for KxNLN with x = 0.01–0.015, while the lowest OT-PTT point appears between 42 °C and 39 °C for KNyLN with y = 0.02–0.025. Similar to the OT-PTT results, d33 values are very sensitive to a very small deviation from stoichiometry for both KxNLN and KNyLN ceramics as shown in Fig. 9. However, the dependence of OT-PPT and d33 is more significant for KxNLN ceramics. When excess content of Na increases up to x = 0.02, a significantly enhanced d33 (~167 pC/N) is attained as compared with the d33 value (~116 pC/N) of uncompensated KNN ceramics. Further compensation of Na up to x = 0.025 results in a decrease of d33. Overall, the samples with higher d33 and lower OT-PTT are usually achieved in KxNLN ceramics with x = 0.010–0.015 and KNyLN ceramics with x = 0.020–0.025. These observations imply that the compositions closest to stoichiometry are attained by compensating Na or K in a very narrow range of x = 0.010–0.015 or y = 0.020–0.025. These results are consistent with previous reports that the sodium evaporates more dramatically than the potassium at the same sintering temperature [33].
In a combination with results in Fig. 3, the close relationship between OT-PTT, d33, and WRP of orthorhombic, tetragonal and monoclinic phase in KxNLN and KNyLN ceramics emerges. A common trend noticed is that ceramics with the lower WRP of monoclinic phase present a lower OT-PTT point and a higher d33. Our findings further confirm that an optimized weight ratio percentage exists in order to attain enhanced dielectric and electrical properties of KNN ceramics. It is also believed there is an optimum ratio between orthorhombic and tetragonal phase in these compositions. Subsequently, the OT-PPT shifts to near room temperature and the enhanced piezoelectric properties are attained for these ceramics.
It is important to point out that the amount of A-site nonstoichiometry is dependent on the sintering temperature. It has been shown [37, 38] that the optimum electrical properties can be attained by adjusting the sintering temperature. Wu et al. [39] reported enhanced piezoelectric properties were achieved in K0.48Na0.535NbO3 when only excess A-site Na was added as well. Sung et al. [40] reported that the piezoelectric properties of (Bi0.5K0.5+xLiy)TiO3 ceramics by K nonstoichiometry and Li addition were significantly enhanced. We believe that these phenomena are actually ascribed to high sensitiveness of structures to A-site K or Na nonstoichiometry. However, it has been suggested that the dielectric properties can be significantly influenced by induced strain upon doping rather than chemical inhomogeneities and structural disorder, which are common occurrences in doped ferroelectrics [41]. It is clear that it is necessary to carry out further detailed phase structure study of KNN with slight deviation from stoichiometry.
4 Conclusions
The coexistence of three phases, orthorhombic, tetragonal, and monoclinic phases, in 0.94K0.5+xNa0.5NbO3–0.06LiNbO3 and 0.94K0.5Na0.5+yNbO3–0.06LiNbO3 lead-free ceramics (x, y = 0–0.025) at room temperature was observed. The weight ratio percentages of three phases along with the orthorhombic-tetragonal phase transition temperature and d33 were highly sensitive to A-site K or Na non-stoichiometry. Excess A-site Na played a dominant role in decreasing the weight ratio percentage of monoclinic phase and enhancing the electrical properties of KNN by shifting the orthorhombic-tetragonal phase transition temperature to around room temperature. As compared with uncompensated KNN ceramics of d33 ~ 116pc/N, significantly d33 ~ 167pc/N was attained for 0.94K0.5Na0.5+yNbO3–0.06LiNbO3 with y = 0.02.
References
A. J. Moulson, J. M. Herbert, Electroceramics: materials, properties, applications, Second edn. (John Wiley & Sons, West Sussex, 2003)
R. Guo, L. E. Cross, S. E. Park, B. Noheda, D. E. Cox, G. Shirane, Phys. Rev. Lett. 84, 5423 (2000)
J. A. Perez, M. R. Soares, P. Q. Mantas, A. M. R. Senos, J. Eur. Ceram. Soc. 25, 2207 (2005)
Y. Saito, H. Takao, T. Tani, T. Nonoyama, K. Takatori, T. Homma, T. Nagaya, M. Nakamura, Nature (London) 432, 84 (2004)
