Abstract
Purpose
Preimplantation genetic testing (PGT) using Karyomapping is used to screen embryos for single gene disorders prior to implantation. While Karyomapping is not designed to screen for abnormalities in chromosome copy number, this testing is based upon a genome-wide analysis of single nucleotide polymorphisms (SNP) and, as such, some chromosome abnormalities are detected. The aim of this study was to validate whether Karyomapping could provide reliable and accurate PGT for a paternal 46,XY,t(10;19)(p15;p13.3) reciprocal translocation.
Methods
Feasibility/validation for PGT was performed using DNA from the couple, as well as DNA from the paternal parents and from a previous unbalanced pregnancy. Karyomapping was performed using Illumina’s HumanKaryomap-12 BeadChip microarray technology. SNP analysis was performed using BlueFuse Multi software (Illumina). Transmission of the translocation was assessed through the analysis of SNP markers on the chromosome regions of interest.
Results
PGT-SR was determined to be feasible as chromosomal SNP analysis could reliably distinguish normal/balanced outcomes from all unbalanced outcomes. The couple transferred a normal/balanced embryo in an elective single embryo transfer procedure following 2 IVF/PGT-SR cycles. A clinical pregnancy was achieved.
Conclusion
This is the first report of PGT-SR test validation using Karyomapping for a 46,XY,t(10;19)(p15;p13.3) reciprocal translocation. Karyomapping may offer a means of detecting unbalanced forms of chromosome rearrangements when other PGT platforms fail.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Preimplantation genetic testing (PGT) (previously preimplantation genetic diagnosis) is an alternative to invasive prenatal diagnosis for couples who are at risk of producing children with a single gene condition [1]. Karyomapping is a comprehensive linkage-based test used to screen embryos for single gene disorders prior to implantation. PGT involves an assessment of the single nucleotide polymorphism (SNP) profile of both partners, a reference (close relative of known genetic status) and the cells from the embryo. Analysis of the single gene disorder is performed by studying the SNPs that surround the gene of interest. By comparing the SNP profile of each partner against the SNP profile of the reference, it is possible to identify a unique SNP fingerprint that can be used to indirectly detect the affected and unaffected gene copies in the embryo [2]. While Karyomapping has been validated for the detection of single gene disorders using haplotype analysis [2, 3] as this technology is based upon genome-wide analysis of SNPs, it is also capable of detecting meiotic trisomy, triploidy and uniparental heterodisomy; it has not been validated for the detection of post-zygotic abnormalities in chromosome copy number that can lead to mosaicism [4]. The use of Karyomapping for the detection of segmental chromosome abnormalities has also been reported [3]. Given that Karyomapping is capable of detecting segmental chromosome abnormalities, this testing platform may provide a viable PGT option for couples with structural chromosome rearrangements (PGT-SR).
Here, we present a case study using Karyomapping for a paternal reciprocal translocation, 46,XY,t(10;19)(p15;p13.3).
Case details
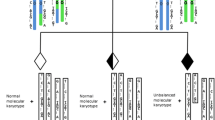
This case involved a 27-year-old female and a 31-year-old male who were referred for IVF treatment with PGT-SR. The couple had had three previous pregnancies of which two had unfortunately resulted in miscarriage and the third a 15/40-week termination. Genetic testing of chorionic villus sampling (CVS) revealed an unbalanced molecular karyotype. A G-banded karyotype of both parents indicated that the father carried an apparently balanced reciprocal translocation involving chromosomes 10 and 19 (Table 1).
Based on the karyotype results, the couple were deemed at risk of suffering reproductive difficultly due to malsegregation of the paternal reciprocal translocation. The couple requested a PGT-SR test that would allow normal/balanced embryos to be differentiated from unbalanced embryos that have resulted from the paternal translocation.
Test validation/feasibility
The couple underwent genetic counselling. An initial theoretical assessment was performed to determine which PGT-SR platform/s were potentially viable for this couple. It was determined that the PGT-SR platforms typically used for reciprocal translocations (i.e. next-generation sequencing (NGS) or array comparative genomic hybridisation) did not have the resolution required to detect the smaller segments that would result from this translocation (i.e. 659 kb and 7.8 Mb) [5]. As a result, Karyomapping was investigated. Given that the distribution of SNPs on the HumanKaryomap-12 BeadChip (Illumina) is not uniform (i.e. with lower coverage in the telomere and centromere regions [6]), BlueFuse Multi V4.0 (Illumina) was initially used to assess whether there were enough SNPs in the region/s of interest (i.e. on chromosomes 10 and 19) to proceed with feasibility testing. This assessment determined that there were an adequate number of SNPs in the regions of interest (52 SNPs in the 10p15.3 region and 1187 SNPs in the 19p13.3p13.2 region) (Table 2).
