Abstract
It is well known that for successful fertilization, oocyte activation is required, which involves a signal transduction cascade leading to the conversion of the oocyte to a diploid embryo. During oocyte activation, intracellular calcium levels oscillate repetitively causing exocytosis of cortical granules, the enzymes which the latter contain are released into the perivitelline space, leading to modifications of the zona pellucida (ZP), which prevent the penetration of the ZP by further spermatozoa. Τhe necessary element that initiates oocyte activation is apparently the release of intracellular calcium (Ca2+) stored in the endoplasmic reticulum (ER). The exact mechanism via which Ca2+ is released within the oocyte has not been yet clarified, and has been a matter of an ongoing debate. Today, the sperm factor hypothesis has gained general acceptance, according to which a sperm molecule, either phospholipase C (PLCζ) or a post-acrosomal sheath WW domain-binding protein (PAWP), diffuses into the ooplasm initiating a molecular cascade involving mainly the phosphoinositide pathway. Mounting evidence now indicates that these calcium oscillations are caused by a testis-specific PLC termed PLCζ, released into the oocyte following gamete fusion. Also, recently, PAWP has been proposed as an alternative sperm factor candidate. These different sperm candidates have led to a significant debate. This raises important questions as regards to the relative importance of these two proteins as diagnostic tools in reproductive medicine with therapeutic potential, indicating the need for further research. In the present mini review, the phenomenon of oocyte activation during fertilization as well as the existing controversy will be highlighted and the possible mechanisms that are involved in this process will be discussed. Finally, an explanation of the existing debate will be attempted.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
The hypothesis of PLCζ for oocyte activation
It has been suggested that intracellular Ca+2 rise is crucial for oocyte activation during fertilization [1]. Many hypotheses have been proposed to explain the mechanism of this rise but the most plausible is the one involving the spermatozoon itself, called “sperm hypothesis.” This hypothesis is supported by experiments involving the injection of sperm extracts into the cytoplasm of oocytes (via the ICSI procedure). Among the sperm factors (SFs) that are included in the extracts, one, the sperm-specific phospholipase C (PLC) with the property to enhance Ca+2-sensitivity, is likely to be responsible for oocyte activation [2]. The PLCζ factor is localized in the acrosomal and post-acrosomal regions of the spermatozoon during fertilization [3]. The proposed mechanism via which PLCζ induces oocyte activation involves several steps: After the fusion of the equatorial segment of the spermatozoon with the membrane of the oocyte, PLCζ is released into the ooplasm, where it hydrolyzes phosphatidylinositol 4,5-biphosphate (PIP2) for the generation of diacylglycerol (DAG) and inositol triphospate (IP3). The latter binds to own receptors (IP3R) which are present in the membrane compartment of cytoplasmatic calcium stores. The binding of IP3 to receptors triggers the release of calcium from the ooplasmatic stores (Fig. 1). Subsequent to the initial rise of calcium, wave-like calcium oscillations take place in variable periods. This kind of transient increase in calcium activity provides a positive-feedback mechanism for further calcium production. This mechanism is reversed upon a critical calcium concentration, where the receptors are losing their sensitivity to the ligand IP3. Abnormal frequency and duration of calcium release results in abnormal oocyte activation, while high or low calcium oscillations disturb completion of the second meiosis. Specifically, when 1 mg/ml of sperm cytosolic factor (SCF) was injected into freshly ovulated mouse metaphase II oocytes, the result was Ca2+ oscillations with low frequency and short duration which consequently induced normal activation and cleavage to the two-cell stage. On the other hand, injection of 15 mg/ml SCF triggered high-frequency and persistent Ca2+ oscillations which resulted in abnormal activation that was characterized by abnormal chromatin configurations, inhibition of DNA synthesis, and lack of first mitotic spindle assembly [4–6]. It seems that there are distinct calcium requirements for the initiation and the subsequent completion of oocyte activation. There are several experimental studies that have demonstrated the PLCζ utility on oocyte activation. By the help of ICSI, sperm extracts injected into the ooplasm without the PLCζ content failed to induce the calcium oscillations [7]. Moreover, mounting evidence has revealed a correlation between certain types of male infertility and abnormal function and localization of PLCζ in human spermatozoa. Specifically, PLC-deficient sperm samples from patients who experienced fertilization failure after ICSI failed to produce Ca+2 oscillations when these sperm samples were injected into mouse oocytes. These results provide evidence that PLC deficiency correlates with subfertility [8]. This has been also noticed in cases of recurrent ICSI failure and oocyte activation deficiency (OAD), indicating the crucial role of PLCζ to trigger oocyte activation and embryo development in mammals [9]. Nevertheless, the mouse and human expression profile of PLC seems not to correlate with oocyte activation, questioning in that way the role of PLCζ as sperm activating factor [10].
