Abstract
Purpose
To study whether a new combination of different warming kits is clinically effective for vitrified human blastocysts.
Methods
This is a longitudinal cohort study analysing two hundred fifty-five blastocysts warming cycles performed between January and October 2018. Embryos were vitrified using only one brand of ready-to-use kits (Kitazato), whereas the warming procedure was performed with three of the most widely used vitrification/warming kits (Kitazato, Sage and Irvine) after patient stratification for oocyte source. The primary endpoint was survival rate, while the secondary endpoints were clinical pregnancy, live birth and miscarriage rates.
Results
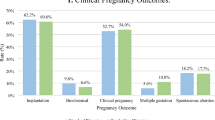
We observed a comparable survival rate across all groups of 100% (47/47) in KK, 97.6% (49/50) in KS, 97.6% (41/42) in KI, 100% (38/38) in dKK, 100% (35/35) in dKS and 100% (43/43) in dKI. Clinical pregnancy rates were also comparable: 38.3% (18/47) in KK, 49% (24/49) in KS, 56.1% (23/ 41) in KI, 47.4% (18/38) in dKK, 31.4% (11/35) in dKS and 48.8% (21/ 43) in dKI. Finally, live birth rates were 29.8% (14/47) in KK, 36.7% (18/49) in KS, 46.3% (19/41) in KI, 36.8% (14/38) in dKK, 25.7% (9/35) in dKS and 41.9% (18/43) in dKI, showing no significant differences.
Conclusion
This study confirmed the efficacy of applying a single warming protocol, despite what the “industry” has led us to believe, supporting the idea that it is time to proceed in the cryopreservation field and encouraging embryologists worldwide to come out and reveal that such a procedure is possible and safe.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Currently in vitro fertilization (IVF) clinics face the need to transfer gametes or embryos between centres. Initially, the application of the slow-freezing (SF) method to both oocytes and embryos provided reasonably good outcomes [1], but IVF laboratories rapidly switched their practice towards vitrification (VT) when this protocol was proven simpler, faster and safer than slow freezing [2] and as efficient as fresh transfers in terms of pregnancy, miscarriage and live birth rates [3,4,5,6]. Vitrification, indeed, represents the gold standard worldwide using different commercial brands of ready-to-use solutions [7]. Among the thawing media approved for human reproduction, each brand producing cryopreservation kits usually recommends the use of its own thawing kit. However, for the IVF laboratory, it can be quite expensive or difficult to source the same brand solution for every cryopreservation kit. The vitrification/warming (VIT/WARM) solutions contained in the cryopreservation kits present only small differences in their composition, although the exact amount and type of the single components are rarely declared [8]. In order to minimize inter-laboratory variability in the freezing/thawing procedures, it is possible to consider combining different VIT/WARM kits based on the use of a single WARM solution of a given brand, to warm specimens vitrified with another brand. As a matter of fact, a potential “Universal Warming” protocol based on subsequent steps with 1 M and 0.5 M concentration of any extracellular cryoprotectant (ECCP) has already been proposed, irrespective of the freezing method originally applied [9]. This strategy is based on the combination of different VIT/WARM solutions and proved effective in handling specimens cryopreserved by both slow freezing and vitrification. The procedure has been successfully tested on some VIT/WARM kit combinations on human oocytes [8,9,10,11,12,13] and cleavage stage embryos and blastocysts [14, 15]. Interestingly, the clinical impact of the combinations of Kitazato (Kitazato, Japan), Sage (Origio, Denmark) and Irvine (FujiFilm Irvine, US) vitrification/warming solutions have been tested on human oocytes, showing comparable cryo-survival, blastulation and implantation rates in both own and donor oocyte cycles [8,9,10,11,12,13]. However, the data available on human blastocysts are limited to the combinations of a restricted number of commercial kits, thus requiring further studies to reinforce the evidence for the application of this strategy in thawed blastocyst transfer cycles. In particular, Kitazato and Sage were previously tested and showed comparable cryo-survival and implantation rates [14]. The aim of the present study was to test the flexibility of the “Universal Warming” protocol by checking a new combination of VIT/WARM solutions—Kitazato and Irvine, previously tested only on human oocytes [8]—and comparing cryo-survival, pregnancy and live birth rates of vitrified human blastocysts to those obtained with another commercial kits brands. Following the common and often unavoidable combination of different VIT/WARM solutions in the routine of the IVF lab, the current study encourages embryologists to share their expertise and test new kits combinations, in order to reinforce the clinical evidence on the safety and efficacy of this procedure, especially in case of legal controversies.
