Abstract
Purpose
What is the rate of euploidy and clinical viability of embryos resulting from micro 3 pronuclei zygotes?
Methods
Retrospective cohort analysis in a single, academic in vitro fertilization (IVF) center from March 2018 to June 2021. Cohorts were separated by fertilization as either a 2 pronuclear zygote (2PN) or micro 3 pronuclear zygote (micro 3PN). PGT-A was performed to identify embryonic ploidy rates in embryos created from micro 3PN zygotes. The clinical outcomes of all transferred euploid micro 3PN zygotes were evaluated from frozen embryo transfer (FET) cycles.
Results
During the designated study period, 75,903 mature oocytes were retrieved and underwent ICSI. Of these, 60,161 were fertilized as 2PN zygotes (79.3%) and 183 fertilized as micro 3PN zygotes (0.24%). Of the micro 3PN-derived embryos that underwent biopsy, 27.5% (n=11/42) were deemed euploid by PGT-A, compared to 51.4% (n=12,301/23,923) of 2PN-derived embryos, p=0.06. Four micro 3PN-derived embryos were transferred in subsequent single euploid FET cycles, which includes one live birth and one ongoing pregnancy.
Conclusion
Micro 3PN zygotes that develop to the blastocyst stage and meet the criteria for embryo biopsy have the potential to be euploid by preimplantation genetic testing for aneuploidy (PGT-A) and if selected for transfer can achieve a live birth. Although there are a significantly lower number of micro 3PN embryos that make it to blastocyst biopsy, the potential to continue to culture abnormally fertilized oocytes may give these patients a chance at pregnancy that they previously did not have.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
In the in vitro fertilization (IVF) setting, normal oocyte fertilization is commonly confirmed 13–19 hours after conventional insemination or intracytoplasmic sperm injection (ICSI) with the appearance of two pronuclei (2PN) [1, 2]. Abnormal oocyte fertilization is characterized by the presence of any number of pronuclei other than two, such as one pronuclei (1PN) or three pronuclei (3PN), which has been observed in up to ~10% of fertilized oocytes [3]. 1PN or 3PN fertilized gametes are commonly discarded due to the suspected increased risk of abnormal ploidy count [3,4,5,6]. Whether these abnormally fertilized zygotes can be cultured for successful embryo transfer has yet to be thoroughly investigated or published in reproductive medical journals [7].
1PN zygotes were believed to be haploid [5]. However, more recent studies have shown that 1PN zygotes can contain a diploid chromosomal complement, particularly if the single PN underwent a fusion or appeared asynchronously [8]. The origin of 1PN zygotes may be due to parthenogenetic oocyte activation or asynchronous pronuclear formation and have been linked with implantation failure, though more recent data has shown 1PN zygotes may retain the ability to blastulate and can even lead to live births [3, 9,10,11,12,13,14,15].
Tripronucelate zygotes (3PN) may result from dispermic penetration during conventional insemination. When 3PN zygotes are observed after the use of ICSI, their formation is usually assumed to be due to the retention of a second polar body resulting from improper meiotic division [16, 17]. However, a recent study utilizing time lapse technology shows that a majority of these zygotes do extrude the second polar body, calling this hypothesis into question [18]. These zygotes are believed to contain a triploid chromosomal complement, with the one study showing that 3PN formation accounts for 15–18% of cytogenetically abnormal cases among spontaneous abortions [19]. Despite the appearance of abnormal pronuclei counts, literature has demonstrated that the number of pronuclei is not, in fact, a direct predictor of the ploidy state of a zygote [12, 20]. For example, although many 3PN zygotes are triploid, there are documented cases in which these abnormal zygotes spontaneously revert to diploidy and “self-correct” the presumed triploid abnormality after ICSI [21, 22]. This self-correction has been observed to take place early in embryonic development perhaps at the zygotic stage. One speculation is that 3PN zygotes contain an insufficient number of centrioles when compared to the number of pronuclei present; thus, these embryos may not contain adequate microtubules to assemble three pronuclei and instead self-correct to a diploid state [22, 23].
