Abstract
Purpose
Anti Müllerian Hormone (AMH) has a negative and inhibitory role in many functions of human granulosa-lutein cells (hGCs) including notoriously the reduction of the aromatase CYP19A1 expression induced by follicle-stimulating hormone (FSH). No data have been provided on the possible role of AMH in modulating the response to luteinizing hormone (LH) (alone or combined with FSH) as well as its effect on other enzymes involved in steroidogenesis including aromatase P450scc. The aim of this study was to investigate the role of AMH as regulator of the basal and stimulated steroids production by hGCs.
Methods
Primary culture of hGCs were incubated with hormones AMH, LH, and FSH, alone or in combination. The CYP19A1 and P450scc messenger RNA (mRNA) expression, normalized by housekeeping ribosomal protein S7 (RpS7) gene, was evaluated by reverse transcriptase quantitative PCR (RT-qPCR). Each reaction was repeated in triplicate. Negative controls using corresponding amount of vehicle control for each hormone treatment were performed.
Result
AMH did not modulate the basal mRNA expression of both aromatase genes at any of the concentrations tested. Meanwhile, the strong mRNA induction of CYP19A1 and P450scc generated by a 24-h gonadotropin treatment (alone and combined) was suppressed by 20 ng/ml AMH added to culture medium.
Conclusions
These findings contribute in clarifying the relationship between hormones regulating the early phase of steroidogenesis confirming that AMH is playing a suppressive role on CYP19A1 expression stimulated by gonadotropin in hGCs. Furthermore, a similar inhibitory effect for AMH was observed on P450scc gene expression when activated by gonadotropin treatment.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Anti-Müllerian hormone (AMH) is a dimeric glycoprotein, a member of the transforming growth factor-beta (TGF-β) superfamily, which is expressed in the ovary by granulosa cells surrounding the growing follicles, from early antral to the selection stage. In humans, this occurs when the follicles are at the stage of 4–6 mm in diameter [1, 2].
In the mouse, AMH exerts an inhibitory role on the development of ovarian follicles in the early stages of follicular development [3, 4], confirmed by both in vitro and in vivo experiments in which the absence of AMH enhanced the transition from primordial into growing follicles with subsequent early depletion of the primordial follicle pool [5]. This increased rate of recruitment from the primordial pool in AMH knockout mice was already evident before the initiation of the estrous cycle when the ovaries of 4-month-old AMH knockout mice compared with their wild-type littermates showed three times as many small non-atretic growing follicles and a reduced number of primordial follicles [6]. Since AMH null mice have low levels of follicle-stimulating hormone (FSH), and yet increased numbers of growing follicles, it has been hypothesized that follicles are more sensitive to FSH in the absence of AMH. The possible inhibitory effect of AMH on follicular sensitivity to FSH could play a role in the process of follicular selection [6, 7]. Diminished expression of AMH within the growing follicles would reduce the threshold level for FSH, allowing follicles to continue growing and to ovulate in the next cycle [5, 8].
Current theories also suggest a role for AMH as a co-regulator of steroidogenesis in granulosa cells, as AMH is related to estradiol levels in follicular fluid from small antral follicles [9]. This was confirmed by a recent study which showed that polymorphisms in the gene for AMH or AMH receptor type II were related to estradiol levels, demonstrating a role for AMH in human ovarian steroidogenesis [10].
Indeed, several studies have clearly showed a direct effect of AMH in modulating aromatase gene expression [11–13]. In human, AMH significantly decreased FSH-stimulated aromatase expression in granulosa cells (GC) [14, 15] and also reduced FSH receptor messenger RNA (mRNA) expression [15], hence showing a relevant effect of AMH in modulating ovarian follicular responses to gonadotropins.
While studies have largely confirmed the role of AMH in inhibiting basal and FSH induced aromatase expression, no research has been conducted on the possible role of AMH in modulating P450 cholesterol side-chain cleavage enzyme (P450scc), another key enzyme involved in the ovarian steroidogenesis. This enzyme is typically expressed in steroidogenic theca cells and in granulosa-luteinized cells, and its main function is to catalyze the conversion of cholesterol to pregnenolone, a fundamental substrate for successive steroidogenesis [16].
Finally, since the steroidogenesis is influenced not only by FSH but also by luteinizing hormone (LH), we aimed to investigate the role of AMH in modulating the ovarian response to both the gonadotropins.