Material and methods
Study design
This longitudinal cohort study analysed the outcomes of 255 blastocyst warming cycles performed on 236 consecutive patients between January and October 2018 at LIVET Clinic (Turin, Italy). In particular, 139 blastocyst warmings were performed using patients’ own oocytes, whereas other 116 warmings were performed with egg donation-derived embryos. Cycles with female age at freezing > 44 years for patients using own oocytes and > 50 years for patients using donor oocytes were not included in the study. Cycles with embryos biopsied for the preimplantation genetic test (PGT) were also excluded. Egg donation-derived embryos were generated at Marques Clinic (Barcelona, Spain) using donor eggs and own partner frozen sperm and then shipped to Italy, where embryo transfer (ET) was performed. All blastocysts were vitrified with Cryotop carrier using the Vitrification Kit (Kitazato, Japan) [16, 17]. At warming, the embryos, stratified by patients’ oocyte source, were randomly allocated into three arms, corresponding to different warming kits. The six resulting groups were identified as follows: (a) group KK: 47 embryos from own oocytes vitrified with Kitazato and warmed with Kitazato (Kitazato, Japan); (b) group KS: 50 embryos from own oocytes vitrified with Kitazato and warmed with Sage (Origio, Denmark); (c) group KI: 42 embryos from own oocytes vitrified with Kitazato and warmed with Irvine (FujiFilm Irvine, US); (d) group dKK: 38 embryos from donor oocytes vitrified with Kitazato and warmed with Kitazato; (e) group dKS: 35 embryos from donor oocytes vitrified with Kitazato and warmed with Sage; (f) group dKI: 43 embryos from donor oocytes vitrified with Kitazato and warmed with Irvine. Randomization was achieved using a specific software available online (http://www.randomizer.org). The primary endpoint was the survival rate (number of embryos surviving per number of embryos warmed). Blastocysts were scored 2 h after warming and were considered to have survived in case of ≥ 50% of blastocoel re-expansion and in the absence of dark necrotic cytoplasm and of cracked zona pellucida [18]. The secondary endpoints were clinical pregnancy (US confirmed by the presence of a gestational sac with foetal heartbeat 2 weeks after positive hCG testing), live birth and miscarriage rates. The current study has been conducted according to the Declaration of Helsinki for medical research. All the media used for this study are class III CE 93/42 certified, approved and marketed for thawing procedures in human IVF. Signed, written informed consent was obtained from all patients undergoing IVF treatment and embryo cryopreservation, performed with one of the cryopreservation kits available on the market and with a high-performance vitrification technique.
Controlled ovarian stimulation, oocyte retrieval, insemination and embryo culture
Controlled ovarian stimulation, transvaginal ultrasound-guided oocyte retrieval, oocyte decumulation, ICSI and embryo culture were performed according to our clinical practice, as described elsewhere [19]. Embryo culture at low oxygen tension (5%) was performed in BT37 Planer (Origio) benchtop incubators. The blastocysts not transferred during the fresh cycle were vitrified using the Kitazato protocol on day 5 or 6 and their performance at warming was analysed.
Embryos from donor oocytes
Fresh oocytes from young donors were inseminated with their own partner frozen sperm [12, 20]. The blastocysts obtained were vitrified using the Kitazato protocol. The shipping from Spain to Italy was performed by an authorized air courier. Once in Livet, the embryos were warmed in accordance with the “Universal Warming” protocol as described below.
Composition of vitrification/warming kits
As described elsewhere [8, 13, 16], Kitazato vitrification and warming solutions contain trehalose and are supplemented with hydroxypropyl cellulose (HPC); Sage contains sucrose with human serum albumin (HSA); Irvine kits contain sucrose with dextran serum supplement (DSS). The basic medium is TCM199 for Kitazato and Irvine, whereas it is modified HTF with MOPS for Sage. The cryoprotectant cocktail comprises 7.5% dimethyl sulfoxide (DMSO) and 7.5% ethylene glycole (EG) in the equilibration solution, and 15% DMSO and 15% EG in the vitrification solution. All the warming kits involve subsequent steps with 1 M and 0.5 M concentrations of ECCP.