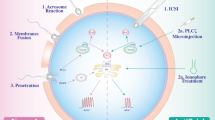
The crossroad of oocyte activation. Either PLCζ or PAWP released from the spermatozoon, as sperm factor (SF), into the ooplasm results in calcium oscillations by different pathways. After hydrolyzing PIP2, the PLCζ factor generates the IP3 molecule that binds to the respective receptor, IP3R. The result of this binding causes calcium release. On the other hand, PAWP seems to bind to oocyte-borne YAP proteins that finally activate the phosphoinositidine signal transduction pathway. The function of both sperm factors leads to the periodically wave-like transient calcium release, which in turn produces the exocytosis of cortical granules that leads to oocyte
The PAWP hypothesis for oocyte activation
There is increasing scientific and clinical evidence that supports the notion that it is not PLCζ but another sperm factor, which plays a key role in the process of oocyte activation [11]. In contrast to the established knowledge of PLCζ for oocyte activation, recently, another sperm protein (PAWP) has gained some attention. In 2007, Dr. Oko and colleagues [12] proposed that an alkaline extractable protein of the sperm head that exclusively resides in the post-acrosomal sheath region of the perinuclear theca (PT) may play a major role in the process of meiotic resumption during fertilization. This protein was named post-acrosomal WW-domain binding protein (PAWP) and it was found in many species, including humans. Subsequently, it was proposed that the same protein was responsible for the initiation of zygotic development in Xenopus eggs and the mechanism involved calcium oscillations [13]. A more recent study has demonstrated that sperm concentrations of PAWP were associated with the fertilization potential in humans undergoing IVF treatment without defining the cellular mechanism of this association [14]. Very recently, it was proposed that PAWP was responsible for human and mice oocyte activation through the mechanism of calcium release [11] (Fig. 1), but this notion was challenged by Nomikos [15]. Certainly, the signal transduction pathway of calcium release through PAWP has not been clarified yet. Nevertheless, Wu and colleagues have suggested that PAWP mediates its effects through other cytoplasmatic proteins, such as the yes-associated protein (YAP), which by activating PLCγ leads to calcium release. According to the above increasing evidence, it is likely that PAWP is a possible candidate sperm factor inducing oocyte activation during fertilization. This is further supported by recent data demonstrating a correlation between the expression of PAWP in bull spermatozoa and sperm quality as well as fertility following artificial insemination [16]. Moreover, semen quality of Chinese Holstein bulls altered when the transcriptional activity of the PLCζ was affected [17].
PLCζ or PAWP: pros and cons. Solutions in relation to IVF
By virtue of the above discrepancies, numerous questions have come up regarding the process of oocyte activation through calcium release. Given that calcium oscillations are generated exclusively from the activation of the PLC pathway, PLCζ has an advantage over PAWP as an oocyte activating factor. Moreover, in vitro and in vivo experiments have confirmed that PLCζ exerts its action directly through the IP3-mediated calcium release [18], while PAWP modulates its effects indirectly, via other molecules that consequently activate the IP3-mediated calcium oscillations [11]. Therefore, PLCζ gains further advantage over PAWP. The PLCζ hypothesis is further supported by the fact that oocyte activation (or calcium oscillations) is driven by candidate factors that are located at least around the pronuclear formation, after plasma membrane fusion. Experimental studies confirmed the localization of PLCζ around that area, while this has not been verified yet for PAWP [19]. Additionally, PAWP was found present in sperm extracts of many species, while its cellular localization is variable. On the other hand, the PLCζ factor has been identified in PT fractions [20]. In contrast to the specific localization of the PAWP factor in the post-acrosomal sheath of the sperm PT, there is conflicting evidence concerning the localization of the PLCζ. Today there are different laboratories that identified PLCζ either in the equatorial or in the post-acrosomal region of the sperm head [21] while it is getting obvious that further experimental work with specified antibodies is needed to recognize the exact location of this factor. Beside all these conflicting data, a very recent study reported the localization of PLCζ in the PT of the equatorial and postacrosomal regions of human spermatozoa confirming in that way the role of PLCζ as a major sperm oocyte activating factor [22].
