Abstract
Purpose
There is clinical evidence that early cleavage timing parameters predictive of blastocyst development also correlate with embryo implantation potential. The aim of this study is to determine the developmental competency of embryos with delayed blastulation.
Methods
Retrospective study performed from 2015 to 2016 at the Division of Reproductive Endocrinology and Infertility at Northwestern University.
Results
A total of 2,292 embryos from 524 patients were included. Day 6 blastocysts had statistically significant longer times for every time point analyzed than day 5 blastocysts (p < 0.001). We found no statistically significant difference in euploidy rates between day 5 (44%) and day 6 (41%) embryos (p = 0.573). t7 and t8 time points were independent predictors of euploidy after controlling for day of biopsy (p < 0.015 and p < 0.014, respectively). Intrauterine pregnancy (IUP) and live birth (LB) were less likely to occur after transferring day 6 embryos (p = 0.0033 and p = 0.0359) without previous genetic testing. However, in embryos that undergo preimplantation genetic testing for aneuploidy (PGT-A), there were no significant differences in IUP or LB rates.
Conclusion
Early time-lapse points can be used to predict embryo development. Day of blastulation may be an independent predictor IUP, with day 6 blastocysts having lower pregnancy and live birth rates. Our data suggests that day 5 and day 6 PGT-A tested embryos show similar rates of euploidy, suggesting that differences in PR seen in the non-PGT-A tested group may be caused by factors other than aneuploidy. Genetic testing technologies in combination with time-lapse microscopy may provide further information to improve IVF outcomes.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
A major goal in reproductive medicine is to maximize live birth (LB) rates with the use of elective single-embryo transfers. Despite efforts made in improving in vitro fertilization (IVF) efficacy, overall implantation rates remain low at 30.1% [1, 2]. Aneuploidy is a main contributor to implantation failure and risk of miscarriage in pregnancies achieved with IVF. There is well-documented evidence of increasing maternal age directly correlating with an increase in embryonic aneuploidy rates [3, 4]. Thus, techniques to determine embryo implantation potential and aneuploidy status are needed. Morphologic evaluation of embryos has historically been the method of choice to choose the best embryo for transfer. However, this method has limitations; the inter-observer consistency between morphology assessments is poor, as is its correlation with the chromosome makeup of the embryo. [5]. Time-lapse microscopy (TLM) is an imaging system that enables continuous monitoring of preimplantation embryo development, offering the opportunity to noninvasively visualize time points and aspects of embryo morphokinetics, with the intent of finding more accurate predictors of embryo developmental competency. These events may provide additional information to improve embryo selection, with the ultimate goal of improving clinical outcomes and promoting single embryo transfer [6, 7]. Past studies have drawn a clear correlation between embryo morphology and viability [8,9,10]. There is increasing clinical evidence that early cleavage timing parameters predictive of blastocyst development also correlate with embryo implantation and establishment of pregnancy [5]. However, several systematic reviews independently conclude that there is currently insufficient evidence to support the clinical use of time-lapse imaging data for predicting live birth [5, 11].
An objective assessment tool to evaluate embryo ploidy status and viability is of critical importance for the selection of the best embryo to be transferred. Blastocyst-stage embryo transfer may enhance embryo selection, but embryo morphology, even at the blastocyst stage, may be misleading [12,13,14,15]. Patients undergoing IVF may choose to undergo preimplantation genetic testing for aneuploidy (PGT-A). The goal is to decrease the chance of having a genetically abnormal pregnancy and to potentially decrease the chance of implantation failure and miscarriage [16]. Studies have shown that when genetically normal embryos are transferred, the effect of maternal age on pregnancy rates is eliminated and pregnancy rates increase 60 to 70% across all age groups [16]. Benefits of PGT-A have been described in two randomized controlled trials [4, 17]. For this reason, PGT-A has generated significant improvements in IVF treatment outcomes, especially for the older patient population. However, this type of testing is expensive and invasive and has the potential to damage the embryo [18, 19].
Even though the chance of any detrimental effect of trophectoderm biopsy on implantation rate is very low, noninvasive assessment of ploidy status with high validity would be very useful and potentially offer cost savings to patients. By combining trophectoderm biopsy with TLM of early embryo development, it may even be possible to preferentially select embryos to be tested [12,13,14,15].
Literature shows heterogeneous results regarding the clinical implications of a delay in development. This study compares clinical outcomes between blastocysts vitrified on day 5 and those on day 6 to assess the clinical implication of a delay in blastulation. In addition, we examined whether euploid compared with aneuploid embryos display differing morphokinetic variables over the preimplantation cleavage period and examined intrauterine pregnancy (IUP) and live birth rates (LBR) in frozen transferred blastocysts, both PGT-A tested and not tested (Fig. 1). Our hypothesis is that early cleavage stage parameters may predict embryo developmental competency.