Materials and methods
Patients selected (n = 7) for this study had a mean age of 36 (±6). They had regular menstrual cycles and were otherwise healthy. They underwent in vitro fertilization (IVF) because of male infertility. Clinical exclusion criteria were as follows: previous ovarian surgery, positivity for Chlamydia antibody testing (CAT), presence of ovarian cysts, history of pelvic inflammatory disease (PID), any known metabolic or endocrinological disease.
The patients underwent IVF cycles according the gonadotropin releasing hormone (GnRH) antagonist protocol. The GnRH antagonist protocol was based on the administration of recombinant FSH (Gonal-f, Merck Serono, Italy or Puregon, MSD Organon, Italy) at a dose of at least 150 IU/day subcutaneously from days 2 or 3 of a spontaneous menstrual cycle. The GnRH antagonist, Ganirelix (Orgalutran, Schering-Plough) or Cetrorelix (Cetrotide, Merck Serono, Italy), was next administered daily by s.c. injection (0.25 mg/day) from the day of the stimulation cycle when the first follicle reached 14 mm in size to the day of human chorionic gonadotropin (hCG) administration. When follicles reached ≥18 mm, 10,000 IU of hCG was administrated intramuscularly and 34–36 h later, the follicles were aspirated under patient sedation.
Granulosa-lutein cells preparation
Human granulosa-lutein cells (hGCs) were isolated from ovarian follicles of women undergoing oocyte retrieval for IVF protocol.
All patients gave written informed consent at the time of the IVF cycle for recording and the use of laboratory and clinical data related to their medical history for clinical research purpose. Institutional review board (IRB) approval for this study was obtained.
Granulosa-lutein cell isolation and primary cell culture
The hGCs were purified through a discontinuous Percoll (Amersham, Sweden) gradient as indicated in Nordhoff et al. [17], and cultured in a 24-well plate (50 × 103 cells/well) in McCoy 5A medium (Carlo Erba, Italy) supplemented with 5 % fetal bovine serum (FBS) South America (EU Approved, Carlo Erba, Italy), 2 mM l-Glutamine, 1 % penicillin/streptomycin, and 1 % amphotericin B (Sigma Aldrich, St. Louis, MO, USA). hGC primary culture were maintained at 37 °C under a controlled atmosphere of 5 % CO2 for 6 days to avoid side effect due to IVF hormones treatment and subjected to medium changes with fresh culture medium daily.
Treatments
The hGC primary cultures were initially incubated in starvation medium for 12 h to synchronize cells before the treatments. The hGCs were then incubated for 24 h with 5 ng/ml of rhFSH (Gonal-f, Merck Serono, Italy) or rhLH (Luveris, Merck Serono, Italy) or both in combination. Gonadotropins were dissolved into a starvation medium (McCoy 5A medium supplemented with 0.1 % FBS South America) without antibiotics. Treated hGCs were further incubated with an increasing dosage from 2 to 200 ng/ml of rhAMH (R&D Systems, Minneapolis, MN, USA) for 24 h. Negative controls using the corresponding amount of vehicle control for each gonadotropin or AMH treatment were performed. Comparison between negative controls performed and untreated cells showed no differences in terms of cells vitality, toxic effects, or impaired gene expression caused directly by the vehicle in which hormones were resuspended.
Evaluation of gene expression by RT-qPCR
Collected hGCs after each treatment were immediately processed for total RNA extraction using commercial product Tri-Reagent® (Sigma Aldrich, St Louis, MO, USA) following the manufacturer protocol. After an optional DNase I (Promega, Madison, WI, USA) digestion of 30 min at 37 °C, the extracted RNA was evaluated and quantified by spectrophotometry using Nanodrop ND-1000 (Thermo Fisher Scientific, Waltham, MA, USA), and 2 μg of RNA of each sample was reverse transcribed to cDNA using iScript™ cDNA synthesis kit (Bio-Rad, Hercules, CA, USA) according to datasheet. Two microliters of cDNA of each sample was tested in triplicate in reverse transcriptase quantitative PCR (RT-qPCR) using SsoAdvanced Universal SYBR® Green Supermix (Bio-Rad, Hercules, CA, USA) following the conditions suggested by the manufacturer’s protocol. The primers used (listed in Table 1) in the qPCR reaction were designed, where possible, across the intron to avoid genomic DNA (gDNA) contaminant amplification. All primers used has similar melting temperature, so the general thermal profile of the reaction is for all the genes tested 30 s at 95 °C to initially activate the enzyme followed by 40 cycles of 95 °C for 5 s and 60 °C for 20 s for each cycle. The Results were normalized by using housekeeping constitutively expressed ribosomal protein S7 (RpS7) gene. The specificity of each assay was validated by dissociation curve analysis, and amplicons were separated by gel electrophoresis and imaged. Assay performance was validated by evaluating amplification efficiencies by means of calibration curves, and ensuring that the plot of log input amount versus DCq (also known as DCT) has a slope. Each reaction was repeated in triplicate. Negative control reactions omitted templates.
