Abstract
Tungsten doped CaBi4Ti4O15 (CBT) ceramics was prepared by solid-state reaction method. A pure single phase of layer-structured ferroelectric with m = 4 was formed when x ≤ 0.025. The effect of W doping on dielectric, ferroelectric and piezoelectric properties was investigated. W6+ doping increased the temperature stability of dielectric constant and decreased dielectric loss. W6+ doping decreased the remanent polarization, while the coercive field decreased as well, as a result, the piezoelectric constant was increased. AC conductivity measurement showed that W6+ doping increased the conductivity of CBT ceramics and showed n-type conducting mechanism. W6+ doped ceramics has smaller activation energy due to many defects introduced in the crystal lattice.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Bismuth layer-structured ferroelectrics (BLSFs) are extensively studied for its potential use in non-volatile memory. They are also good candidate for high temperature piezoelectric applications due to their high Curie temperature. BLSFs compounds have the general formula of (Bi2O2)2+(A m−1B m O3m+1)2−, where A-site can be occupied by a variety of mono-, di- or trivalent ions with a large ionic radius, and B denotes tetra- or pentavalent ions with a small ionic radius. The integer m ranges from 2 to 5 [1, 2]. Bi4Ti3O12 (BIT) is one of the most studied compounds among BLSFs [3, 4]. During the sintering of BIT ceramic at high temperature, Bi2O3 shows a volatile nature. Therefore, oxygen vacancies are formed in pure BIT, which result in a p-type conductor [5]. Donor dopants such as V5+, W6+ in BIT can decrease the conductivity and enhance the ferroelectric and piezoelectric properties [6–9]. Nb5+ and W6+ doped BIT ceramics increased the piezoelectric constant d 33 to as high as 20 pC/N [9, 10]. CaBi4Ti4O15 (CBT) is a very promising candidate for high temperature piezoelectric applications due to its high Curie temperature (790 °C) and high electrical resistance. It has a very similar crystal structure to BIT. A-site doping is usually used to enhance the piezoelectric properties of CBT, but A-site doping often causes a decrease of Curie temperature [11, 12]. B-site doping in CBT has rarely been studied. It is still unknown whether donor dopant can decrease the oxygen vacancies and conductivity. In this paper, the effects of W6+ dopant on the dielectric, ferroelectric and piezoelectric properties of CBT are studied.
Experimental
CaBi4Ti4−x W x O15 (x = 0, 0.025, 0.050, 0.075) (CBTW x ) ceramics were prepared by the solid-state reaction method. The starting raw materials were analytical purity grade oxides and carbonate powders: Bi2O3, TiO2, WO3, and CaCO3. Chemical stoichiometric amounts of the starting powders were thoroughly mixed with ethanol in a ball mill for 4 h, dried and calcined at 900 °C for 3 h in an alumina crucible. After calcination, the ground and ball-milled powders were pressed into disks of 15 mm in diameter and about 2 mm in thickness. The pressed samples were finally sintered at temperatures of 1,000–1,180 °C for 4 h in a sealed alumina crucible. Pt electrodes were sputtered on both sides of the ceramic specimens. The samples were poled in a silicon oil bath at 200 °C under electric field of 8–9 kV/mm to measure piezoelectric properties.
Bulk densities of the sintered ceramics were measured by the Archimedes method. The crystal phases of CBTW x ceramics were determined by X-ray diffraction (XRD) analysis (D/max 2,550 V) using Cu-Kα radiation with a scan speed of 2°/min and a step width of 0.02°. The ferroelectric hysteresis loops were measured by a TF Analyzer 2000 FE-Module ferroelectric tester at a frequency of 1 Hz. Dielectric constants as a function of temperature were measured with an HP4284A LCR meter at 100 kHz. AC impedance of the specimens was measured by Agilent 4294A impedance analyzer. Piezoelectric constant d 33 was measured by a quasi-static d 33 meter (Model ZJ-3A, Institute of Acoustics, Beijing).