Universal Warming procedure
The following solutions were used for warming: 4 ml of 1 M ECCP warming solution, 500 μl of 0.5 M ECCP warming solution and 500 μl + 500 μl of washing solution (basic medium). The first warming step was performed with the 1 M ECCP warming solution at 37 °C. The vial(s) containing the solution was pre-warmed to 37 °C at least 60 min before use and kept closed throughout. The other solutions were brought to room temperature (20–25 °C) at least 60 min before use; the content of each vial was well mixed by repeated gentle inversion before use and aseptically dispensed into a one-well dish (BioCare, Europe): 500 μl of 0.5 M into well 1, 500 μl of washing solution into well 2 and another 500 μl into well 3. The entire warming procedure was described by Parmegiani et al. [8].
Endometrial preparation and embryo transfer
Endometrial preparation was performed in a natural cycle, supported with 180 mg/day natural progesterone intravaginally (Crinone 8, MerckSerono, Germany). Progesterone supplementation started 2 days after the detection of the urinary LH peak (LH + 2), whereas embryo transfer (ET) was performed at LH + 5. A single expanded blastocyst was transferred in utero using the soft catheter Sydney Guardia (Cook, Australia) under US guidance.
Statistical analysis
The sample size was calculated to test the kits’ equivalence for the cryo-survival rate. The calculation of sample size was based on our experience with vitrification, assuming the mean survival rate of 98% obtained in our centre in the previous 5 years. A sample size of 35 embryos in each group achieved 76% power to detect a survival rate of 70% as minimum competence and 95% as a benchmark [21] using a 2 degrees of freedom chi-square test with a significance level (alpha) of 0.05 (PASS 2020 Power Analysis and Sample Size Software (2020). NCSS, LLC. Kaysville, Utah, USA, ncss.com/software/pass). Continuous variables are shown as mean ± standard deviation (SD) and median (IQR), and categorical variables as absolute and relative frequencies. Confidence intervals (CI95%) are presented. After assessing the normal distribution of data with the Shapiro–Wilk test, comparison among groups was performed using the analysis of variance ANOVA or the non-parametric Kruskal–Wallis test, as appropriate, for continuous variables, and the chi-square test for categorical variables. All statistical tests were two-sided and a p-value of 0.05 or less was considered statistically significant. All the analyses were performed with Stata16 (StataCorp. 2019. Stata Statistical Software: Release 16. College Station, TX: StataCorp LLC).
Results
The clinical characteristics of the 236 consecutive patients, stratified according to oocyte source, are summarized in Table 1. No significant differences were observed between the three kits used to perform blastocyst warming. Table 2 reports data about the study outcomes. In patients using their own oocytes, a comparable survival rate among the three study groups was observed: 100% (47/47) in group KK, 97.6% (49/50) in group KS and 97.6% (41/42) in group KI. Clinical pregnancy rates were also comparable: 38.3% (18/47) in group KK, 49% (24/49) in group KS and 56.1% (23/ 41) in group KI. Live birth rates were 29.8% (14/47) in group KK, 36.7% (18/49) in group KS and 46.3% (19/41) in group KI respectively, with higher but not statistically significant values in group KI. Finally, no significant differences were observed for miscarriage rates.
We observed similar results, with no significant differences among groups, with egg donation-derived embryos (Table 2).
The percentage of transferred day 5 blastocysts was comparable among the three study groups for both patients using own oocytes and donated eggs. We obtained similar embryological and clinical outcomes even after stratification according to the day of vitrification (data not shown).
