Abstract
In this study, (Na0.465K0.465Bi0.07)(Nb0.93Ti0.07)O3 ceramics with MnO2 addition were prepared by conventional mixed oxide method. The effects of MnO2 addition on the structural and electrical properties of the specimens were investigated for the application of piezoelectric devices. As the results of X-ray diffraction analysis, all specimens showed the typical polycrystalline perovskite structure without presence of the second phase. Sintered densities increased with increasing the addition amount of MnO2 and the specimen added with 0.06 mol% of MnO2 showed the maximum value of 4.87 g/cm3. Average grain size increased and densification increased with an increasing of MnO2 contents. The electromechanical coupling factor, dielectric constant, dielectric loss, d33 and Curie temperature of the 0.06 mol% of MnO2 doped (Na0.465K0.465Bi0.07)(Nb0.93Ti0.07)O3 specimens were 0.32, 1309, 0.016, 122 and 465 °C, respectively.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
1 Introduction
Lead-oxide based ferroelectric ceramics, for example, Pb(Zr,Ti)O3 (PZT), Pb(Mg,Nb)O3-PbTiO3 (PMN-PT) and Pb(Zn,Nb)O3-PbTiO3 (PZN-PT) etc., are widely used for piezoelectric actuators, sensors and transducers due to their excellent electrical properties [1, 2]. Especially, PZT ceramics are widely used due to their excellent piezoelectric properties near the morphotropic phase boundary (MPB), showing a good temperature stability of electrical properties. However, lead-based piezoelectric materials fatally contain large quantities of the toxic element, Pb. Alternative lead-free piezoelectric materials have attracted much attention for environmental issues recently.
In Europe, the legislation on waste electrical electronic equipment has been issued; the use of hazardous substances such as lead in electrical parts is prohibited from 2006. From the viewpoint of sustainable development of world society, the use of toxic substances should be prohibited in many other countries in the field of piezoelectric ceramics. Therefore, it is necessary to develop lead-free ceramics with excellent piezoelectric properties [3]. Among the several lead-free ferroelectric materials, (Na,K)NbO3 (NKN)–based ferroelectric material is a promising candidate materials because it has a high Curie temperature (~ 400 °C) and good piezoelectric properties [4, 5]. NKN composition is a solid solution of ferroelectric KNbO3 and antiferroelectric NaNbO3 [6]. Recently, ferroelectric NaNbO3-BaTiO3 (NN-BT) solid solution has been investigated and showed good ferroelectric and piezoelectric properties [7].
The preparations of alkali niobate ceramics are difficult to control the chemical compositions because the volatilization of the alkali metal elements easily occurs during heat treatment at sintering temperature. Therefore, alkali niobate-base ceramics have a serious problem of their low electrical resistivity or high leakage current characteristic. In order to solve these problems, alkali niobate ceramics have been fabricated by the dopant addition or various sintering methods such as hot-pressing, spark plasma sintering, and hydrothermal synthesis [8, 9]. In previous study, we have investigated “Structural and electrical properties of (Na0.5K0.5)NbO3 ceramics with addition(0 ~ 0.07 mol%) of BiTiO3”. Result of previous experiments showed the specimen doped with 0.07 mol% of BiTiO3 has maximum value. We have investigated the doping of low melting point Bi3+ ion into A-site of NKN-base ceramics in order to increase sintered density and electrical properties and doping of Ti4+ into B-site so as to compensate excess cations of A-site. In this study (Na0.5K0.5)NbO3 specimens doped with 0.07 mol% Bi,Ti [(Na0.465K0.465Bi0.07)(Nb0.93Ti0.07)O3] from the previous experiments were chosen for the basic composition. In this study, we investigated the effects of MnO2 addition on the structural and electrical properties of (Na0.465K0.465Bi0.07)(Nb0.93Ti0.07)O3 (NKB-NT) ceramics.
2 Experimental procedure
(Na0.465K0.465Bi0.07)(Nb0.93Ti0.07)O3-xMnO2 (x = 0 ~ 0.08 mol%) ceramics were fabricated the conventional mixed-oxide method and the basic composition ratio is chosen from the previous experiments [10]. Reagent-grade Na2CO3, K2CO3, Nb2O5, Bi2O3, TiO2 and MnO2 were weighed in accordance with the formula, and mixed with zirconia balls in ethanol for 24 h. The dried powder was calcined at 950 °C for 2 h, and granulated with 3 wt% polyvinyl alcohol (PVA). The granulated powder was pressed into pellet shape with 1000 kg/cm2 pressure and then cold iso-static press (CIP) process was performed with 30 MPa. The specimens were sintered at 1110 °C for 2 h. Ag paste was painted the both side of the specimens as electrodes and specimens were electrical poled in silicon oil at 100–120 °C under a dc field of 3 kV/mm for 30 min. The crystalline structure and the microstructure of the specimens were examined using X-ray diffractometer (XRD) and field emission scanning electron microscopy (FE-SEM). Dielectric and piezoelectric properties with variation of temperature from room temperature to 500 °C were measured using a computer controlled LCR meter (HP 4284) and impedance analyzer (HP 4192A).