The PLCζ theory seems to gain more grounds as compared to the PAWP theory, since PLCζ has more pros in relation to PAWP. Nevertheless, one should keep in mind that PAWP has a positive relation with fertilization rate in patients undergoing IVF/ICSI-ET treatments and PAWP has been found very closely to regions that are involved in oocyte activation. Although the debate about oocyte activation still holds [23–25], identification of the factor that exerts calcium release might be useful in clinical terms regarding fertilization as it may be used as a candidate marker for male infertility. For that reason, further experimental studies should be carried out regarding these two factors. A possibility might be to study sperm PLCζ or PAWP in knockout for these factors transgenic animal models by examining their capacity to elicit calcium release and subsequently how fertilization potential is affected. Further to such experiments, it may be useful to examine for each factor the biochemical pathway that leads to calcium release in the above animal models. Only one study observed aberrant (and not completely loss) calcium oscillations in transgenic PLCζ mouse models [26]. In addition, microinjection of both factors into the ooplasm, with one of them being neutralized by using specific antibodies each time, may help in the understanding of the possible impact of either factor on calcium release. The injection of both factors may lead to extra-release of calcium, while the injection of either PLCζ or PAWP separately may lead to different levels of calcium release and different calcium oscillations. Given that only a specific threshold level of calcium oscillations results in oocyte activation the above experiments may elucidate the role of each factor in oocyte activation. It might be interesting to try to measure the amount of PLC and PAWP in a single spermatozoon and to investigate whether this amount is adequate to trigger Ca changes and egg activation. Due to ethical and legal reasons, these experiments should be done in animal models rather than in humans. Besides, in vitro experiments in bovine oocytes with ghrelin and tissue-type plasminogen showed that these molecules accelerate in vitro maturation and produce embryos of excellent quality, indicating that there are various factors that affect oocyte activation and embryo development [27–29]. Nevertheless, apart from the SFs and other various factors, oocyte factors also seem to underlie oocyte activation deficiency [30]. Theoretically, if any of these factors is oocyte activating sperm factor, fertilization failure in IVF/ICSI cycles may overcome by selecting populations of spermatozoa containing the specific factor, since both SFs have been implicated in male infertility [25].
In conclusion, oocyte activation is in a crossroad since it remains a mystery, while the proposed factors (PLCζ or PAWP) should be confirmed by further research. This may provide useful biological sperm markers for fertilization failure during ICSI.
References
Machaty Z. Signal transduction in mammalian oocytes during fertilization. Cell Tissue Res. 2015;363(1):169–83.
Saunders CM, Larman MG, Parrington J, Cox LJ, Royse J, Blayney LM, et al. PLC zeta: a sperm-specific trigger of Ca(2+) oscillations in eggs and embryo development. Development. 2002;129(15):3533–44.
Young C, Grasa P, Coward K, Davis LC, Parrington J. Phospholipase C zeta undergoes dynamic changes in its pattern of localization in sperm during capacitation and the acrosome reaction. Fertil Steril. 2009;91(5):2230–42.
Miyazaki S, Shirakawa H, Nakada K, Honda Y, Yuzaki M, Nakade S, et al. Antibody to the inositol trisphosphate receptor blocks thimerosal-enhanced Ca(2+)-induced Ca2+ release and Ca2+ oscillations in hamster eggs. FEBS Lett. 1992;309(2):180–4.
Gordo AC, Wu H, He CL, Fissore RA. Injection of sperm cytosolic factor into mouse metaphase II oocytes induces different developmental fates according to the frequency of [Ca(2+)](i) oscillations and oocyte age. Biol Reprod. 2000;62(5):1370–9.
Fissore RA, Gordo AC, Wu H. Activation of development in mammals: is there a role for a sperm cytosolic factor? Theriogenology. 1998;49(1):43–52.
Yoon SY, Fissore RA. Release of phospholipase C zeta and [Ca2+]i oscillation-inducing activity during mammalian fertilization. Reproduction. 2007;134(5):695–704.
Yoon SY, Jellerette T, Salicioni AM, Lee HC, Yoo MS, Coward K, et al. Human sperm devoid of PLC, zeta 1 fail to induce Ca(2+) release and are unable to initiate the first step of embryo development. J Clin Invest. 2008;118(11):3671–81.
Ito J, Parrington J, Fissore RA. PLCζ and its role as a trigger of development in vertebrates. Mol Reprod Dev. 2011;78(10–11):846–53.
Aarabi M, Yu Y, Xu W, Tse MY, Pang SC, Yi YJ, et al. The testicular and epididymal expression profile of PLCζ in mouse and human does not support its role as a sperm-borne oocyte activating factor. PLoS One. 2012;7(3):e33496.
Aarabi M, Balakier H, Bashar S, Moskovtsev SI, Sutovsky P, Librach CL, et al. Sperm-derived WW domain-binding protein, PAWP, elicits calcium oscillations and oocyte activation in humans and mice. FASEB J. 2014;28(10):4434–40.
Wu AT, Sutovsky P, Manandhar G, Xu W, Katayama M, Day BN, et al. PAWP, a sperm-specific WW domain-binding protein, promotes meiotic resumption and pronuclear development during fertilization. J Biol Chem. 2007;282(16):12164–75.