Discussion
To our knowledge, the present study is the first reporting the application a single warming protocol in a new combination of VIT/WARM kits on vitrified human blastocysts, namely Kitazato for vitrification and Irvine for warming. This combination may be considered as “off-label”, according to the recommendation of each brand producing cryopreservation kits to use its own thawing kit. In the current study, this alternative and unconventional use was reported as a common, although covert, combination of kits used in the IVF clinical practice. Importantly, preliminary tests have been performed on research oocytes before designing the current study. In our work, the clinical efficacy of this “Universal Warming” protocol was tested in a large number of embryos thawing cycles, with both own and donor oocytes, using 1 and 0.5 M ECCP, thus allowing to draw robust conclusions. Results recently obtained by Parmegiani et al. [8] reported comparable cryo-survival, blastulation, implantation and live birth rates after thawing of human oocytes with a combination of Kitazato and Irvine kits. The present results confirm the possibility of applying a single warming protocol also to vitrify human blastocysts, using this new combination without affecting survival and implantation rates. In order to minimize potential bias, blastocysts derived from own and donor oocytes were analysed separately. Indeed, the two study groups showed significant differences in patients’ age, years of infertility and number of previous IVF treatments (data not shown). However, no differences in survival rate were observed among the three VIT/WARM combinations across all study groups. Furthermore, the overall survival rate ranged from 97.6 to 100.0%, which conforms to the benchmark of the key performance indicator (KPI) for cryopreservation [18]. The use of the new combination did not show any detrimental effect on clinical outcomes. As a matter of fact, clinical pregnancy rates, live birth rates and miscarriage rates were comparable with those currently obtained in our clinic using Kitazato warming kits only. These results confirm the feasibility of a flexible application of the “Universal Warming” protocol, irrespective of brand, cryoprotectants and basic medium in the kits. This is a relevant fact, as the combinations used are currently considered unconventional. One drawback of our study is surely represented by the limited sample size providing a statistic power slightly higher than 70%. However, our results, although obtained in a single IVF centre, may represent the basis for larger multicentric studies investigating different alternative combinations of vitrification and warming kits whose use is often covert in IVF. In addition, should the present results be confirmed in series from different IVF laboratories, the clinical validity of a single warming protocol with the combination of different VIT/WARM solutions might be considered for routine use. It is of note that we tested the combination of different VIT/WARM kits also in a novel oocyte donor programme, based on the transportation of frozen sperm and blastocysts between two different IVF clinics [22]. This further suggests that the protocol could work in several clinical settings. The kits tested are the most widely used in IVF worldwide, which further supports the relevance and generalization of the present results. From a biological perspective, it is of interest that we tested solutions which differ only slightly in their composition, which contain either sucrose or trehalose as ECCP, supplemented by either human albumin, hydroxypropyl cellulose or dextran serum supplement, but equivalent in terms of safety and efficiency [9]. Our data confirmed that the type of basal medium composition and protein supplementation has no significant impact on the outcome of the vitrification/thawing procedure, as they simply drive oncotic efficiency. Conversely, a crucial role should be conferred to the ECCP, together with the presence of a non-permeating CPA, used in specific conditions such as concentrations and timings, a real practical pillar of the Universal Warming procedure. The encouraging results obtained in the present and previous works [8, 10, 11, 13, 16] potentially allow to extend the concept of “Universal Warming”, which would provide assisted reproduction centres with alternative options for their thawing procedures. Indeed, the controversial use of alternative combinations of different VIT/WARM solutions seems to represent a common, although covert, practice in the IVF lab, due to the recurrent need to thaw oocytes or embryos imported from other clinics. As a consequence, the accumulation of clinical evidence on the application of different combinations of VIT/WARM solutions would represent a call to action for embryologists worldwide to share their laboratory practices and test new combinations, with the aim of building a robust scientific background for the application of the “Universal Warming” procedure on different unconventional combinations of cryopreservation kits, especially in case of legal controversies. Once all the possible combinations have been tested, we would be able to consider the “Universal Warming” protocol reliable, consistent and reproducible in all laboratory settings. In addition, one major problem that IVF labs are currently facing is that of several reproductive samples (oocytes and embryos) which have been stored by slow freezing over a number of years [23,24,25]. Unfortunately, in many cases, the original warming solutions are not available anymore on the market. It would be worth testing the “Universal Warming” protocol also on these samples. Finally, the current study was inspired by the need to find alternative options to thaw cryopreserved embryos when the CE mark for the Kitazato kit was temporarily withdrawn in 2017, when its use was permitted in Spain but not in Italy [8]. As a matter of fact, in some countries, the clinical practice may be highly affected by regulatory/commercial/availability differences when a medium is withdrawn from the market for various reasons, as European regulations specify the use of FDA/CE marked thawing media only approved for human reproduction and specific for its cryopreservation solution [8]. Indeed, IVF clinics currently face the need to thaw gametes or embryos cryopreserved in other centres using vitrification media approved, validated or available only locally. In addition, each brand producing cryopreservation kits usually recommends the use of its own thawing kit, forcing embryologists to perform thawing procedures according to manufacture instructions. In this scenario, the application of “Universal Warming” through the combination of different vitrification/warming kits can solve the immediate clinical problem, thus limiting the arise of legal controverses.