Based on the above assessment, it was determined that the couple could proceed with Karyomapping feasibility testing. Feasibility testing was performed using blood/DNA samples from the following:
-
Female partner
-
Male partner
-
Both the male partners’ parents
-
CVS from a previous pregnancy
All family members consented to feasibility testing. DNA from the CVS was provided by the prenatal laboratory. DNA extraction from EDTA peripheral blood from all family members was performed within 1 week of collection using the ReliaPrep blood gDNA Miniprep System as per the manufacturer’s instructions (Promega, USA). Extracted DNA was stored at − 20 °C in preparation for Karyomapping.
Karyomapping
Each DNA sample was quantitated using the Qubit Fluorometer and dsDNA High-Sensitivity (HS) Assay Kits (ThermoFisher Scientific, AUS) and diluted to 50 ng/μl in preparation for Karyomapping. Karyomapping was performed on the extracted DNA of all family members using the HumanKaryomap-12 BeadChip Kit (Illumina, USA) as per the manufacturer’s instructions [7] with the initial whole genome amplification step performed overnight (< 24 h) at 37 °C to increase the yield of DNA samples. The Karyomapping data for each gDNA sample was imported into BlueFuse Multi V4.0 (Illumina) to assess the number of ‘informative’ SNPs for the family (i.e. the number of SNPs that can potentially be used in PGT-SR to detect segmental chromosome imbalances resulting from the translocation).
Analysis
Analysis was independently performed by two genetic scientists using BlueFuse Multi V4.0 software (Illumina, USA) according to the analysis guidelines recommended by the manufacturer [6]. At the completion of feasibility test development, the following was known:
-
The SNP profiles for each family member
-
The number of informative SNPs (within and flanking the region/s of interest)
-
The QC data for each gDNA sample (Table 3)
Table 3 QC values obtained following Karyomapping feasibility testing
The success of the Karyomapping procedure was determined based upon a review of the QC values for each DNA sample. A review of the data indicated that the QC values obtained for all DNA samples were within the acceptable range.
As a guide, in order to accept a case, there should be ≥ 10 informative SNPs flanking the area of interest. If < 10 informative SNPs are available in one or both of these regions, the case may be accepted; however, acceptability will vary depending on the distance the informative SNPs are to the area of interest and if additional family samples are available in order to obtain sufficient SNP data for phasing.
Figure 1 shows the Bluefuse Multi data for the male partner, reference and CVS. Transmission of the reciprocal translocation was assessed through the analysis of SNP markers on the chromosome regions of interest. PGT-SR was determined to be feasible based upon the fact that the detailed haploblock chart reliably detected segmental copy number changes involving the translocated segments (and thus is presumed capable of distinguishing normal/balanced outcomes from all unbalanced outcomes). The most appropriate reference to use for clinical PGT-SR was determined to be the paternal grandfather (due to the highest number of SNPs within the chromosome regions involved in the translocation) as shown in Table 4.