Another possible abnormality in fertilization is the appearance of an additional micro pronucleus, termed a micro 3PN or 2.1PN. These zygotes are characterized by the development of a third “micro” pronucleus that is physically smaller than the pronuclei seen in a typical 2PN or 3PN zygote. Similar to 1PN and 3PN zygotes, there is an inference that micro 3PN zygotes are aneuploid—again likely triploid—which traditionally results in their abandonment as a potential viable embryo. However, it has been demonstrated in micro 3PN zygotes that morphology alone is not a sufficient predictor of chromosomal status. A recent study assessed the ploidy state of abnormally fertilized zygotes and observed 12/14 (85.7%) blastocysts derived from micro 3PN, which they termed 2.1PN, zygotes were diploid, while the remaining blastocysts (2/14, 14.3%) were triploid [3]. Furthermore, the study found that two of the three live births derived from abnormally fertilized oocytes resulted from micro 3PN zygotes; the third live birth was from a monopronucleated (1PN) zygote [3]. An additional recent study investigated the blastulation rate and biopsy results of 15/32 embryos derived from 2.1PN embryos and found no euploid embryos [24].
While micro 3PN zygotes account for less than 1% of fertilized oocytes, there is limited evidence to support that they retain reproductive potential [12]. Screening for ploidy status poses an opportunity to use abnormally fertilized zygotes by morphological standards, especially when a patient has no other embryos available for embryo transfer. There is a paucity of data specifically examining the chromosomal complement of micro 3PN zygotes; thus, the objective of the study is to assess the rate of euploidy and the clinical viability of micro 3PN zygotes by looking at the largest cohort of micro 3PN embryos analyzed by preimplantation genetic testing for aneuploidy (PGT-A).
Materials and methods
This retrospective cohort study included all infertile patients who presented to a single academic center from March 2018 to June 2021 and underwent in vitro fertilization with ICSI and preimplantation genetic testing for aneuploidy (PGT-A) for any indication. All embryos developed from fresh in vitro fertilization cycles from both 2PN and micro 3PN fertilized zygotes were analyzed. Embryos were excluded if they were developed from cryopreserved and thawed oocytes, from surgical sperm, and previously cryopreserved gametes which were subsequently thawed for PGT-A only. Euploid embryos that developed from micro 3PN zygotes were considered suitable for embryo transfer if no other euploid embryos were available that developed from 2PN zygotes. Our study was approved by an academic institutional review board (IRB#18-00441).
Clinical protocols
Ovarian stimulation
Patients underwent controlled ovarian hyperstimulation for IVF as previously described [25] using a protocol determined by their physician. Transvaginal ultrasound was used to monitor follicular growth until at least two follicles were deemed mature (reaching at least 18 mm in diameter). The trigger type for final maturation of the oocytes was determined by individual physicians. Final maturation was induced by either purified or recombinant human chorionic gonadotropin (HCG) alone (Ovidrel®, EMD Serono, Rockland, MA, USA; Novarel®, Ferring Pharmaceuticals, Parsippany, NJ, USA; or Pregnyl®, Schering-Plough, Kenilworth, NJ, USA), leuprolide acetate alone (Lupron®, AbbVie Laboratories, Chicago, IL, USA) alone, or a “dual trigger” with both HCG and leuprolide acetate. 36 hours after surge, patients underwent ultrasound-guided vaginal oocyte retrieval.
Laboratory procedures
Oocytes were examined for maturity between 4 and 6 hours post retrieval. All oocytes reaching the Metaphase II (M2) stage were then inseminated by intracytoplasmic sperm injection (ICSI) due to planned preimplantation genetic testing for aneuploidy (PGT-A). Embryo media culture conditions were as previously described [25]. Of note, embryos were cultured in bench top incubators, and time lapse was not used. Fertilization check was performed 16–18 hours post ICSI, and any embryo with an abnormal number of pronuclei was noted. Zygotes which had two evenly sized pronuclei were defined as 2PN zygotes, whereas zygotes which showed one additional smaller pronucleus in addition to two evenly size pronuclei as annotated by the embryologist were defied as micro 3PN zygotes (Fig. 1). On day 3 of development, laser-assisted hatching was performed on all embryos to increase herniation of the trophectoderm (TE) for preimplantation genetic testing by using a ZILOS-tk laser (Hamilton Thorne Biosciences, Beverly, MA) to form a small 25–30 μm opening in the zona pellucida. All embryos were cultured to the blastocyst stage as previously described [26].