Statistical analysis
Statistical analysis was performed using Software GraphPad Prism applying student t test, as appropriate (p < 0.05) set for statistical significance. The relative expression of each gene has been evaluated using the 2−ΔΔCt method [3] and was calculated as the relative ratio in comparison to the first control sample, set arbitrarily to 1.
Results
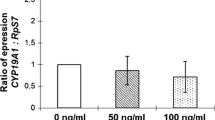
P450scc and CYP19A1 basal gene expression is not affected by rhAMH
The hGCs retrieved from seven patients who underwent IVF were purified and individually cultured then incubated for 24 h with increasing concentration of rhAMH, showing no changes in AMH gene expression (data not shown) at any of the concentrations tested.
To investigate a possible role of rhAMH as modulator of P450scc and CYP19A1 expressions, the hGC primary culture were treated with different concentrations of rhAMH in the absence of gonadotropins for 24 h and the gene activation, expressed as ratio normalized by a housekeeping gene on qPCR assay, was studied (Fig. 1). Of all concentrations tested, rhAMH alone did not produced changes in both aromatase genes expression.
LH and FSH alone or combined induced P450scc and CYP19A1 gene expression
The addition of FSH (5 ng/ml) or LH (5 ng/ml) was associated to a significant activation of gene expression of both CYP19A1 and P450scc. Expectantly, the LH was significantly more effective in inducing the two genes when compared to FSH (Fig. 2a, b).
The combination of the two gonadotropins led to a significant increase of the two gene expressions as per LH stimulation (Fig. 2c).
Gonadotropin-induced expression of CYP19A1 and P450scc is prevented by rhAMH addition to the culture
The addition of rhAMH at a concentration of 20 ng/ml prevented the activation of the P450scc and CYP19A1 induced by FSH and LH (Fig. 3).
Effect of rhAMH (20 ng/ml) on gonadotropin-induced aromatase expression (a) and on gonadotropin-induced P450scc expression (b). Significant differences were marked by *p < 0.05 versus the respective untreated cells controls or **p < 0.05 versus respective gonadotropin-treated cells, Student’s t test
Discussion
The present study showed that AMH may modulate ovarian response to both the gonadotropins, namely, FSH and LH. In particular, AMH prevent the activation of key genes for steroidogenesis such as CYP19A1 and P450scc.
The finding that AMH regulates ovarian steroidogenesis has been recently reported. In the study by Grossman et al., it has been shown that AMH inhibits FSH-dependent aromatase expression and estradiol production [12]. The negative relationship between AMH and aromatase activity has also been confirmed in studies reporting that AMH gene expression and follicular fluid concentration of AMH were negatively related to estradiol concentration [18] and to CYP19A1 mRNA expression in the corresponding granulosa cells [19].
AMH inhibits FSH activity by binding its specific receptor since knockdown of the AMH type II receptor reversed the negative effect of AMH on aromatase enzyme [14].
The recent study by Pellat and coworkers [15] largely contributed in clarifying the pathway of AMH regulation of steroidogenesis. Indeed, they showed that AMH reduced GC sensitivity to FSH by decreasing the FSH-stimulated aromatase expression and activity through inhibition of promoter II activity and through a reduction in FSH receptors number.
P450scc is a member of the superfamily of cytochrome P450 enzymes (family 11, subfamily A, polypeptide 1). The gene name is CYP11A1. The cytochrome P450 proteins are monooxygenases which catalyze many reactions involved in synthesis of cholesterol and steroids. It is localized in the mitochondrial inner membrane and catalyzes the conversion of cholesterol to pregnenolone. Together with aromatase CYP19A1, cytochrome P450scc is necessary to regulate estradiol and progesterone production within the ovary.