Conclusions
In conclusion, the present study supports the use of a single warming protocol not only for vitrified oocytes, but also for blastocysts and in a new combination of kits, here tested for the first time. The use of this laboratory procedure is encouraged irrespectively of kit brands and any manufacture instructions. If further confirmed, our findings may represent a call to action encouraging embryologists worldwide to test alternative combinations of VIT/WARM kits and to step out of the conventional comfort zone of cryobiology.
References
Vajta G, Nagy ZP. Are programmable freezers still needed in the embryo laboratory? Review on vitrification. Reprod Biomed Online. 2006;12(6):779–96.
Rienzi L, Gracia C, Maggiulli R, LaBarbera AR, Kaser DJ, Ubaldi FM, et al. Oocyte, embryo and blastocyst cryopreservation in ART: systematic review and meta-analysis comparing slow-freezing versus vitrification to produce evidence for the development of global guidance. Hum Reprod Update. 2017;23(2):139–55.
Cobo A, de los Santos MJ, Castello D, Gamiz P, Campos P, Remohi J. Outcomes of vitrified early cleavage-stage and blastocyst-stage embryos in a cryopreservation program: evaluation of 3,150 warming cycles. Fertil Steril. 2012;98(5):1138-46 e1.
Zaat T, Zagers M, Mol F, Goddijn M, van Wely M, Mastenbroek S. Fresh versus frozen embryo transfers in assisted reproduction. Cochrane Database Syst Rev. 2021;2:CD011184.
Roy TK, Bradley CK, Bowman MC, McArthur SJ. Single-embryo transfer of vitrified-warmed blastocysts yields equivalent live-birth rates and improved neonatal outcomes compared with fresh transfers. Fertil Steril. 2014;101(5):1294–301.
Feng G, Zhang B, Zhou H, Shu J, Gan X, Wu F, et al. Comparable clinical outcomes and live births after single vitrified-warmed and fresh blastocyst transfer. Reprod Biomed Online. 2012;25(5):466–73.
Nagy ZP, Shapiro D, Chang CC. Vitrification of the human embryo: a more efficient and safer in vitro fertilization treatment. Fertil Steril. 2020;113(2):241–7.
Parmegiani L, Minasi MG, Arnone A, Casciani V, Cognigni G, Vinoles R, Varricchio VT, Quintero LA, Greco E, Filicori M. “Universal Warming” protocol for vitrified oocytes to streamline cell exchange for transnational donation programs: a multi-center study. J Assist Reprod Genet. 2020;37:1379–85.
Parmegiani L, Tatone C, Cognigni GE, Bernardi S, Troilo E, Arnone A, Maccarini AM, Di Emidio G, Vitti M, Filicori M. Rapid warming increases survival of slow-frozen sibling oocytes: a step towards a single warming procedure irrespective of the freezing protocol? Reprod BioMed Online. 2014;28:614–23.
Parmegiani L, Bernardi S, Garello C, Granella F, Criscuoli L, Dabizzi S, Filicori M. Testing the efficiency of the “universal warming protocol”. Multicenter randomised controlled study on human slow frozen oocytes. Fertil Steril 2014;102(3):e122.
Parmegiani L, Garello C, Revelli A, Criscuoli L, Dabizzi S, Gualtieri R, Talevi R, Filicori M. Efficacy and efficiency of the “universal warming protocol”: multicenter randomized controlled study on human slow frozen oocytes. Fertil Steril. 2014;102:e122.
Parmegiani L, Maccarini AM, Rastellini A, Bernardi S, Troilo E, Arnone A, Lanzillotti S, Filicori M. Oocyte vitrification/storage/handling/ transportation/warming, effect on survival and clinical results in donation programmes. Curr Trends Clin Embryol. 2017;4:34–40.