Embryos were examined on days 5, 6, and 7 of development for eligibility for biopsy. All blastocysts were graded using a modified Gardner system to evaluate the expansion, inner cell mass, and trophectoderm, and subsequent TE biopsy was performed on eligible embryos as previously described [27]. The morphological grading of the embryos was used to classify them into three groups by comparing the expansion, inner cell mass, and trophectoderm grades at the time of vitrification as previously described [28]. The three groups included good quality embryos (expansion grade of 4 or greater and ICM and TE grading of AA, AB, BA, or BB), moderate quality embryos (expansion grade 4 or greater and ICM and TE grading of AC, CA, BC, or CB), and fair quality (any expansion grade and an ICM and TE grade of CC). TE biopsy cells were sent to a commercial lab (Invitae) for PGT-A evaluation using FAST-SeqS next-generation sequencing (NGS) platform [29], and all embryos were then cryopreserved using vitrification and the Cryotop™ method (Kitazato Corp., Shizuoka, Japan).
Embryo selection
Euploid embryos by PGT-A were considered for subsequent FET. Embryos with initially abnormal fertilization pattern were only considered for transfer if there were no euploid embryos available from 2PN zygotes. Cryopreserved euploid embryos with the best morphologic grade were selected for transfer and thawed on the day of transfer as previously described [25]. Synthetic preparation of the endometrium was used for subsequent FET cycles as previously described [27]. Pregnancy rates from subsequent frozen-thawed single euploid embryo transfer (SEET) cycles were examined.
Statistical analysis
For statistical analysis, comparative statistics were performed with t-test and Kruskal Wallis after testing for normality. Categorical data was compared using chi-squared test. Results were expressed as percentages, means, and standard deviations as appropriate. Logistic regression was performed for both embryo biopsy rate and euploidy rate and adjusted for confounding variables which were decided a priori based on prior studies which included oocyte age, AMH, partner age, BMI, and semen analysis parameters. All p values are set with a clinical significance level determined at p ≤ 0.05.
Results
During the designated study period, 75,903 mature oocytes were retrieved and underwent ICSI. Of these, 60,161 were fertilized as 2PN zygotes (79.3%) and 183 fertilized as micro 3PN zygotes (0.24%). Demographic characteristics of the cycles the embryos derived from were compared and included in Table 1.
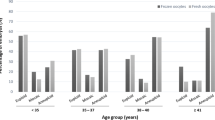
The number of embryos that developed to the blastocyst stage and met biopsy criteria was significantly different among the groups (39.8% of 2PNs (n=23,923/60,161) vs. 23.0% of micro 3PNs (n=42/183), p <0.00001) (Fig. 2). A logistic regression was then performed and adjusted for oocyte age, BMI, AMH, partner age, and semen analysis parameters. There was still a lower odds of blastocyst biopsy with embryos derived from micro 3PN embryos versus 2PN embryos with an OR of 0.09 and 95% CI 0.06, 0.14 (p=<0.0001).
Of the micro 3PN-derived embryos that underwent biopsy, 27.5% (n=11/42) were deemed euploid by PGT-A, compared to 51.4% (n=12,301/23,923) of 2PN-derived embryos, p=0.06 (Fig. 2). Of the micro 3PN-derived embryos that underwent biopsy, 2.4% (n=1/42) had a mosaic component by PGT-A compared to 6.0% (n=1438/23,923) of 2PN embryos in this cohort, p=0.98. A logistic regression was again performed and adjusted for oocyte age, BMI, AMH, partner age, and semen analysis parameters. There was a lower odds of euploidy with embryos derived from micro 3PN embryos versus 2PN embryos with an OR 0.04 and 95% CI 0.02, 0.09 (p=<0.0001). PGT-A analysis of each micro 3PN biopsied embryo is shown in Table 2, specifically noted is there were no embryos with a triploidy complement. The distribution of types of aneuploidies in micro 3PN embryos is relatively similar to embryos tested in the validation study by the commercial lab using FAST-SeqS NGS [28].