Log R chart: The Log R chart represents the logged ratio of observed probe intensity to expected intensity (Y axis represents fluorescent intensity, X axis represents the chromosome) and is expected to show zero when two chromosome copies are present. Any deviation from zero is indicative of a chromosome copy number change (e.g. a Log R ratio > 0 indicates more than two copies while a Log R ratio < 0 indicates less than two copies). B-Allele chart: The B-Allele chart is expected to show three tracts (AA,AB,BB) for the autosomes. Deviations from this are indicative of an abnormal number of chromosomes (e.g. loss of the AB tract is indicative of monosomy). Detailed haploblock chart: The Karyomaps for each parental chromosome are displayed as two rows of informative SNPs. The region of interest is identified by the blue area. The 2-Mb flanking regions are displayed by the grey shading either side of the blue area. The SNPs are represented by the coloured dots. The haplotypes displayed in BlueFuse Multi are relative to the reference sample. Blue and yellow represent the paternal and maternal haplotypes inherited by the reference and red and green represent the paternal and maternal haplotypes that were not inherited by the reference. The detailed haploblock charts are used to analyse the number of key and non-key SNPs in the region of interest. Key SNPs are indicated above the allele and non-key SNPs are indicated below the allele. A loss of key SNPs is indicative of a loss of chromosome material and a loss of non-key SNPs is indicative of a gain in chromosome material. In this figure, the paternal grandfather has been imported as a reference to phase for the paternal reciprocal translocation and the CVS has been entered as a mock embryo. The reciprocal translocation was not visible on the Log R, B-allele and detailed haploblock charts for the male partner and the paternal grandfather as there was no visible changes on the charts (i.e. the reciprocal translocation is balanced and the karyotype of the paternal grandfather is either balanced or not a carrier). However, the following was observed for the CVS (identified by the red boxes):
- Log R chart < 0 for chromosome 10 indicating a deletion of 10pter-p15.3
- Loss of the AB tract on the B-allele chart for chromosome 10 indicating a deletion of 10pter-p15.3
- Loss of paternal key SNPs and non-key SNPs vary phase for chromosome 10 on the detailed haploblock chart indicating a deletion of 10pter-p15.3
- Log R chart > 0 for chromosome 19 indicating a duplication of 19pter-p13.3
- A dispersed AB tract on the B-allele chart for chromosome 19 indicating a duplication of 19pter-p13.3
- Loss of paternal non-key SNPs and key SNPs vary phase for chromosome 19 on the detailed haploblock chart indicating a duplication of 19pter-p13.3
In order to mimic the diagnosis of an embryo, the SNP data for the CVS was imported into BlueFuse Multi as a mock embryo (sibling) with each of the selected references to confirm that detection of the reciprocal translocation was possible. Detailed haploblock charts were used to analyse the number of key and non-key SNPs in the regions of interest. Figure 1 shows the BlueFuse Multi data for the male partner, paternal grandfather (reference) and the CVS (sibling). The data confirms that identification of segmental copy number changes as a result of malsegregation of the paternal reciprocal translocation was feasible.
A feasibility report was issued to the IVF specialist and referring clinic. This report outlined that Karyomapping could be used to screen embryos for the paternal reciprocal translocation. The report noted that Karyomapping is unable to distinguish between apparently normal and balanced outcomes. However, if the couple wished and the paternal grandparents were willing to undertake a karyotype, Karyomapping may be able to distinguish between all outcomes.
Limitations, reliability and accuracy
Karyomapping is not designed to screen for segmental chromosome imbalances and, unless specifically validated during feasibility testing (e.g. the paternal translocation in this case), it is possible that these types of chromosome errors will not be detected. If a chromosome abnormality unrelated to the indication for testing is detected, these embryos would be diagnosed as abnormal and not considered suitable for transfer.
The estimated error rates for Karyomapping are reported to be as low as 0.3% [4, 8, 9]. Patient-specific factors such as consanguinity, the quality of the biopsied materials, the number of biopsied cells retrieved from the embryo, the number of informative SNPs and the quality of the resulting data could affect the accuracy of the test.
Karyomapping does not exclude the presence of non-targeted single gene mutations, chromosomal aneuploidy, mosaicism or balanced chromosomal rearrangements. Karyomapping may identify chromosome abnormalities that are unexpected and unrelated to the original reason for performing the test (i.e. incidental findings). Incidental findings may be detected in gDNA (during feasibility testing) and/or embryos (during PGT-SR). If incidental findings are identified, our clinic policy is that they are
-
Only disclosed if they are believed to be linked to a potential health problem or if they are likely to impact on the health of a child to be born following PGT-SR.
-
Disclosed by a genetic counsellor/clinical geneticist/IVF specialist.
-
Confirmed by an accredited diagnostic laboratory.
The couple was fully informed of the described test limitations.