Parmegiani L, Arnone A, Cognigni G, Quintero L, Vinoles R, Filicori M. Universal warming protocol” for a transnational egg donation program with vitrified oocytes. Fertil Steril. 2018;110:e230.
Parmegiani L, Beilby KH, Arnone A, Bernardi S, Maccarini AM, Nardi E, Cognigni GE, Filicori M. Testing the efficacy and efficiency of a single “universal warming protocol” for vitrified human embryos: prospective randomized controlled trial and retrospective longitudinal cohort study. J Assist Reprod Genet. 2018;35(10):1887–95.
Serdarogullari M, Coban O, Boynukalin FK, Bilgin EM, Findikli N, Bahceci M. Successful application of a single warming protocol for embryos cryopreserved by either slow freezing or vitrification techniques. Syst Biol Reprod Med. 2019;65(1):12–9.
Kuwayama M, Vajta G, Kato O, Leibo SP. Highly efficient vitrification method for cryopreservation of human oocytes. Reprod BioMed Online. 2005;11:300–8.
Kuwayama M. Highly efficient vitrification for cryopreservation of human oocytes and embryos: the Cryotop method. Theriogenology. 2007;67(1):73–80.
Alpha Scientists In Reproductive Medicine. The Alpha consensus meeting on cryopreservation key performance indicators and benchmarks: proceedings of an expert meeting. Reprod BioMed Online. 2012;25:146–67.
Revelli A, Pettinau G, Basso G, Carosso A, Ferrero A, Dallan C, Canosa S, Guidetti D, Benedetto C. Controlled ovarian stimulation with recombinant-FSH plus recombinant-LH vs. human menopausal gonadotropin according to the number of retrieved oocytes: results from a routine clinical practice in a real-life population. Reprod Biol Endocrinol 2015;25(13):77.
Nunez R, Parmegiani L, Olaya Vila E, Cortés Gallego S, Caballero PP. Oocyte donation in Italy: effect on Spanish scenario. Curr Trends Clin Embryol. 2017;4:41–4.
ESHRE Special Interest Group of Embryology and Alpha Scientists in Reproductive Medicine. The Vienna consensus: report of an expert meeting on the development of ART laboratory performance indicators. Reprod Biomed Online. 2017;35:494–10.
La Marca A, Capuzzo M, Bartolucci S, Schirinzi F, Dal Canto MB, Buratini J, Mignini Renzini M, Rodriguez A, Vassena R. Exploring the pros and cons of new approaches for gamete cross-border donation based on fresh and vitrified oocytes. Facts Views Vis Obgyn. 2020;12(2):111–8.
Edgar DH, Gook DA. A critical appraisal of cryopreservation (slow cooling versus vitrification) of human oocytes and embryos. Hum Reprod Update. 2012;18(5):536–54.
Shi Q, Xie Y, Wang Y, Li S. Vitrification versus slow freezing for human ovarian tissue cryopreservation: a systematic review and meta-analysis. Sci Rep. 2017;7:8538.
Silvestris E, De Palma G, Canosa S, Palini S, Dellino M, Revelli A, Paradiso AV. Human ovarian cortex biobanking: a fascinating resource for fertility preservation in cancer. Int J Mol Sci. 2020;21(9):3245.
Acknowledgements
We sincerely thank Elisa Pace for providing language help.
Author information
Authors and Affiliations
Contributions
S.C. and L.P. conceived the study and contributed to the writing and editing of the manuscript; S.C., C.G., F.G., F.E. and G.M. performed warming procedures; S.C. collected the data; L.C. performed statistical analysis; G.G., C.G., D.G., A.R., M.F and F.B. contributed to the final interpretation of data and the editing of the manuscript. All authors gave their final approval.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
S. Canosa and L. Parmegiani consider that the first two authors should be regarded as joint first authors
Rights and permissions
About this article
Cite this article
Canosa, S., Parmegiani, L., Charrier, L. et al. Are commercial warming kits interchangeable for vitrified human blastocysts? Further evidence for the adoption of a Universal Warming protocol. J Assist Reprod Genet 39, 67–73 (2022). https://doi.org/10.1007/s10815-021-02364-1
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10815-021-02364-1