Materials and methods
Patients and data collection
Our study was approved by the Northwestern Institutional Review Board. This was a single center retrospective clinical comparative study performed from 2015 to 2016 in the Division of Reproductive Endocrinology and Infertility at Northwestern University. All embryos that became blastocysts on day 5 and day 6 grown in the time-lapse incubators (EmbryoScope) were included. A sub-analysis of embryos that were PGT-A tested was done to compare TLM and euploidy rates in day 5 vs day 6 blastocysts. In addition, a sub-analysis of frozen embryo transfers (FET), both PGT-A tested and not tested, was done to evaluate pregnancy rates and LBR after transferring day 5 and day 6 embryos.
All patients who had IVF and whose embryos were cultured in the time-lapse incubator until blastocyst stage from 2015 to 2016 were included (Table 1). The following information was retrieved from the patients’ medical records: age, stimulation protocol, time-lapse points, day of blastocyst development, PGT-A results (if done), and pregnancy and live birth rates (if an embryo was transferred).
Controlled ovarian hyperstimulation protocols
Patients underwent one of the following ovarian stimulation protocols: luteal phase leuprolide acetate suppression (Lupron; Abbott Laboratories) with or without oral contraceptive pretreatment, microdose leuprolide acetate flare, or an antagonist protocol. Controlled ovarian hyperstimulation was achieved by administration of once-daily injections of follicle-stimulating hormone (FSH) (Follistim; Organon USA, Rose-land, NJ or Gonal-F; EMD Serono) and FSH þ luteinizing hormone (LH) (Menopur; Ferring Pharmaceuticals) at total daily doses of FSH ranging from 75 through 600 IU depending on age, ovarian reserve, and infertility diagnosis. In the antagonist protocol, the GnRH antagonist was added when a lead follicle measured 13 mm or the estradiol concentration exceeded 300 pg/mL. Cycles were monitored with serum estradiol levels and transvaginal ultrasounds beginning on stimulation day 4 or 5 and every 1 to 2 days thereafter. When at least three follicles had reached a mean diameter of 16 mm, 250 mg of recombinant human chorionic gonadotropin (hCG, Ovidrel; EMD Serono) was administered subcutaneously. Ultrasound-guided oocyte retrieval was performed 36 h later [20].
Frozen embryo transfer
To more accurately control the uterine environment for ET, patients underwent endometrial priming with estrogen and progesterone [21, 22]. Ovarian steroid supplementation consisted of 4 mg of oral estradiol starting on day 2–3 of the menstrual cycle. If the uterine lining was noted to be less than 7 mm on day 14, an additional 2–4 mg of estradiol was administrated vaginally. If the uterine lining was greater than 7 mm, once-daily intramuscular progesterone and vaginal progesterone (Endometrin or Crinone) were started. ET was performed on the morning of the 6th day after starting progesterone. Pregnancies were initially detected by serum β-hCG concentrations and confirmed by transvaginal ultrasound. All pregnant women continued estradiol and progesterone until 9 weeks of gestation [23].
Laboratory protocols
Controlled ovarian stimulation was performed as previously described. The cumulus oocyte complexes were aspirated 36 h after the trigger of ovulation. Oocytes were denuded of cumulus cells 38 h after the trigger of ovulation by enzymatic digestion with hyaluronidase (80 IU/mL). Insemination of oocytes by means of intracytoplasmic sperm injection (ICSI) was carried out 1–2 h after denudation. Fertilization was verified by the presence of two pronuclei 15 to 18 h later. All embryos were cultured in the EmbryoScope® incubator (Fertilitech). Single-step culture medium was used to culture embryos in the culture dish (embryo slide). Prolonged culture was used without media changes. Embryos were cultured at 37 °C, 6% CO2, and 5% O2 in either SAGE 1-step pre-supplemented with HAS (CooperSurgical) or G-TL (Vitrolife). During the biopsy process, embryos were placed in a HEPES buffered media supplemented with 10% serum substitute supplement (SSS, Irvine Scientific). Embryos were vitrified using the Sage Vit kit. Warming of embryos was done using Irvine Vit Kit-Thaw.