Preimplantation genetic testing
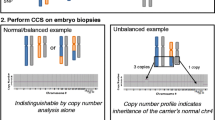
To date, the couple have undertaken two IVF/PGT-SR cycles. Ovarian stimulation, embryo culture and embryo biopsy were performed through an external IVF clinic. The embryo biopsy procedure was performed on day 5/6 post oocyte collection. Embryos were considered suitable for biopsy if they had developed to the blastocyst stage and contained a clearly defined inner cell mass (i.e. the inner cell mass was identified as a tightly compacted ball of cells clearly separate from the trophectoderm cells) and an appropriate number of trophectoderm cells to enable the removal of approximately 3–7 cells for PGT while retaining an adequate number of trophectoderm cells to support ongoing embryo development. The biopsied blastocysts were vitrified post biopsy. Each biopsy sample was washed through a series of 20 μl 1× PBS drops (Cell Signalling Technologies, USA) before being transferred to a sterile 0.2-μl PCR tube containing 2.5 μl of 1× PBS. Whole genome amplification (WGA) was performed using the RepliG Single Cell Kit (Qiagen, Netherlands). Following WGA, Karyomapping was performed using the HumanKaryomap-12 BeadChip Kit (Illumina, USA) as per the manufacturer’s instructions [7] with the initial whole genome amplification step performed overnight (< 24 h) at 37 °C to increase the yield of DNA samples. Samples were analysed independently by two genetic scientists using BlueFuse Multi V4.0 (Illumina). Analysis was performed using the manufacturer’s recommendations published in ‘A technical guide to Karyomapping – Phasing single gene defects’ [6]. The results of both IVF/PGT-SR cycles are presented in Table 5. Overall, 11 embryos were biopsied and tested using Karyomapping over two egg collections. A conclusive genetic result was obtained for 10/11 embryo biopsy samples. An unbalanced chromosome complement was obtained for 7/11 embryos, 3/11 embryos showed a normal/balanced chromosome complement and one embryo did not obtain a result due to amplification failure. Figure 2 shows the Karyomapping profiles for embryo 6 from IVF cycle 1. This embryo was diagnosed as unbalanced for the paternal reciprocal translocation (deletion 10pter-p15.3, duplication 19pter-p13.2). Overall, this couple had three cryopreserved embryos suitable for frozen embryo transfer. The couple transferred a normal/balanced embryo in an elective single embryo transfer procedure. A clinical pregnancy (ultrasound confirmation of foetal heart beat) was achieved which was ongoing at the time of publication (12 weeks).
Log R chart: The Log R chart represents the logged ratio of observed probe intensity to expected intensity (Y axis represents fluorescent intensity, X axis represents the chromosome) and is expected to show zero when two chromosome copies are present. Any deviation from zero is indicative of a chromosome copy number change (e.g. a Log R ratio > 0 indicates more than two copies while a Log R ratio < 0 indicates less than two copies).
B-Allele chart: The B-Allele chart is expected to show three tracts (AA,AB,BB) for the autosomes. Deviations from this are indicative of an abnormal number of chromosomes (e.g. loss of the AB tract is indicative of monosomy). Detailed haploblock chart: The Karyomaps for each parental chromosome are displayed as two rows of informative SNPs. The region of interest is identified by the blue area. The 2 Mb flanking regions are displayed by the grey shading either side of the blue area. The SNPs are represented by the coloured dots. The haplotypes displayed in BlueFuse Multi are relative to the reference sample. Blue and yellow represent the paternal and maternal haplotypes inherited by the reference and red and green represent the paternal and maternal haplotypes that were not inherited by the reference. The detailed haploblock charts are used to analyse the number of key and non-key SNPs in the region of interest. Key SNPs are indicated above and non-key SNPs are indicated below. A loss of key SNPs is indicative of a loss of chromosome material and a loss of non-key SNPs is indicative of a gain in chromosome material. This figure shows the Karyomapping results for embryo number 6 from IVF cycle 1. This embryo was diagnosed as unbalanced (deletion 10pter-p15.3, duplication 19pter-p13.2). In this figure, the paternal grandfather has been imported as the reference to phase for the paternal reciprocal translocation in embryos. The following was observed (identified by the red boxes):
- Log R chart < 0 for chromosome 10 indicating a deletion of 10pter-p15.3
- Loss of the AB tract on the B-allele chart for chromosome 10 indicating a deletion of 10pter-p15.3
- Loss of paternal key SNPs and non-key SNPs vary phase for chromosome 10 on the detailed haploblock chart indicating a deletion of 10pter-p15.3
- A dispersed AB tract on the B-allele chart for chromosome 19 indicating a duplication of 19pter-p13.3
- Loss of paternal non-key SNPs and key SNPs vary phase for chromosome 19 on the detailed haploblock chart indicating a duplication of 19pter-p13.3
Discussion