3 Results and discussion
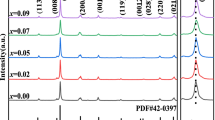
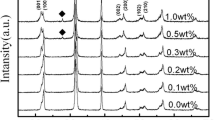
Figure 1 shows the XRD patterns of NKB-NT + x MnO2 (x = 0 ~ 0.08 mol%) specimens sintered at 1110 °C for 2 h. The NKB-NT + xMnO2 ceramics with dopant concentrations in the range from 0 to 0.08 showed a perovskite phase. The diffraction pattern was similar to that of randomly oriented pseudo cubic perovskite dielectric, all specimens were crystallized in the perovskite single phase without any formation of a second phase such as pyrochlore. The NKB-NT + MnO2 ceramics had a typical perovskite structure with a pseudo cubic structure, and no anomalous change with MnO2 contents was observed.
Figure 2 shows the surface micrographs of NKB-NT + MnO2 specimens with variation of the amount of MnO2 sintered at 1110 °C for 2 h and thermally etched at 1010 °C. Average grain size and densification increase with increasing MnO2 amount. But, the average grain size of the specimens doped with more than 0.08 mol% MnO2 decreases because some excess Mn is deposited at grain boundaries which inhibits grain growth. The average grain size in the NKB-NT + 0.06 mol% MnO2 was about 1.3 μm.
Figure 3 shows the sintered density of NKB-NT + MnO2 specimens with variation of the amount of MnO2. The sintered density increases with increasing MnO2 amount because the grain size is increased. Specimens had high density and the value of the specimen doped with 0.06 mol% MnO2 was 4.87 g/cm3. From amount of MnO2 above 0.08 mol% the density start to decrease and increase the grain boundary layer with low density and porosity, as shown in Fig. 2.
Figure 4 shows the electromechanical coupling factor of NKB-NT + MnO2 specimens with variation of the amount of MnO2. Electromechanical coupling factor increases with increasing MnO2 amount due to increase the grain size with high density and the value of the specimen doped with 0.06 mol% MnO2 was 0.32. From amount of MnO2 above 0.08 mol% the electromechanical coupling start to decrease.
Figure 5 shows the relative dielectric constant and dielectric loss of NKB-NT + MnO2 specimens with variation of the amount of MnO2. Relative dielectric constant increases with increasing MnO2 amount and the value of the specimens doped with more than 0.08 mol% MnO2 decreases. Contrary, dielectric loss decreases with increasing MnO2 amount and the value of the specimens doped with more than 0.08 mol% MnO2 increases. Mn ions exist as Mn2+ and Mn3+ ions at sintering temperature, and act as substitutions on the B-site of the ABO3 unit lattice. The Mn2+ and Mn3+ ions are associated with charged oxygen vacancies formed to maintain the neutrality condition in the same perovskite cell. In this way axial defect centers are formed, and these axial defects are switched in the direction of the applied poling field [11, 12]. Thus the dielectric constant increased and dielectric loss decreased with different amount of MnO2. In the specimens doped with more than 0.08 mol% MnO2, dielectric constant decreased and dielectric loss increased due to decrease the grain size and densification. Relative dielectric constant and dielectric loss of the specimen doped with 0.06 mol% MnO2 were 1309 and 0.016, respectively.
Figure 6 shows the piezoelectric properties of NKB-NT + MnO2 specimens with variation of the amount of MnO2. The piezoelectric properties of d33 increases with increasing MnO2 amount due to increase the grain size with high density and the value of the specimen doped with 0.06 mol% MnO2 was 122. From amount of MnO2 above 0.08 mol% the d33 start to decrease.
Figure 7 shows the relative dielectric constant of NKB-NT + MnO2 specimens with variation of the temperature. The NKB-NT ceramics have two phase transition temperatures, near about 200 °C and about 450 °C around, corresponding to the transition temperatures for the pseudo-cubic to tetragonal and tetragonal to cubic (Tc) transition, respectively
Figure 8 shows the Curie temperature of NKB-NT + MnO2 specimens with variation of the amount of MnO2. Curie temperature increases with increasing MnO2 amount, the value of the specimen doped with 0.06 mol% MnO2 was 465 °C. This suggests that the axial defect centers, which are preferentially oriented parallel to the direction of the spontaneous polarization [13], act as stabilizers to inhibit switching of the domain, as mentioned in Fig. 5. Curie temperature of the specimen doped with more than 0.08 mol% MnO2 decreased due to pores and small grain size.