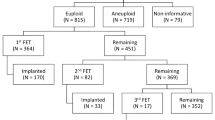
Four micro 3PN-derived embryos were transferred in subsequent frozen-thawed SEET cycles. One transfer resulted in a live birth, one resulted in a clinical loss after a crown rump length was observed (without testing of products of conception), one resulted in an ongoing pregnancy, and the last transfer did not result in a pregnancy.
Discussion
Micro 3PN zygotes that develop to blastocyst stage and meet the criteria for embryo biopsy have the potential to be euploid and if selected for transfer can achieve a live birth. This study’s finding is contrary to prior clinical inference that abnormally fertilized oocytes develop to be aneuploid and would not be suitable for potential embryo transfer. Notably, as a first-line choice, physicians should offer genetic testing of and potential transfer normally fertilized 2PN embryos and precautions should be taken when transferring embryos that originated from micro 3PN zygotes. In this study, we observed significantly less micro 3PN embryos available for biopsy when compared to normally fertilized 2PN embryos. Despite this difference, the study showed that micro 3PN zygotes can be used by patients as a second-line choice if they have no other embryos available for transfer.
Micro 3PN zygotes in particular, like 3PN zygotes, were thought to be triploid and were not expected to result in a healthy pregnancy if selected for transfer. When abnormal fertilization is confirmed in the laboratory by the presence of anything other than two pronuclei, it had been common practice to discard these “abnormal” zygotes [10]. Recent data suggests that 1PN, 3PN, and micro 3PN (also referred to as 2.1PN) zygotes are capable of forming viable embryos with normal chromosomal configuration, despite initial abnormal morphology in the fertilization assessment [12, 17]. Beyond development of viable embryos, abnormally fertilized zygotes have been shown to result in clinical pregnancy and live births [12, 16, 19].
This data is of particular importance to patients with diminished ovarian reserve who may only have embryos from abnormally fertilized oocytes available for biopsy and transfer. Previous studies have shown that 1PN, 3PN, and micro 3PN embryos can all have reproductive potential [3, 5,6,7, 9, 12,13,14, 19, 20, 30,31,32,33]. Although there are a significantly lower number of micro 3PN embryos that make it to blastocyst biopsy, the potential to continue to culture abnormally fertilized oocytes may give these patients a chance at pregnancy that they previously did not have.
The results of our study confirm that morphology alone is not a sufficient predictor of ploidy, particularly that two pronuclei plus one “micro” pronucleus as shown in micro 3PN zygotes do not predict triploidy. Similar to 1PN and 3PN zygotes, genetic testing by PGT-A on micro 3PN zygotes should be considered to understand their chromosomal configuration and potential for euploid blastocyst embryo transfer [3]. In this study, we biopsied blastocysts developed from micro 3PN zygotes and show that a number of micro 3PNs are in fact diploid and euploid by PGT-A and capable of producing viable embryos that result in clinical pregnancy. Interestingly, none of the micro 3PN embryos showed a triploid chromosomal complement; thus, this prior belief should not dissuade extended culture of these abnormally fertilized oocytes.
Furthermore, of the 4 embryos that were transferred, 75% resulted in a clinical pregnancy (n = 3/4), although one encountered an early clinical loss. In the context of the starting number of micro 3PNs in this study, 1.1% (n = 2/183) led to ongoing pregnancy/live birth. Although a small proportion, this demonstrates the possibility for abnormally fertilized embryos to develop into normal embryos and give patients an opportunity for transfer and live birth.
However, this study is not without limitations. The retrospective nature of this study restricts variables that we can study. The use of time lapse imaging could give more information on the formation or early correction of micro 3PN embryos, which could not be assessed due to our lab protocols. This study does not answer the questions of how or why micro 3PN embryos form and the hypotheses remain including fragmented nucleus from fertilization, failed cytokinesis, diandry, and delayed fertilization. This study is limited by not using an objective measurement of a “micro” 3PN and instead relies on the expertise of the embryologist to annotate this fertilization pattern which could add in potential bias. However, senior embryologists confirm all fertilization checks on abnormally fertilized oocytes which decreases this bias. In addition, PGT-A by NGS in abnormally fertilized oocytes could benefit from the addition of karyomapping to increase accuracy.