As Karyomapping is a relatively new PGT technology, there are limited reports on its clinical usage for indications other than familial single gene conditions. Sarasa et al. [10] presented a case study to assess the use of Karyomapping for PGT-SR to determine whether this technology could distinguish between apparently normal versus balanced embryos (given that other methods of PGT-SR (such as NGS and array-CGH) do not have the diagnostic capability to do this). They assessed 29 embryos from three patients who had different reciprocal translocations. Their results showed that 8/29 embryos were euploid and 20/29 embryos were abnormal. The remaining 1/29 embryos did not obtain a conclusive result. Of the euploid embryos, 3/8 carried a balanced form of the familial translocation and 5/8 were apparently normal (i.e. translocation not detected). Of the abnormal embryos, 15/20 had abnormalities attributed to the familial translocation and 5/20 had chromosomal aneuploidies unrelated to the translocation. Three patients elected to have a transfer and all three obtained a successful pregnancy. Sarasa et al. concluded that Karyomapping is a reliable PGT-SR test for the detection of apparently normal, balanced and unbalanced forms of inherited chromosome rearrangements. In addition, they estimated that the technology is able to detect at least ¾ of chromosome aneuploidies in embryos and that the accuracy for inherited abnormalities is > 95%. An advantage of Karyomapping is that it can be used for chromosome rearrangements with small segments or complex rearrangements in which other PGT platforms may not be able to be used. This was reinforced by the same group when they successfully used Karyomapping technology for a couple carrying a deletion in the gene responsible for tuberous sclerosis (TSC2) in which there was no family history of the condition [11].
Another group, Stock-Myer et al. [12], investigated the use of Karyomapping to detect pathogenic copy number variants (CNVs) below ~ 5 Mb in size. They also hoped to clarify whether this technology could be used to distinguish between apparently normal versus balanced embryos from chromosome rearrangement carriers. They performed PGT-SR using Karyomapping for various duplications and deletions (sizes ranged from 0.2 to 2.5 Mb) from 13 patients and one translocation patient. They determined that Karyomapping was a reliable PGT test for CNVs below the level of detection of other PGT technologies and that Karyomapping is able to be used for the detection of apparently normal, balanced and unbalanced PGT embryos for chromosome rearrangement carriers.
In order to use Karyomapping for clinical testing, an appropriate reference sample is required. An appropriate reference can be DNA from a child, prenatal sample, products of conception or another family member that has been shown to have an unbalanced form of the chromosome rearrangement to be investigated. While this couple had access to a DNA sample from an unbalanced CVS in a previous pregnancy, in some families, DNA from an appropriate reference may not be available. Under this circumstance, DNA from at least one unbalanced embryo from a previous IVF/PGT-SR cycle may be able to be used (if DNA quality is good enough) as a reference [3]. If none of the above reference options are available, then PGT-SR using Karyomapping will not be an option.
Both array-CGH and NGS have been designed for calling whole chromosome abnormalities and even though the manufacturer states the limit of detection to be 20 Mb, other groups have reportedly validated the technology for the detection of segmental aneuploidies < 20 Mb [13,14,15]. Our group has similarly validated the detection of segmental aneuploidies below 10 Mb [5]. Therefore, for patients with chromosome rearrangements involving translocated segments < 20 Mb (such as the current case study), the best platform to use will depend on the chromosome regions involved and the results of in-house validation. Other occasions when alternate PGT-SR technologies may need to be investigated include if there has been quality issues during the manufacturing of a platform (for example the product quality notifications released in July 2017 for Illumina’s 24Sure+ array-CGH platform) or if a platform is removed from the market (for example the obsolescence of Illumina’s 24Sure+ array-CGH platforms).
The couple transferred a normal/balanced embryo in an elective single embryo transfer procedure. A clinical pregnancy (ultrasound confirmation of foetal heart beat) was achieved which was ongoing at the time of publication (12 weeks). While confirmatory prenatal diagnosis would confirm the overall clinical validity of this test, we believe that the benefits of PGT-SR outweigh the risks of this procedure. The case study presented here is the first report of PGT-SR test validation/feasibility using Karyomapping for a 46,XY,t(10;19)(p15;p13.3) reciprocal translocation and shows that for some patient indications (when DNA from an appropriate reference is available), Karyomapping can provide a means of detecting unbalanced forms of rearranged chromosomes when other PGT-SR platforms fail.