Results and discussion
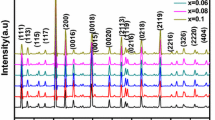
Figure 1 shows the XRD patterns of CBTW x . The inset in the figure is the enlarged of 2θ from 25 to 30°. The XRD pattern was indexed referring to JCPDS Card no. 43-0973 (SrBi4Ti4O15) because no JCPDS Card for CBT until now. The X-ray diffraction analysis indicated that pure single phase of layered perovskite ferroelectrics with m = 4 was obtained when x ≤ 0.025, while further increase of W6+, an unknown impurity phase emerged on the left of (119) peak for CBTW0.075, and a should can be seen still for sample CBTW0.050. It indicates that the solid solution capacity of W6+ substitute of Ti4+ is about 0.025 moles. The low solubility limit of W6+ can be ascribed mainly to the higher valence of W6+ compared to Ti4+. The tolerance factor for the perovskite structure is given by \( t = {{\left( {r_{A} + r_{o} } \right)}} \mathord{\left/ {\vphantom {{{\left( {r_{A} + r_{o} } \right)}} {{\sqrt 2 }}}} \right. \kern-\nulldelimiterspace} {{\sqrt 2 }}{\left( {r_{B} + r_{o} } \right)} \) where r A, r B and r o are ionic radii of an A-site cation, a B-site cation, and an oxygen ion, respectively. Since W6+ has smaller ionic radius than Ti4+ (r W = 0.60 Å, r Ti = 0.605 Å, coordinate number (C.N. = 6)), incorporation of W6+ into B-site will increase the tolerance factor of CBT. We previously studied the V5+ doped CBT ceramics [13]. V5+ has smaller ionic radius (r V = 0.54 Å, C.N. = 6) than W6+, but the solid solution capacity of V5+ in CBT (>0.1 mole) is about two times that of W6+. The levels of W dopants are too low to influence the tolerance factor in the present study. Therefore, in higher valence ion doped CBT, the valence of dopant is a more important factor to the solubility limit of B-site doping in CBT.
Figure 2 shows the temperature dependence of dielectric constant of the CBTWx ceramics. The Curie temperatures almost remain constant for all W6+ doped ceramics because the dopant content was very low, but the dielectric constant at Curie temperature decreased. The temperature stability of dielectric constant increased especially for CBTW0.025.
Figure 3 shows the temperature dependence of dielectric loss of CBTW x ceramics. The dielectric loss at room temperature is very low, but it increased fast above 400 °C. W6+ doping decreased the dielectric loss at high temperature. It can be noticed that the curves of W6+ doped CBT crossed with that of CBT, which means the W6+ doping decreases the dielectric loss only below a certain temperature, and the temperature decreased with the increase of W6+ dopant. The result indicates that there are different dielectric loss mechanisms. The oxygen vacancy is an important source in BLSFs at lower temperatures. W6+ doping decreases the oxygen vacancies in CBT ceramics, so the dielectric loss decreased. Other mechanisms such as conductivity play major role in higher temperature and W6+ doped CBT ceramics have higher dielectric loss than undoped CBT ceramics.
The hysteresis loops of CBTW x ceramics were measured at various applied voltage. Since CBTW x piezoelectric ceramics have a high Curie temperature and coercive electric field, in order to get better saturated P–E curves, all ceramic specimens were tested at 150 °C. The ferroelectric hysteresis loops are plotted as shown in Fig. 4. The dependence of 2Pr as function of x is shown in Fig. 5. It can be seen that 2Pr decreases with the increase of x, the coercive field decreased at the same time. This is different with vanadium doped BIT ceramics, 2Pr and Ec increased with the increase of V5+ content. The difference may be caused by different crystal structure and defects in both structures.
Figure 6 shows the piezoelectric constant d 33 as a function of x value. For undoped CBT, the piezoelectric constant is rather low (d 33 = 7 pC/N), and it slightly increased at x = 0.025. Further increase of W6+ doping content decreases the piezoelectric property. Although the W6+ doping decreases the remanent polarization, the coercive filed decreased as well. The improved piezoelectric property for CBTW0.025 is mainly due to its lower dielectric loss tan δ, which makes better polarization easier.