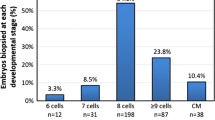
Fertilization and embryo development were assessed by making annotations on the EmbryoViewer. All embryos were individually cultured in a time-lapse incubator from time of fertilization to the blastocyst stage. PGT-A tested embryos were biopsied on day 5 or day 6. Laser-assisted hatching was performed with the removal of 3 to 5 trophectoderm cells that were sent for genetic testing. Genetic analysis was performed at outside companies using either NGS or SNP-arrays. Embryos were not hatched prior to biopsy on day 5 or 6. All blastocysts were cryopreserved using vitrification and stored in liquid N2 at − 196 °C.
Embryo culture time points
The time point was assigned to each image and reported as hours after time zero (t0), where t0 is defined as the time of injecting the sperm into the oocyte [24]. The following early time points after t0 were recorded: time to pronuclear appearance (tPNa), time to pronuclear fading (tPNf), 2 cells (t2), 4 cells (t4), five cells (t5), 6 cells (t6), 7 cells (t7), and 8 cells (t8). The analysis was stopped on t8 to focus on early cleavage developmental events as predictive of blastocyst formation and ploidy.
Outcome parameters
IUP was confirmed by visualization of an intrauterine gestational sac with fetal heart activity on ultrasound 5–6 weeks after the embryo transfer. LBR were defined as the proportion of transferred embryos that implanted resulting in live birth beyond 24 weeks of gestation.
Statistical analyses
The data was entered into a STATA data file to compare variables between groups. A value of p < 0.05 was considered to be significant. The chi-square test was used for categorical variables, and Student’s t test was used for continuous variables that were normally distributed. Crude odds ratios (OR) and 95% confidence intervals (95% CI) were determined. Logistic regression was performed to examine the association of variables with confounding factors.
Results
A total of 2,292 blastocysts from 608 cycles and 524 patients were included for analysis (Fig. 1). Demographic data of this cohort is shown in Table 1. The mean age and BMI for this cohort were 35.8 years old (95% CI, 35.3–36.0) and 25.8 (95% CI, 23.3–31.4). We found that 1,815 (79%) became blastocysts on day 5 and 477 (21%) on day 6. Our data shows that day 6 blastocysts had statistically significant longer times for every time point analyzed than day 5 embryos: tPNf, t2, t3, t4, t5, t6, t7, t8 (p < 0.001) (Table 2; Fig. 2). Results persisted after controlling for AMH and fertilization rates (these were the only 2 variables that showed significant differences between the groups which is why they were controlled for; other variables including age were similar in both groups).
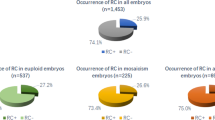
We then performed a sub-analysis examining all the PGT-A tested embryos. A total of 754 blastocysts from 152 patients and 212 cycles of IVF with PGT-A were analyzed for euploidy rates on day 5 (n = 586) or day 6 (n = 168). Table 3 shows the demographic data of this cohort of patients. The average age and BMI for all PGT-A tested patients were 36.8 years old (CI 95%, 36.2–37.4) and 24.0 (CI 95%, 23.4–24.6), respectively. Overall euploidy rate was 43.5% (255 out of 586) for day 5 and 41% (69 out of 168) for day 6 blastocysts. Our data demonstrated no statistically significant difference in euploidy rates between embryos biopsied on day 5 or day 6 (p = 0.573). We then performed a logistic regression comparing TLM between PGT-A normal and abnormal while controlling for day of biopsy (5 vs 6) and interestingly found that t7 (p = 0.015) and t8 (p = 0.013) remained as independent predictors of aneuploidy.
We then examined all frozen-thawed single blastocyst transfer cycles (both PGT-A tested and non-tested). A total of 302 transferred blastocysts from 239 patients were included: 252 days 5 blastocysts (83.4%) and 50 days 6 (16.6%). LBR were significantly higher after transferring day 5 embryos (56% vs 38%; p = 0.0295). IUP was also higher when transferring day 5 embryos but this difference was not significant (58% vs 46%) (Fig. 3).
Differences in pregnancy outcomes between PGT-A tested and non-tested blastocysts are shown in Table 4. Of the 302 transferred blastocysts (both day 5 and day 6), 112 (37.1%) were PGT-A tested and 190 (62.9%) embryos were non-tested. As expected, both PR and LB were significantly higher after transferring euploid embryos (IUP (69.6%), p = 0.0003; LB (63.4%), p = 0.0043). These differences persisted after controlling for AMH and number of fertilized oocytes.
Breaking down the 302 transferred embryos, 252 blastocysts were day 5 embryos (93 PGT-A tested and 159 non-tested) and 50 were day 6 embryos (19 euploid and 31 non-tested). Although IUP rates were not significantly different (58% vs 46%; p = 0.16), we found that LBR (56% vs 38%; p = 0.0295) were less likely to occur after transferring a day 6 embryo (Table 4).