References
Katz MG, Fitzgerald L, Bankie A, Savulescu J, Cram DS. Issues and concerns of couples presenting for preimplantation genetic diagnosis (PGD). Prenat Diagn. 2002;22:1117–22.
Handyside AH, Harton GL, Mariani B, Thornhill AR, Affara N, Shaw MA, et al. Karyomapping: a universal method for genome wide analysis of genetic disease based on mapping crossovers between parental haplotypes. J Med Genet. 2010;47:651–8.
Natesan S, Bladon A, Coskun S, Qubbaj W, Prates R, Munne S, et al. Genome-wide karyomapping accurately identifies the inheritance of single-gene defects in human preimplantation embryos in vitro. Genet Med. 2014;16:838–45.
Griffin D, Gould R. What is Karyomapping and where does it fit in the world of preimplantation genetic diagnosis (PGD)? Med Res Arch. 2017;5(6):1–18.
Cuman C, Beyer CE, Brodie D, Fullston T, Lin JI, Willats E, et al. Defining the limits of detection for chromosome rearrangements in the preimplantation embryo using next generation sequencing. Hum Reprod. 2018;33(8):1566–76.
A technical guide to Karyomapping – phasing single gene defects. Illumina; 2015.
Infinium Karyomapping Assay Protocol Guide. 2015.
Kuliev A, Verlinsky O, Rechitsky S. Safety, accuracy and reproductive outcome of preimplantation genetic diagnosis. J IVF Reprod Med Genet. 2013;1:116.
Natesan S, Handyside A, Thornhill C, Ottolini K, Sage M, Summers M, et al. Live birth after PGD with confirmation by a comprehensive approach (Karyomapping) for simultaneous detection of monogenic and chromosomal disorders. Reprod BioMed Online. 2014;29:600–5.
Sarasa J, Wheeler K, Lansdowne L, Raberi A, Babariya D, Wells D. Clinical experience using single nucleotide polymorphism (SNP) arrays for preimplantation genetic diagnosis (PGD) of chromosomal translocations. Conferene paper P-658. Hum Reprod. 2015;30:403.
Giménez C, Sarasa J, Arjona C, Vilamajó E, Martínez-Pasarell O, Wheeler K, et al. Karyomapping allows preimplantation genetic diagnosis of a de-novo deletion undetectable using conventional PGD technology. Reprod BioMed Online. 2015;31(6):770–5.
Stock-Myer S, Twomey A, Tang P, Kohfahl A, Martic M. PGD for reciprocal translocation and CNVs using Karyomapping. Australian Society of Diagnostic Genomics Abstract, 2017.
Colls P, Escudero T, Fischer J, Cekleniak NA, Ben-Ozer S, Meyer B, et al. Validation of array comparative genome hybridization for diagnosis of translocations in preimplantation human embryos. Reprod BioMed Online. 2012;24(6):621–9.
Lai H, Chuang T, Wong L, Lee M, Hsieh C, Wang H, et al. Identification of mosaic and segmental aneuploidies by next-generation sequencing in preimplantation genetic screening can improve clinical outcomes compared to array-comparative genomic hybridization. Mol Cytogenet. 2017;10:14.
Vera-Rodriguez M, Michel C, Mercader A, Bladon A, Rodrigo L, Kokocinski F, et al. Distribution patterns of segmental aneuploidies in human blastocysts identified by next-generation sequencing. Fertil Steril. 2016;105(4):1047–55.
Acknowledgements
The authors would like to thank all the staff at Monash IVF clinic for referring and coordinating the couple and the genetics staff at Monash IVF for their genetic analysis and contributions towards this paper.
Authors’ roles
Claire E Beyer—case study concept and design, data acquisition, study execution, analysis, manuscript drafting, interpretation of data.
Amy Lewis—data acquisition, study execution, analysis, manuscript drafting, interpretation of data.
Elissa Willats—Manuscript drafting, interpretation of data.
Jayne Mullen—Manuscript review.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Beyer, C.E., Lewis, A., Willats, E. et al. Preimplantation genetic testing using Karyomapping for a paternally inherited reciprocal translocation: a case study. J Assist Reprod Genet 36, 951–963 (2019). https://doi.org/10.1007/s10815-019-01413-0
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10815-019-01413-0