In conclusion, given modern sequencing advances, we now provide further evidence that micro 3PN zygotes can develop into euploid blastocysts and result in ongoing pregnancies. The study advances understanding of the genetic heterogeneity within abnormally fertilized oocytes and increases assurance in achieving a clinical pregnancy when 2PN embryos are absent, even though micro 3PNs account for a small proportion of all retrieved oocytes. This study adds to the scientific literature on abnormally fertilized oocytes by including a larger sample size of micro 3PN zygotes and using all NGS for PGT-A. Further studies are needed to increase the number of transfers with abnormally fertilized oocytes and to determine if other “abnormal” PN counts identified during the fertilization assessment could also yield viable euploid embryos for transfer.
References
Alpha Scientists in Reproductive Medicine and European Society for Human Reproduction and Embryology Special Interest Group of Embryology. The Istanbul consensus workshop on embryo assessment: proceedings of an expert meeting. Hum Reprod. 2011;26:1270–83.
Nagy ZP, Janssenswillen C, Janssens R, De Vos A, Staessen C, Van de Velde H, Van Steirteghem AC. Timing of oocyte activation, pronucleus formation and cleavage in humans after intracytoplasmic sperm injection (ICSI) with testicular spermatozoa and after ICSI or in-vitro fertilization on sibling oocytes with ejaculated spermatozoa. Hum Reprod. 1998 ;13(6):1606–12. https://doi.org/10.1093/humrep/13.6.1606.
Capalbo A, Treff N, Cimadomo D, Tao X, Ferrero S, Vaiarelli A, Colamaria S, Maggiulli R, Orlando G, Scarica C, Scott R, Ubaldi FM, Rienzi L. Abnormally fertilized oocytes can result in healthy live births: improved genetic technologies for preimplantation genetic testing can be used to rescue viable embryos in in vitro fertilization cycles. Fertil Steril. 2017;108(6):1007–1015.e3. https://doi.org/10.1016/j.fertnstert.2017.08.004.
Plachot M, de Grouchy J, Junca AM, Mandelbaum J, Salat-Baroux J, Cohen J. Chromosome analysis of human oocytes and embryos: does delayed fertilization increase chromosome imbalance? Hum Reprod. 1988 ;3(1):125–7. https://doi.org/10.1093/oxfordjournals.humrep.a136644.
Rosenbusch B. The chromosomal constitution of embryos arising from monopronuclear oocytes in programmes of assisted reproduction. Int J Reprod Med. 2014;2014:418198.
Staessen C, Van Steirteghem AC. The chromosomal constitution of embryos developing from abnormally fertilized oocytes after intracytoplasmic sperm injection and conventional in-vitro fertilization. Hum Reprod. 1997;12(2):321–7. https://doi.org/10.1093/humrep/12.2.321.
Feenan K, Herbert M. Can ‘abnormally’ fertilized zygotes give rise to viable embryos? Hum Fertil (Camb). 2006;9(3):157–69. https://doi.org/10.1080/14647270600636269.
Munné S, Tang YX, Grifo J, Cohen J. Origin of single pronucleated human zygotes. J Assist Reprod Genet. 1993;10(4):276–9. https://doi.org/10.1007/BF01204942.
Chen X, Shi S, Mao J, Zou L, Yu K. Developmental potential of abnormally fertilized oocytes and the associated clinical outcomes. Front Physiol. 2020;4:528424. https://doi.org/10.3389/fphys.2020.528424.
Gras L, Trounson AO. Pregnancy and birth resulting from transfer of a blastocyst observed to have one pronucleus at the time of examination for fertilization. Hum Reprod. 1999;14(7):1869–71. https://doi.org/10.1093/humrep/14.7.1869.
Itoi F, Asano Y, Shimizu M, Honnma H, Murata Y. Birth of nine normal healthy babies following transfer of blastocysts derived from human single-pronucleate zygotes. J Assist Reprod Genet. 2015 ;32(9):1401–7. https://doi.org/10.1007/s10815-015-0518-y.