We then did a sub-analysis looking at PGT-A tested and non-tested blastocysts (Tables 5 and 6). In the PGT-A non-tested group, we found that overall IUP and LB were less likely to occur after transferring day 6 embryos (p = 0.0033 and p = 0.0359, Table 5). For PGT-A tested embryos, there were no differences in IUP rate when transferring day 5 or day 6 blastocysts (p = 0.9180) (Table 6). Differences in clinical outcomes were not reported between day 5 and day 6 blastocysts, which supports the hypothesis that day 6 blastocyst transfers are influenced by intrinsic characteristics when the whole cohort developed slower. When comparing outcomes after transferring euploid embryos vs non PGT-A tested, both IUP and LBR were significantly higher after transferring euploid embryos (Table 7). The PR and LBR of all 302 transfer cycles were 56% and 53%, respectively.
Discussion
In this current study, we have combined two advanced technologies, time-lapse and PGT-A, and examined both PR and LBR. We found that day 6 blastocysts had statistically significant longer division times for every time point analyzed compared with day 5 blastocysts, even after controlling for AMH and fertilization rates. In the PGT-A tested subgroup, we found no statistically significant difference in euploidy rates between embryos biopsied on day 5 or day 6, suggesting that genetics did not play a role in these longer divisions. After controlling for day of biopsy, we found that t7 and t8 remained as independent predictor factors for aneuploidy, suggesting that these time points may help differentiate genetically normal from abnormal embryos.
As expected, IUP and LB were more likely to occur after transferring PGT-A normal embryos. Although we found that overall IUP and LB were less likely to occur after transferring day 6 embryos, this did not hold true for the subset of patients who had PGT-A. For PGT-A tested embryos, we found no difference in IUP when transferring day 5 or day 6 blastocysts. It should be noted, however, that we had a small number of transferred day 6 PGT-A normal embryos.
Although time-lapse technology may not replace PGT-A, it does represent an excellent selection tool for good prognosis patients for whom PGT-A is not indicated or for patients who do not wish or cannot have PGT-A performed. In our study, we found that t7 and t8 time points were predictive of aneuploidy status, suggesting that when PGT-A is not performed, these time points may be particularly helpful in selecting embryos for transfer.
Several authors have tried to assess the link between blastocyst morphology and chromosomal abnormality [25,26,27,28], built a time-lapse deselection model [29], and created generally applicable morphokinetic algorithms [30]. Currently, there are ten studies that aimed to predict the ploidy status of preimplantation embryos with the use of TLM parameters. Six of these studies reported significant associations between certain TLM parameters and ploidy status [28, 31,32,33], while the remaining four did not find any such association [24, 34,35,36,37]. Some of these previous reports [38,39,40,41] have shown higher implantation and pregnancy rates when transferring embryos on day 5 compared with day 6, suggesting that the viability may be higher for faster developing embryos [42].
Campbell et al. [12] published a study where the morphokinetic variables were compared with ploidy. They found that embryos with multiple aneuploidies had delayed initiation of compaction as well as time to reach full blastulation compared with euploid embryos. Embryos with either single or multiple aneuploidies had delayed initiation of blastulation compared with euploid embryos. No significant differences were observed in first or second cell-cycle length, synchrony of the second or third cell cycles, duration of blastulation, multinucleation at the 2-cell stage, and irregular division patterns between euploid and aneuploid embryos [12]. Chawla et al. [32] published a time-lapse imaging study where they performed a morphokinetic analysis of cleavage stage embryos and its relationship to aneuploidy. They showed that time durations for tPNf, t2, and t5 differed significantly between normal and abnormal embryos, specifically that tPNf and t2 duration for normal embryos was significantly less than that of the abnormal embryos (p < 0.05). They concluded that time-lapse imaging morphokinetics may play a role in early prediction of aneuploidy [32]. Minasi et al. [28] reported that the timing of cleavage from t3 to t4, reaching t4, starting blastulation, reaching full blastocyst stage, blastocyst expansion, and hatching were longer in aneuploid blastocysts (p < 0.05 for early stages and p < 0.0001 for later stages of development, respectively). No statistically significant differences were found between euploid and aneuploid blastocysts for the remaining morphokinetic parameters [28]. In our study, we found that t7 and t8 time points were predictive of aneuploidy. A possible explanation is that embryo genome activation starts at the four-to-eight cell stage. After the third day of culture, the embryo gradually express its own genes and the potential genetic abnormalities would start having an effect on embryo development [25].