Aarabi M, Qin Z, Xu W, Mewburn J, Oko R. Sperm-borne protein, PAWP, initiates zygotic development in Xenopus laevis by eliciting intracellular calcium release. Mol Reprod Dev. 2010;77(3):249–56.
Aarabi M, Balakier H, Bashar S, Moskovtsev SI, Sutovsky P, Librach CL, et al. Sperm content of postacrosomal WW binding protein is related to fertilization outcomes in patients undergoing assisted reproductive technology. Fertil Steril. 2014;102(2):440–7.
Nomikos M, Sanders JR, Theodoridou M, Kashir J, Matthews E, Nounesis G, et al. Sperm-specific post-acrosomal WW-domain binding protein (PAWP) does not cause Ca2+ release in mouse oocytes. Mol Hum Reprod. 2014;20(10):938–47.
Kennedy CE, Krieger KB, Sutovsky M, Xu W, Vargovič P, Didion BA, et al. Protein expression pattern of PAWP in bull spermatozoa is associated with sperm quality and fertility following artificial insemination. Mol Reprod Dev. 2014;81(5):436–49.
Pan Q, Ju Z, Huang J, Zhang Y, Qi C, Gao Q, et al. PLCz functional haplotypes modulating promoter transcriptional activity are associated with semen quality traits in Chinese Holstein bulls. PLoS One. 2013;8(3):e58795.
Nomikos M, Swann K, Lai FA. Starting a new life: sperm PLC-zeta mobilizes the Ca2+ signal that induces egg activation and embryo development: an essential phospholipase C with implications for male infertility. Bioessays. 2012;34(2):126–34.
Miyazaki S, Ito M. Calcium signals for egg activation in mammals. J Pharmacol Sci. 2006;100(5):545–52.
Kurokawa M, Yoon SY, Alfandari D, Fukami K, Sato K, Fissore RA. Proteolytic processing of phospholipase Czeta and [Ca2+]i oscillations during mammalian fertilization. Dev Biol. 2007;312(1):407–18.
Kashir J, Deguchi R, Jones C, Coward K, Stricker SA. Comparative biology of sperm factors and fertilization-induced calcium signals across the animal kingdom. Mol Reprod Dev. 2013;80(10):787–815.
Escoffier J, Yassine S, Lee HC, Martinez G, Delaroche J, Coutton C, et al. Subcellular localization of phospholipase Cζ in human sperm and its absence in DPY19L2-deficient sperm are consistent with its role in oocyte activation. Mol Hum Reprod. 2015;21(2):157–68.
Aarabi M, Sutovsky P, Oko R. Re: Is PAWP the 'real' sperm factor? Asian J Androl. 2015;17(3):446–9.
Nomikos M, Swann K, Lai FA. Is PAWP the "real" sperm factor? Asian J Androl. 2015;17(3):444–6.
Amdani SN, Yeste M, Jones C, Coward K. Sperm factors and oocyte activation: current controversies and considerations. Biol Reprod. 2015;93(2):50.
Knott JG, Kurokawa M, Fissore RA, Schultz RM, Williams CJ. Transgenic RNA interference reveals role for mouse sperm phospholipase Czeta in triggering Ca2+ oscillations during fertilization. Biol Reprod. 2005;72(4):992–6.
Dovolou E, Messinis IE, Periquesta E, Dafopoulos K, Gutierrez-Adan A, Amiridis GS. Ghrelin accelerates in vitro maturation of bovine oocytes. Reprod Domest Anim. 2014;49(4):665–72.
Dovolou E, Periquesta E, Messinis IE, Tsiligianni T, Dafopoulos K, Gutierrez-Adan A, et al. Daily supplementation with ghrelin improves in vitro bovine blastocysts formation rate and alters gene expression related to embryo quality. Theriogenology. 2014;81(4):565–71.
Krania F, Dovolou E, Rekkas CA, Theodosiadou EK, Pappas I, Amiridis GS. Effects of addition of tissue-type plasminogen activator in in vitro fertilization medium on bovine embryo development and quality. Reprod Domest Anim. 2015;50(1):112–20.
Yeste M, Jones C, Amdani SN, Patel S, Coward K. Oocyte activation deficiency: a role for an oocyte contribution? Hum Reprod Update. 2016;22(1):23–47.
Author information
Authors and Affiliations
Corresponding author
Additional information
Capsule
Controversy lingers as to the role of PLCζ, PAWP, or other factors responsible for eliciting oocyte activation.
Rights and permissions
About this article
Cite this article
Anifandis, G., Messini, C.I., Dafopoulos, K. et al. Sperm contributions to oocyte activation: more that meets the eye. J Assist Reprod Genet 33, 313–316 (2016). https://doi.org/10.1007/s10815-016-0653-0
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10815-016-0653-0