Macas E, Imthurn B, Roselli M, Keller PJ. Chromosome analysis of single- and multipronucleated human zygotes proceeded after the intracytoplasmic sperm injection procedure. J Assist Reprod Genet. 1996;13(4):345–50. https://doi.org/10.1007/BF02070150.
Mateo S, Parriego M, Boada M, Vidal F, Coroleu B, Veiga A. In vitro development and chromosome constitution of embryos derived from monopronucleated zygotes after intracytoplasmic sperm injection. Fertil Steril. 2013;99(3):897–902.e1. https://doi.org/10.1016/j.fertnstert.2012.11.014. Epub 2012 Dec 14
Mateo S, Vidal F, Parriego M, Rodríguez I, Montalvo V, Veiga A, Boada M. Could monopronucleated ICSI zygotes be considered for transfer? Analysis through time-lapse monitoring and PGS. J Assist Reprod Genet. 2017;34(7):905–11. https://doi.org/10.1007/s10815-017-0937-z.
van der Heijden GW, van den Berg IM, Baart EB, Derijck AA, Martini E, de Boer P. Parental origin of chromatin in human monopronuclear zygotes revealed by asymmetric histone methylation patterns, differs between IVF and ICSI. Mol Reprod Dev. 2009;76(1):101–8. https://doi.org/10.1002/mrd.20933.
Flaherty SP, Payne D, Swann NJ, Matthews CD. Assessment of fertilization failure and abnormal fertilization after intracytoplasmic sperm injection (ICSI). Reprod Fertil Dev. 1995;7(2):197–210. https://doi.org/10.1071/rd9950197.
Sachs AR, Politch JA, Jackson KV, Racowsky C, Hornstein MD, Ginsburg ES. Factors associated with the formation of triploid zygotes after intracytoplasmic sperm injection. Fertil Steril. 2000;73(6):1109–14. https://doi.org/10.1016/s0015-0282(00)00521-5.
Ezoe K, Takahashi T, Shimazaki K, Miki T, Tanimura Y, Amagai A, Sawado A, Akaike H, Mogi M, Kaneko S, Kato M, Kato K, Tarozzi N, Borini A, Coticchio G. Human 1PN and 3PN zygotes recapitulate all morphokinetic events of normal fertilization but reveal novel developmental errors. Hum Reprod. 2022;37(10):2307–19. https://doi.org/10.1093/humrep/deac177.
Li M, Zhao W, Xue X, Zhang S, Shi W, Shi J. Three pro-nuclei (3PN) incidence factors and clinical outcomes: a retrospective study from the fresh embryo transfer of in vitro fertilization with donor sperm (IVF-D). Int. J Clin Exp Med. 2015;8(8):13997–4003.
Mutia K, Wiweko B, Iffanolida PA, Febri RR, Muna N, Riayati O, Jasirwan SO, Yuningsih T, Mansyur E, Hestiantoro A. The frequency of chromosomal euploidy among 3PN embryos. J Reprod Infertil. 2019;20(3):127–31.
Gu C, Li K, Li R, Li L, Li X, Dai X, He Y. Chromosomal aneuploidy associated with clinical characteristics of pregnancy loss. Front Genet. 2021;15(12):667697. https://doi.org/10.3389/fgene.2021.667697.
Grau N, Escrich L, Martín J, Rubio C, Pellicer A, Escribá MJ. Self-correction in tripronucleated human embryos. Fertil Steril. 2011;96:951–6.
Joergensen MW, Labouriau R, Hindkjaer J, Stougaard M, Kolevraa S, Bolund L, Agerholm IE, Sunde L. The parental origin correlates with the karyotype of human embryos developing from tripronuclear zygotes. Clin Exp Reprod Med. 2015 ;42(1):14–21. https://doi.org/10.5653/cerm.2015.42.1.14.
Takahashi H, Hirata R, Otsuki J, Habara T, Hayashi N. Are tri-pronuclear embryos that show two normal-sized pronuclei and additional smaller pronuclei useful for embryo transfer? Reprod Med Biol. 2022;21(1):e12462. https://doi.org/10.1002/rmb2.12462.