On the other hand, Rienzi et al. [36] published a longitudinal cohort study to investigate whether blastocyst aneuploidy was detectable by specific morphokinetic parameters in patients at increased risk of aneuploidy because of advanced maternal age, history of unsuccessful IVF treatments, or both. No statistical correlation between 16 commonly detected morphokinetic characteristics (including t2 to t8) or in vitro embryo development and aneuploidy was found. Their results suggested that morphokinetic characteristics could not be used to select euploid blastocysts in poor-prognosis patients who may be regarded as candidates for pre-implantation genetic screening [36].
Regarding delayed blastulation, Barrenetxea et al. [42] published a study where they compared transfers of day 5 and day 6 embryos. Similar to our findings, these authors showed that blastocysts transferred on day 5 implanted at almost five times the rate of those transferred on day 6 (23% vs 5%). Pregnancy rates were three times as high among the day 5 patients compared with the day 6 transfer patients (38% vs 11%) [42]. Shapiro et al. [38] published a study where they compared day 5 and day 6 blastocyst transfers and pregnancy outcomes. They found that day 5 embryos were approximately twice as likely to implant compared with day 6 [38]. Kovalevsky et al. [43] compared pregnancy and implantation rates after transferring day 5, 6, and 7 embryos. Implantation rates and PR were significantly higher after transferring day 5 embryos [43].
We found overall lower IUP and LBR in patients who had a day 6 blastocyst transferred. Multiple studies suggested that blastocysts developing by day 5 of culture give rise to higher pregnancy rates than embryos reaching the blastocyst stage by day 6 [38, 42]. Unlike our study findings, Barash et al. [19] found that embryos available for biopsy on day 5 had higher euploidy rates than embryos available for biopsy on day 6 in all age groups. In our study, we found similar rates of euploidy in day 5 and day 6 blastocysts, suggesting that genetics may not explain slower development. It is not clear whether this lower PR and LBR is due to impaired embryo quality of the slower to develop day 6 blastocysts or an asynchronous uterine environment with poor endometrial receptivity [44, 45]. Of note, our clinic transfers day 6 blastocysts when there are no day 5 blastocysts available. Further analysis of outcomes of blastocysts vitrified on day 5 or day 6 may provide a better understanding of the impact of delayed development on subsequent embryo competency [46]. Several recent papers suggested that in some cases, extended culture up to day 7 may be necessary to assess genetic status of the embryos [43, 47]. At the same time, reported pregnancy rates after transferring embryos biopsied on day 7 are relatively low and long-term outcomes are unknown [19].
Our study has a number of important strengths. The data comes from a single, high-volume center experienced in TE biopsy and the use of time-lapse incubators. In addition, we have a large sample size. The main limitation is that the number of day 6 blastocysts PGT-A tested and transferred was low. In addition, the time-lapse points were not automated and thus subject to human error. A weakness is that blastocysts were divided into two groups according to the day of development at the blastocyst stage. This selection implies that the same patients may have embryos in both the day 5 and day 6 cohort. In our practice, day 5 embryos are preferentially chosen over day 6, unless the only euploid embryos are day 6.
Our data shows similar rates of euploid embryos between day 5 and day 6, suggesting that genetics may not explain slow development, but PR and LB are lower when non-PGT-A day 6 blastocysts are transferred. However, this result does not hold true after transferring day 6 embryos which have undergone PGT-A (Table 6). We found that early time-lapse points can be used to predict which embryos will become blastocysts on day 5 vs day 6, and that t7 and t8 time points were independent predictors of euploidy. Genetic testing technologies in combination with time-lapse microscopy may provide further information to improve IVF outcomes.
References
Nyboe Andersen A, Goossens V, Ferraretti AP, Bhattacharya S, Felberbaum R, de Mouzon J, et al. Assisted reproductive technology in Europe, 2004: results generated from European registers by ESHRE. Hum Reprod. 2008;23(4):756–71. https://doi.org/10.1093/humrep/den014.
Speroff L. Female infertility. In: Fritz MA, Speroff L, editors. Clinical gynecologic endocrinology and infertility. 8th ed; 2012.
Handyside AH. Molecular origin of female meiotic aneuploidies. Biochim Biophys Acta. 2012;1822(12):1913–20. https://doi.org/10.1016/j.bbadis.2012.07.007.
Coates A, Kung A, Mounts E, Hesla J, Bankowski B, Barbieri E, et al. Optimal euploid embryo transfer strategy, fresh versus frozen, after preimplantation genetic screening with next generation sequencing: a randomized controlled trial. Fertil Steril. 2017;107(3):723–30 e3. https://doi.org/10.1016/j.fertnstert.2016.12.022.