4 Summary
In this work, we fabricated (Na0.465K0.465Bi0.07)(Nb0.93Ti0.07)O3–xMnO2 (x = 0 ~ 0.08 mol%) ceramics using the mixed-oxide method. And the structural and dielectric properties were investigated with variation of MnO2 amount. All specimens had a typical perovskite structure with a pseudo cubic structure. Electromechanical coupling factor, dielectric constant and dielectric loss properties were greatly affected by the structural properties such as grain size and porosity. Dielectric constant and Curie temperature properties were enhanced because of the Mn-Vo association formed to maintain the neutrality condition in the perovskite structure.
References
H.N. Al-Shareef, A.I. Kingon, X. Chen, K.R. Bellur, O. Auciello, Contribution of electrodes and microstructures to the electrical properties of Pb(Zr0.53Ti0.47)O3 thin film capacitors. J. Mater. Res. 9, 2968–2975 (1994)
R. Dat, D.J. Lichtenwalner, O. Auciello, A.I. Kingon, Polycrystalline La0.5Sr0.5CoO3/ PbZr0.53Ti0.47O3/La0.5Sr0.5CoO3 ferroelectric capacitors on platinised silicon with no polarization fatigue. Appl. Phys. Lett. 64, 2673–2675 (1994)
Y. Guo, K. Kakimoto, H. Ohsato, Dielectric and piezoelectric properties of lead-free (Na0.5K0.5)NbO3 –SrTiO3 ceramics. Solid State Commun. 129, 279–284 (2004)
K. Suenaga, K. Shibata, K. Watanabe, A. Nomoto, F. Horikiri and T. Mishima, Effect of Lattice Strain and Improvement of the Piezoelectric Properties of (K,Na)NbO3 Lead-Free Film, Jpn. J. Appl. Phys. 49, 09MA05(5 pages) (2010)
B. Malic, J. Bemard, J. Holc, D. Jenko, M. Kosec, Alkaline-earth doping in (K, Na)NbO3 based piezoceramics. J. Eur. Ceram. Soc. 25, 2707–2711 (2005)
G. Shirane, R. Newham, R. Pepinsky, Dielectric properties and phase transitions of NaNbO3 and (Na, K)NbO3. Phys. Rev. 96, 581–588 (1954)
J.T. Zeng, K.W. Kwok, W.K. Tam, H.Y. Tian, X.P. Jiang, H.L.W. Chan, Plate-like Na0.5Bi0.5TiO3 template synthesized by a topochemical method. J. Am. Ceram. Soc. 89, 3850–3853 (2006)
J.F. Li, K. Wang, B.P. Zhang, L.M. Zhang, Ferroelectric and piezoelectric properties of fine-grained Na0.5K0.5NbO3 lead-free piezoelectric ceramics prepared by spark plasma sintering. J. Am. Ceram. Soc. 89(2), 706–709 (2006)
R.E. Jaeger, L. Egerton, Hot pressing of potassium-sodium niobates. J. Am. Ceram. Soc. 45, 209–213 (1962)
T.H. Lee, D.Y. Kim, S.H. Jo, G.H. Jeong, S.G. Lee, Structural and electrical properties of (Na0.5K0.5)NbO3 ceramics with addition of BiTiO3. Trans. KIEE. 60, 2093–2096 (2011)
H.J. Hegemann, Loss mechanisms and domain stabilisation in doped BaTiO3. J. Phys. C Solid State Phys. 11, 3333–3344 (1978)
S.G. Lee, Y.H. Lee, C.Y. Park, Pyroelectric properties of lead antimony stannate –lead titanate-lead zirconate ceramics modified with La and Mn. Jpn. J. Appl. Phys. 32, 2014–2019 (1993)
Y. Matsuo, M. Fujimura, H. Sasaki, Lead titanate ceramics doped with manganese oxide. J. Am. Ceram. Soc. 48, 111–112 (1965)
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Lee, TH., Lee, SG., Yeo, JH. et al. Piezoelectric properties of (Na0.465K0.465Bi0.07)(Nb0.93Ti0.07)O3 ceramics with MnO2 addition. J Electroceram 30, 213–216 (2013). https://doi.org/10.1007/s10832-013-9786-z
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10832-013-9786-z