Rodriguez-Purata J, Lee J, Whitehouse M, Duke M, Grunfeld L, Sandler B, Copperman A, Mukherjee T. Reproductive outcome is optimized by genomic embryo screening, vitrification, and subsequent transfer into a prepared synchronous endometrium. J Assist Reprod Genet. 2016;33(3):401–12. https://doi.org/10.1007/s10815-016-0647-y.
Nazem TG, Sekhon L, Lee JA, Overbey J, Pan S, Duke M, Briton-Jones C, Whitehouse M, Copperman AB, Stein DE. The correlation between morphology and implantation of euploid human blastocysts. Reprod Biomed Online. 2019;38(2):169–76. https://doi.org/10.1016/j.rbmo.2018.10.007.
Hernandez-Nieto C, Lee JA, Slifkin R, Sandler B, Copperman AB, Flisser E. What is the reproductive potential of day 7 euploid embryos? Hum Reprod. 2019;34(9):1697–706. https://doi.org/10.1093/humrep/dez129.
Hernandez-Nieto C, Lee JA, Alkon-Meadows T, Luna-Rojas M, Mukherjee T, Copperman AB, Sandler B. Late follicular phase progesterone elevation during ovarian stimulation is not associated with decreased implantation of chromosomally screened embryos in thaw cycles. Hum Reprod. 2020;35(8):1889–99. https://doi.org/10.1093/humrep/deaa123.
Walters-Sen L, Neitzel D, Bristow SL, Mitchell A, Alouf CA, Aradhya S, Faulkner N. Experience analysing over 190,000 embryo trophectoderm biopsies using a novel FAST-SeqS preimplantation genetic testing assay. Reprod Biomed Online. 2022;44(2):228–38. https://doi.org/10.1016/j.rbmo.2021.06.022.
Barak Y, Kogosowski A, Goldman S, Soffer Y, Gonen Y, Tesarik J. Pregnancy and birth after transfer of embryos that developed from single-nucleated zygotes obtained by injection of round spermatids into oocytes. Fertil Steril. 1998;70(1):67–70. https://doi.org/10.1016/s0015-0282(98)00106-x.
Chen Z, Yan J, Feng HL. Aneuploid analysis of tripronuclear zygotes derived from in vitro fertilization and intracytoplasmic sperm injection in humans. Fertil Steril. 2005;83(6):1845–8. https://doi.org/10.1016/j.fertnstert.2004.11.076.
Kola I, Trounson A, Dawson G, Rogers P. Tripronuclear human oocytes: altered cleavage patterns and subsequent karyotypic analysis of embryos. Biol Reprod. 1987;37(2):395–401. https://doi.org/10.1095/biolreprod37.2.395.
Yalçınkaya E, Özay A, Ergin EG, Öztel Z, Özörnek H. Live birth after transfer of a tripronuclear embryo: an intracytoplasmic sperm injection as a combination of microarray and time-lapse technology. Turk. J Obstet Gynecol. 2016;13(2):95–8. https://doi.org/10.4274/tjod.45144.
Acknowledgements
The authors thank all the physicians, fellows, embryologists, and research and staff members for the valuable work and help in the realizing of this manuscript.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Ethics approval
This retrospective chart review study involving human participants was in accordance with the ethical standards of the institutional and national research committee and with the 1964 Helsinki Declaration and its later amendments or comparable ethical standards. The Human Investigation Committee (IRB# 18-00441) of the Icahn School of Medicine at Mount Sinai approved this study.
Consent to participate
Not applicable.
Consent for publication
Not applicable.
Conflict of interest
Alan Copperman is a board member of Sema4 Genomics and Progyny and possesses stock/stock options in Sema4 Genomics and Progyny. The other coauthors declare no conflicts of interest.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Canon, C., Thurman, A., Li, A. et al. Assessing the clinical viability of micro 3 pronuclei zygotes. J Assist Reprod Genet 40, 1765–1772 (2023). https://doi.org/10.1007/s10815-023-02830-y
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10815-023-02830-y