Weinerman R, Feng R, Ord TS, Schultz RM, Bartolomei MS, Coutifaris C, et al. Morphokinetic evaluation of embryo development in a mouse model: functional and molecular correlates. Biol Reprod. 2016;94(4):84. https://doi.org/10.1095/biolreprod.115.134080.
Wong CC, Loewke KE, Bossert NL, Behr B, De Jonge CJ, Baer TM, et al. Non-invasive imaging of human embryos before embryonic genome activation predicts development to the blastocyst stage. Nat Biotechnol. 2010;28(10):1115–21. https://doi.org/10.1038/nbt.1686.
Swain JE. Could time-lapse embryo imaging reduce the need for biopsy and PGS? J Assist Reprod Genet. 2013;30:1081–90. https://doi.org/10.1007/s10815-013-0048-4.
Neuber E, Mahutte NG, Arici A, Sakkas D. Sequential embryo assessment outperforms investigator-driven morphological assessment at selecting a good quality blastocyst. Fertil Steril. 2006;85(3):794–6. https://doi.org/10.1016/j.fertnstert.2005.08.064.
Finn A, Scott L, O’Leary T, Davies D, Hill J. Sequential embryo scoring as a predictor of aneuploidy in poor-prognosis patients. Reprod BioMed Online. 2010;21(3):381–90. https://doi.org/10.1016/j.rbmo.2010.05.004.
Meseguer M, Rubio I, Cruz M, Basile N, Marcos J, Requena A. Embryo incubation and selection in a time-lapse monitoring system improves pregnancy outcome compared with a standard incubator: a retrospective cohort study. Fertil Steril. 2012;98(6):1481–9.e10. https://doi.org/10.1016/j.fertnstert.2012.08.016.
Kaser DJ, Farland LV, Missmer SA, Racowsky C. Prospective study of automated versus manual annotation of early time-lapse markers in the human preimplantation embryo. Hum Reprod. 2017;32(8):1604–11. https://doi.org/10.1093/humrep/dex229.
Campbell A, Fishel S, Bowman N, Duffy S, Sedler M, Hickman CF. Modelling a risk classification of aneuploidy in human embryos using non-invasive morphokinetics. Reprod BioMed Online. 2013;26(5):477–85. https://doi.org/10.1016/j.rbmo.2013.02.006.
Adler A, Lee HL, McCulloh DH, Ampeloquio E, Clarke-Williams M, Wertz BH, et al. Blastocyst culture selects for euploid embryos: comparison of blastomere and trophectoderm biopsies. Reprod BioMed Online. 2014;28(4):485–91. https://doi.org/10.1016/j.rbmo.2013.11.018.
Basile N, Nogales Mdel C, Bronet F, Florensa M, Riqueiros M, Rodrigo L, et al. Increasing the probability of selecting chromosomally normal embryos by time-lapse morphokinetics analysis. Fertil Steril. 2014;101(3):699–704. https://doi.org/10.1016/j.fertnstert.2013.12.005.
Barrie A, Homburg R, McDowell G, Brown J, Kingsland C, Troup S. Examining the efficacy of six published time-lapse imaging embryo selection algorithms to predict implantation to demonstrate the need for the development of specific, in-house morphokinetic selection algorithms. Fertil Steril. 2017;107(3):613–21. https://doi.org/10.1016/j.fertnstert.2016.11.014.
Harton GL, Munné S, Surrey M, Grifo J, Kaplan B, McCulloh DH, et al. Diminished effect of maternal age on implantation after preimplantation genetic diagnosis with array comparative genomic hybridization. Fertil Steril. 2013;100(6):1695–703. https://doi.org/10.1016/j.fertnstert.2013.07.2002.
Scott RT Jr, Upham KM, Forman EJ, Hong KH, Scott KL, Taylor D, et al. Blastocyst biopsy with comprehensive chromosome screening and fresh embryo transfer significantly increases in vitro fertilization implantation and delivery rates: a randomized controlled trial. Fertil Steril. 2013;100(3):697–703. https://doi.org/10.1016/j.fertnstert.2013.04.035.
Munne S, Wells D, Cohen J. Technology requirements for preimplantation genetic diagnosis to improve assisted reproduction outcomes. Fertil Steril. 2010;94(2):408–30. https://doi.org/10.1016/j.fertnstert.2009.02.091.
Barash OO, Ivani KA, Willman SP, Rosenbluth EM, Wachs DS, Hinckley MD, et al. Association between growth dynamics, morphological parameters, the chromosomal status of the blastocysts, and clinical outcomes in IVF PGS cycles with single embryo transfer. J Assist Reprod Genet. 2017;34(8):1007–16. https://doi.org/10.1007/s10815-017-0944-0.