The stimulation of GC with FSH caused a P450scc mRNA accumulation in both porcine [4] and human [20] granulosa cells, presumably mediated by increased P450scc gene transcription. Indeed, incubation with hCG, FSH, and dibutyryl cAMP increased mRNA accumulation and progesterone secretion [20].
The two gonadotropins seem to act differently at the two gene levels. Indeed, it has been shown that both FSH and LH strongly stimulated CYP19A1 mRNA expression and estradiol production in mature granulosa cells, while P450scc mRNA expression and progesterone synthesis were only weakly induced by FSH. Indeed, the maximal synthesis occurred only in the presence of LH [21]. However, the mature granulosa lutein cells may not reflect the in vivo condition since these cells do not secrete AMH anymore, and they are only exposed to low levels of AMH from the preovulatory follicle fluid.
In the present study, we found a similar action of FSH and LH in activating genes, especially the P450scc gene. Moreover, AMH prevent the gonadotropin-induced expression of CYP19A1 and P450scc.
The relevant effect of AMH on steroidogenesis supports that AMH may have a strong role in modulating folliculogenesis itself.
Modification in AMH production may in some way be related to abnormal folliculogenesis, as in the case as polycystic ovary syndrome (PCOS). The high concentration of AMH observed in anovulatory PCOS women would be the cause of the exaggerated inhibitory effect on the follicular growth [15].
From a physiological point of view, the rapid decline in AMH expression that occurs at the time of follicular selection for dominance has been put in casual relation with the transition of follicle from a low to high estrogen producing state. The high estradiol content is a necessary condition for a follicle in order to become dominant, because of the positive effect of estradiol on FSH receptor expression. In other words, AMH seems to act as a gatekeeper, reducing the rate of follicles developing to the stage of selected follicles [22].
In conclusion, in our study, the primary cultures of hGCs were used to study the direct effect of AMH and gonadotropins on steroidogenic gene expression. Our results showed that AMH alone did not modulate the basal mRNA expression of AMH gene and of both aromatase genes at any of the concentrations tested. Meanwhile, the strong mRNA induction of CYP19A1 and of P450scc after 24 h of treatment with gonadotropins (alone and combined) was suppressed when AMH was added to the culture medium.
References
La Marca A, Broekmans FJ, Volpe A, Fauser BC, Macklon NS, ESHRE Special Interest Group for Reproductive Endocrinology—AMH Round Table. Anti-Mullerian hormone (AMH): what do we still need to know? Hum Reprod. 2009;24:2264–75.
Weenen C, Laven JS, Von Bergh AR, et al. Anti-Mullerian hormone expression pattern in the human ovary: potential implications for initial and cyclic follicle recruitment. Mol Hum Reprod. 2004;10:77–83.
Livak KJ, Schmittgen TD. Analysis of relative gene expression data using real-time quantitative PCR and the 2(−Delta Delta C(T)) method. Methods. 2001;25(4):402–8.
Urban RJ, Garmey JC, Shupnik MA, Veldhuis JD. Follicle-stimulating hormone increases concentrations of messenger ribonucleic acid encoding cytochrome P450 cholesterol side-chain cleavage enzyme in primary cultures of porcine granulosa cells. Endocrinology. 1991;128(4):2000–7.
Durlinger AL, Gruijters MJ, Kramer P, Karels B, Kuman TR, Matzuk MM, et al. Anti-Müllerian hormone attenuates the effects of FSH on follicle development in the mouse ovary. Endocrinology. 2001;142:4891–9.
Durlinger AL, Kramer P, Karels B, de Jong FH, Uilenbroek JT, Grootegoed JA, et al. Control of primordial follicle recruitment by anti-Müllerian hormone in the mouse ovary. Endocrinology. 1999;140:5789–96.
McGee EA, Hsueh AJ. Initial and cyclic recruitment of ovarian follicles. Endocr Rev. 2000;21:200–14.
Visser JA, Durlinger AL, Peters IJ, van den Heuvel ER, Rose UM, Kramer P, et al. Increased oocyte degeneration and follicular atresia during the estrous cycle in anti-Müllerian hormone null mice. Endocrinology. 2007;148:2301–8.
Andersen CY, Byskov AG. Estradiol and regulation of anti-Müllerian hormone, inhibin-A, and inhibin-B secretion: analysis of small antral and preovulatory human follicles’ fluid. J Clin Endocrinol Metab. 2006;91:4064–9.