Figure 7 shows the temperature dependence of AC conductivity of CBT and CBTW0.025 ceramics. V5+ doped CBT ceramics CBTV0.05 is also used for comparison. The conductivity follows the Arrhenius law: \( \sigma = \sigma _{0} \exp ^{{ - {Ea} \mathord{\left/ {\vphantom {{Ea} {kT}}} \right. \kern-\nulldelimiterspace} {kT}}} \), where σ 0 is a pre-exponential factor and a characteristic of the material, Ea, k and T are the activation energy for conduction, Boltzmann’s constant, and the absolute temperature, respectively. It can be seen that both V5+ and W6+ doping increase the conductivity, which indicate that the CBT shows the n-type conductivity. It is different from the conductivity mechanism of BIT. S. Kim et al. studied BaBi4Ti4O15 (BBT) single crystal and found its conduction type was n-type [14]. Both CBT and BBT belong to the BLSFs with m = 4, so they show the same conductivity mechanism. V5+ and W6+ dopants in CBT have higher valence that act as donor and provide more conducting carriers. W6+ doped ceramics have higher conductivity than that of V5+ doped ceramics. The activation energy for conduction E a can be calculated from the slope of the straight line. The activation energy for CBT, CBTV0.05 and CBTW0.025 is 1.25, 1.06 and 0.95 eV, respectively. W6+ doping provides more defects into the crystal lattice and decreases the activation energy. V5+ doping can decrease the sintering temperature markedly, and decrease the concentration of oxygen vacancies caused by Bi2O3 volatilization, hence, V5+ doped ceramics have larger activation energy.
Conclusion
W6+ doped CBT ceramics was prepared by solid-state reaction method. A pure single phase of BLSF with m = 4 was formed when x ≤ 0.025. High valence of W6+ is the major factor for limit of its solid solubility. W6+ doping could increase the temperature stability of dielectric constant and decrease dielectric loss below a certain temperature. W6+ doping decreased the remanent polarization and the coercive field. W6+ doping increased the conductivity of CBT ceramics and showed an n-type conducting mechanism. The activation energy of W6+ doped ceramics decreased. Though the crystal structure of CBT is similar to BIT, the effects of donor doping on the electrical properties are different. Further investigations are required to clarify the nature of the defects.
References
B. Aurivillius, Arkiv 1, 463–480 (1949)
J.F. Dorrian, R.E. Newnham, D.K. Smith, Ferroelectrics 3, 17–27 (1971)
S.E. Cummins, L.E. Cross, J. Appl. Phys. 39, 2268–2274 (1968)
A. Fouskova, L.E. Cross, J. Appl. Phys. 41, 2834–2838 (1970)
H.S. Shulman, M. Testorf, D. Damjanovic, N. Setter, J. Am. Ceram. Soc. 79, 3124–3128 (1996)
Y. Noguchi, M. Miyayama, Appl. Phys. Lett. 78, 1903–1905 (2001)
J.S. Kim, S.S. Kim, J.K. Kim, Jpn. J. Appl. Phys. 41, 6451–6454 (2002)
X. Wang, H. Ishiwara, Appl. Phys. Lett. 82, 2479–2481 (2003)
M. Villegas, T. Jardiel, G. Farias, J. Eur. Ceram. Soc. 24, 1025–1029 (2004)
R.Q. Chu, L.N. Zhang, Z.J. Xu, Phys. Status Solidi A, Appl. Res. 201, R45–R48 (2003)
L. Zheng, G. Li, W. Zhang, Mater. Sci. Eng. B, Solid State Mater. Adv. Technol. 99, 363–365 (2003)
M. Yokosuka, Jpn. J. Appl. Phys. 41, 7123–7126 (2002)
J. Zeng, Y. Li, Q. Yang, Q. Yin, Mater. Sci. Eng. B, Solid State Mater. Adv. Technol. 117, 241–245 (2005)
S. Kim, M. Miyayama, H. Yanagida, J. Ceram. Soc. Jpn. 102, 722–726 (1994)
Acknowledgements
This work was supported by the Ministry of Sciences and Technology of China through 973-project (2002CB613307), National Natural Science Foundation China (NSFC No. 50572113), and the Innovation Project of Shanghai Institute of Ceramics (SCX200409).
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Zeng, J., Wang, Y., Li, Y. et al. Ferroelectric and piezoelectric properties of tungsten doped CaBi4Ti4O15 ceramics. J Electroceram 21, 305–308 (2008). https://doi.org/10.1007/s10832-007-9144-0
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10832-007-9144-0