Eaton JL, Zhang X, Kazer RR. First-trimester bleeding and twin pregnancy outcomes after in vitro fertilization. Fertil Steril. 2016;106(1):140–3. https://doi.org/10.1016/j.fertnstert.2016.03.027.
Pantos K, Stefanidis K, Pappas K, Kokkinopoulos P, Petroutsou K, Kokkali G, et al. Cryopreservation of embryos, blastocysts, and pregnancy rates of blastocysts derived from frozen-thawed embryos and frozen-thawed blastocysts. J Assist Reprod Genet. 2001;18(11):579–82.
Veeck LL, Bodine R, Clarke RN, Berrios R, Libraro J, Moschini RM, et al. High pregnancy rates can be achieved after freezing and thawing human blastocysts. Fertil Steril. 2004;82(5):1418–27. https://doi.org/10.1016/j.fertnstert.2004.03.068.
Pavone ME, Innes J, Hirshfeld-Cytron JE, Kazer R, Zhang J. Comparing thaw survival, implantation and live birth rates from cryopreserved zygotes, embryos and blastocysts. J Hum Reprod Sci. 2011;4(1):23–8. https://doi.org/10.4103/0974-1208.82356.
Mumusoglu S, Yarali I, Bozdag G, Ozdemir P, Polat M, Sokmensuer LK, et al. Time-lapse morphokinetic assessment has low to moderate ability to predict euploidy when patient- and ovarian stimulation-related factors are taken into account with the use of clustered data analysis. Fertil Steril. 2017;107(2):413–21 e4. https://doi.org/10.1016/j.fertnstert.2016.11.005.
Alfarawati S, Fragouli E, Colls P, Stevens J, Gutierrez-Mateo C, Schoolcraft WB, et al. The relationship between blastocyst morphology, chromosomal abnormality, and embryo gender. Fertil Steril. 2011;95(2):520–4. https://doi.org/10.1016/j.fertnstert.2010.04.003.
Fragouli E, Alfarawati S, Spath K, Wells D. Morphological and cytogenetic assessment of cleavage and blastocyst stage embryos. Mol Hum Reprod. 2014;20(2):117–26. https://doi.org/10.1093/molehr/gat073.
Capalbo A, Rienzi L, Cimadomo D, Maggiulli R, Elliott T, Wright G, et al. Correlation between standard blastocyst morphology, euploidy and implantation: an observational study in two centers involving 956 screened blastocysts. Hum Reprod. 2014;29(6):1173–81. https://doi.org/10.1093/humrep/deu033.
Minasi MG, Colasante A, Riccio T, Ruberti A, Casciani V, Scarselli F, et al. Correlation between aneuploidy, standard morphology evaluation and morphokinetic development in 1730 biopsied blastocysts: a consecutive case series study. Hum Reprod. 2016;31(10):2245–54. https://doi.org/10.1093/humrep/dew183.
Liu Y, Chapple V, Feenan K, Roberts P, Matson P. Time-lapse deselection model for human day 3 in vitro fertilization embryos: the combination of qualitative and quantitative measures of embryo growth. Fertil Steril. 2016;105(3):656–62 e1. https://doi.org/10.1016/j.fertnstert.2015.11.003.
Petersen BM, Boel M, Montag M, Gardner DK. Development of a generally applicable morphokinetic algorithm capable of predicting the implantation potential of embryos transferred on day 3. Hum Reprod. 2016;31(10):2231–44. https://doi.org/10.1093/humrep/dew188.
Campbell A, Fishel S, Laegdsmand M. Aneuploidy is a key causal factor of delays in blastulation: author response to ‘A cautionary note against aneuploidy risk assessment using time-lapse imaging’. Reprod BioMed Online. 2014;28(3):279–83. https://doi.org/10.1016/j.rbmo.2013.11.016.
Chawla M, Fakih M, Shunnar A, Bayram A, Hellani A, Perumal V, et al. Morphokinetic analysis of cleavage stage embryos and its relationship to aneuploidy in a retrospective time-lapse imaging study. J Assist Reprod Genet. 2015;32(1):69–75. https://doi.org/10.1007/s10815-014-0372-3.
Basile N, Barriere P, Meseguer M, Freour T. Time-lapse in the IVF lab: how should we assess potential benefit? Hum Reprod. 2015;30(5):1276. https://doi.org/10.1093/humrep/dev045.
Yang Z, Zhang J, Salem S, Liu X, Kuang Y, Salem R, et al. Selection of competent blastocysts for transfer by combining time-lapse monitoring and array CGH testing for patients undergoing preimplantation genetic screening: a prospective study with sibling oocytes. BMC Med Genet. 2014;7(38).