M. R. Bafandeh, R. Gharahkhani, M. H. Abbasi, A. Saidi, J. S. Lee, H. S. Han, J. Electroceram. 33, 128 (2014)
S. Swain, P. Kumar, J. Electroceram. 32, 102 (2014)
G. Lévêque, P. Marchet, F. Levassort, L. P. Tran-Huu-Hue, J. R. Duclere, J. Eur. Ceram. Soc. 31, 577 (2011)
R. López, F. González, M. E. Villafuerte-Castrejón, J. Eur. Ceram. Soc. 30, 1549 (2010)
F. Rubio-Marcos, P. Ochoa, J. F. Fernandez, J. Eur. Ceram. Soc. 27, 4125 (2007)
E. Hollenstein, M. Davis, D. Damjanovic, N. Setter, Appl. Phys. Lett. 87, 182905 (2005)
F. Rubio-Marcos, M. G. Navarro-Rojero, J. J. Romero, P. Marchet, J. F. Fernández, IEEE Trans. Ultrason. Ferroelectr. Freq. Control 56, 1835 (2009)
Y. J. Dai, X. W. Zhang, K. P. Chen, Appl. Phys. Lett. 94, 042905 (2009)
Y. Guo, K. I. Kakimoto, H. Ohsato, Appl. Phys. Lett. 85, 4121 (2004)
Y. H. Lee, J. H. Cho, B. I. Kim, D. K. Choi, J. Appl. Phys. 47, 4620 (2008)
L. Zheng, J. Wang, J. Electroceram. 32, 192 (2014)
C. Miclea, C. Tanasoiu, C. F. Miclea, L. Amarande, A. Gheorghiu, I. Spanulescu, C. Plavitu, C. T. Miclea, M. C. Cioangher, L. Trupina, A. Iuga, J. Eur. Ceram. Soc. 27, 4055 (2007)
M. S. Kim, D. S. Lee, E. C. Park, S. J. Jeong, J. S. Song, J. Eur. Ceram. Soc. 27, 4121 (2007)
J. G. Wu, D. Q. Xiao, Y. Y. Wang, J. G. Zhu, L. Wu, Y. H. Jiang, Appl. Phys. Lett. 91, 252907–252909 (2007)
P. Zhao, B. P. Zhang, J. F. Li, Scripta Mater. 58, 429 (2008)
D. M. Lin, K. W. Kwok, H. L. Chan, J. Am. Ceram. Soc. 92, 2765 (2009)
N. Zhang, A. M. Glazer, D. Baker, P. A. Thomas, Acta Crystallogr. Sect. B: Struct. Sci. 65, 291 (2009)
S. J. Liu, B. B. Wan, P. F. Wang, S. H. Song, Scripta Mater. 63, 124 (2010)
F. Rubio-Marcos, P. Marchet, T. Merle-Méjean, J. F. Fernández, Mater. Chem. Phys. 123, 91 (2010)
N. Klein, E. Hollenstein, D. Damjanovic, H. J. Trodahl, N. Setter, M. Kuball, J. Appl. Phys. 102, 014112 (2007)
T. A. Skidmore, T. P. Comyn, S. J. Milne, Appl. Phys. Lett. 94, 222902 (2009)
D. W. Baker, P. A. Thomas, N. Zhang, A. M. Glazer, Appl. Phys. Lett. 95, 091903 (2009)
D. W. Baker, P. A. Thomas, N. Zhang, A. M. Glazer, Acta Crystallogr. Sect. B: Struct. Sci. 65, 22 (2009)
F. Rubio-Marcos, M. A. Bañares, J. J. Romero, J. F. Fernández, J. Raman Spectrosc. 42, 639 (2011)
H. J. Trodahl, N. Klein, D. Damjanovic, N. Setter, B. Ludbrook, D. Rytz, M. Kuball, Appl. Phys. Lett. 93, 262901 (2008)
R. J. C. Lima, W. Paraguassu, P. T. C. Freire, J. M. Sasaki, F. E. A. Melo, J. Mendes Filho, S. Lanfredi, J. Raman Spectrosc. 42, 639 (2011)
H. R. Xia, H. C. Chen, H. Yu, K. X. Wang, B. Y. Zhao, Phys. Stat. Sol. (b) 210, 47 (1998)
K. H. Cho, H. Y. Park, C. W. Ahn, S. Nahm, J. Am. Ceram. Soc. 90, 1946 (2007)
Y. H. Zhen, J. F. Li, J. Am. Ceram. Soc. 90, 3496 (2007)
L. J. Cheng, L. Liu, Q. Ma, S. J. Liu, Scripta Mater. 111, 102 (2016)
K. H. Härdtl, D. Hennings, J. Am. Ceram. Soc. 55, 230 (1972)
S. J. Liu, V. Zenou, I. Sus, T. Kotani, M. Schilfgaarde, N. Newman, Acta Mater. 55, 2647 (2007)
P. Zhao, B. P. Zhang, J. F. Li, J. Am. Ceram. Soc. 91, 1690 (2008)
P. Zhao, B. P. Zhang, J. F. Li, Appl. Phys. Lett. 90, 242909 (2007)
J. G. Wu, D. Q. Xiao, Y. Y. Wang, L. Wu, Y. H. Jiang, J. G. Zhu, J. Am. Ceram. Soc. 91, 2385 (2008)
Y. S. Sung, H. M. Lee, W. Du, H. G. Yeo, S. C. Lee, J. H. Cho, T. K. Song, M. H. Kim, Appl. Phys. Lett. 94, 062901 (2009)
K. Datta, K. Roleder, P. A. Thomas, Phys. Rev. B 82, 224105 (2010)
Acknowledgments
This work was financially supported by the Program for the National Natural Science Foundation of China (Grant no. 51172053 and 51302150) and the Science Research Funding of Shenzhen Municipality (Grant no. JC201105160545A and JCYJ20140509142357196).
Author information
Authors and Affiliations
Corresponding authors
Rights and permissions
About this article
Cite this article
Ma, Q., Wan, B., Cheng, L. et al. Structure and piezoelectric properties of K0.5Na0.5NbO3 based lead-free piezoceramics with slight deviation from A-site K or Na stoichiometry. J Electroceram 36, 30–39 (2016). https://doi.org/10.1007/s10832-016-0013-6
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10832-016-0013-6