Kevenaar ME, Themmen AP, Laven JS, Sonntag B, Fong SL, Uitterlinden AG, et al. Anti-Müllerian hormone and anti-Müllerian hormone type II receptor polymorphisms are associated with follicular phase estradiol levels in normo-ovulatory women. Hum Reprod. 2007;22:1547–54.
Di Clemente N, Goxe B, Remy JJ, Cate R, Josso N, Vigier B, et al. Inhibitory effect of AMH upon the expression of aromatase and LH receptors by cultured granulosa cells of rat and porcine immature ovaries. Endocrine. 1994;2:553–8.
Grossman MP, Nakajima ST, Fallat ME, Siow Y. Müllerian-inhibiting substance inhibits cytochrome P450 aromatase activity in human granulosa lutein cell culture. Fertil Steril. 2008;89(5 Suppl):1364–70.
Kim JH, Seibel MM, Maclaughlin DT, Donahoe PK, Ransil BJ, Hametz PA, et al. The inhibitory effects of Müllerian-inhibiting substance on epidermal growth factor induced proliferation and progesterone production of human granulose-luteal cells. J Clin Endocrinol Metab. 1992;75:911–7.
Chang HM, Klausen C, Leung PC. Antimüllerian hormone inhibits follicle-stimulating hormone-induced adenylyl cyclase activation, aromatase expression, and estradiol production in human granulosa-lutein cells. Fertil Steril. 2013;100(2):585–92.
Pellatt L, Rice S, Dilaver N, Heshri A, Galea R, Brincat M, et al. Anti-Mullerian hormone reduces follicle sensitivity to follicle-stimulating hormone in human granulosa cells. Fertil Steril. 2011;96(5):1246–51.
Hanukoglu I. Steroidogenic enzymes: structure, function, and role in regulation of steroid hormone biosynthesis. J Steroid Biochem Mol Biol. 1992;43(8):779–804.
Nordhoff V, Sonntag B, von Tils D, Gotte M, Schüring AN, Gromoll J, et al. Effects of the FSH receptor gene polymorphism p.N680S on cAMP and steroid production in cultured primary human granulosa cells. Reprod Biomed Online. 2011;23:196–203.
Jeppesen JV, Anderson RA, Kelsey TW, Christiansen SL, Kristensen SG, Jayaprakasan K, et al. Which follicles make the most anti-Müllerian hormone in humans? Evidence for an abrupt decline in AMH production at the time of follicle selection. Mol Hum Reprod. 2013;19(8):519–27.
Nielsen ME, Rasmussen IA, Fukuda M, Westergaard LG, Andersen CY. Concentrations of anti-Müllerian hormone in fluid from small human antral follicles show a negative correlation with CYP19 mRNA expression in the corresponding granulosa cells. Mol Hum Reprod. 2010;16(9):637–43.
Voutilainen R, Tapanainen J, Chung BC, Matteson KJ, Miller WL. Hormonal regulation of P450scc (20,22-desmolase) and P450c17 (17 alpha-hydroxylase/17,20-lyase) in cultured human granulosa cells. J Clin Endocrinol Metab. 1986;63(1):202–7.
Yong EL, Hillier SG, Turner M, Baird DT, Ng SC, Bongso A, et al. Differential regulation of cholesterol side-chain cleavage (P450scc) and aromatase (P450arom) enzyme mRNA expression by gonadotropins and cyclic AMP in human granulosa cells. J Mol Endocrinol. 1994;12(2):239–49.
Dewailly D, Andersen CY, Balen A, Broekmans F, Dilaver N, Fanchin R, et al. The physiology and clinical utility of anti-Müllerian hormone in women. Hum Reprod Update. 2014;20(3):370–85.
Author information
Authors and Affiliations
Corresponding author
Additional information
Capsule In granulosa cells in vitro, the AMH prevented the stimulatory effect of FSH and LH on the expression of the key enzymes of steroidogenesis, aromatase and P450scc, hence confirming a general inhibitory role for this glycoprotein on ovarian function.
Sandro Sacchi and Giovanni D’Ippolito contributed equally to this work.
Rights and permissions
About this article
Cite this article
Sacchi, S., D’Ippolito, G., Sena, P. et al. The anti-Müllerian hormone (AMH) acts as a gatekeeper of ovarian steroidogenesis inhibiting the granulosa cell response to both FSH and LH. J Assist Reprod Genet 33, 95–100 (2016). https://doi.org/10.1007/s10815-015-0615-y
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10815-015-0615-y