Kramer YG, Kofinas JD, Melzer K, Noyes N, McCaffrey C, Buldo-Licciardi J, et al. Assessing morphokinetic parameters via time lapse microscopy (TLM) to predict euploidy: are aneuploidy risk classification models universal? J Assist Reprod Genet. 2014;31(9):1231–42. https://doi.org/10.1007/s10815-014-0285-1.
Rienzi L, Capalbo A, Stoppa M, Romano S, Maggiulli R, Albricci L, et al. No evidence of association between blastocyst aneuploidy and morphokinetic assessment in a selected population of poor-prognosis patients: a longitudinal cohort study. Reprod BioMed Online. 2015;30(1):57–66. https://doi.org/10.1016/j.rbmo.2014.09.012.
Patel DV, Shah PB, Kotdawala AP, Herrero J, Rubio I, Banker MR. Morphokinetic behavior of euploid and aneuploid embryos analyzed by time-lapse in embryoscope. J Hum Reprod Sci. 2016;9(2):112–8.
Shapiro BS, Richter KS, Harris DC, Daneshmand ST. A comparison of day 5 and day 6 blastocyst transfers. Fertil Steril. 2001;75(6):1126–30.
Wilson M, Hartke K, Kiehl M, Rodgers J, Brabec C, Lyles R. Integration of blastocyst transfer for all patients. Fertil Steril. 2002;77(4):693–6. https://doi.org/10.1016/S0015-0282(01)03235-6.
Behr B, Gebhardt J, Lyon J, Milki AA. Factors relating to a successful cryopreserved blastocyst transfer program. Fertil Steril. 2002;77(4):697–9.
Levron J, Shulman A, Bider D, Seidman D, Levin T, Dor J. A prospective randomized study comparing day 3 with blastocyst-stage embryo transfer. Fertil Steril. 2002;77(6):1300–1.
Barrenetxea G, Lopez de Larruzea A, Ganzabal T, Jimenez R, Carbonero K, Mandiola M. Blastocyst culture after repeated failure of cleavage-stage embryo transfers: a comparison of day 5 and day 6 transfers. Fertil Steril. 2005;83(1):49–53. https://doi.org/10.1016/j.fertnstert.2004.06.049.
Kovalevsky G, Carney SM, Morrison LS, Boylan CF, Neithardt AB, Feinberg RF. Should embryos developing to blastocysts on day 7 be cryopreserved and transferred: an analysis of pregnancy and implantation rates. Fertil Steril. 2013;100(4):1008–12. https://doi.org/10.1016/j.fertnstert.2013.06.021.
Richter KS, Shipley SK, McVearry I, Tucker MJ, Widra EA. Cryopreserved embryo transfers suggest that endometrial receptivity may contribute to reduced success rates of later developing embryos. Fertil Steril. 2006;86(4):862–6. https://doi.org/10.1016/j.fertnstert.2006.02.114.
Shapiro BS, Daneshmand ST, Garner FC, Aguirre M, Ross R. Contrasting patterns in in vitro fertilization pregnancy rates among fresh autologous, fresh oocyte donor, and cryopreserved cycles with the use of day 5 or day 6 blastocysts may reflect differences in embryo-endometrium synchrony. Fertil Steril. 2008;89(1):20–6. https://doi.org/10.1016/j.fertnstert.2006.08.092.
Desai N, Ploskonka S, Goodman L, Attaran M, Goldberg JM, Austin C, et al. Delayed blastulation, multinucleation, and expansion grade are independently associated with live-birth rates in frozen blastocyst transfer cycles. Fertil Steril. 2016;106(6):1370–8. https://doi.org/10.1016/j.fertnstert.2016.07.1095.
Su Y, Li JJ, Wang C, Haddad G, Wang WH. Aneuploidy analysis in day 7 human blastocysts produced by in vitro fertilization. Reprod Biol Endocrinol. 2016;14:20. https://doi.org/10.1186/s12958-016-0157-x.
Funding
This work was financially supported by the Northwestern Memorial Foundation Evergreen Grant (to MEP) and P50 HD076188 (MEP, PI: T. Woodruff).
Author information
Authors and Affiliations
Corresponding author
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
ESM 1
(DOCX 44 kb)
Rights and permissions
About this article
Cite this article
Kimelman, D., Confino, R., Okeigwe, I. et al. Assessing the impact of delayed blastulation using time lapse morphokinetics and preimplantation genetic testing in an IVF patient population. J Assist Reprod Genet 36, 1561–1569 (2019). https://doi.org/10.1007/s10815-019-01501-1
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10815-019-01501-1