Abstract
Purpose
A physiological balance exists between seminal reactive oxygen species (ROS) and antioxidant capacity. An overproduction of ROS which exceeds the seminal plasma antioxidant capacity results in oxidative stress (OS). The aim of the present study was to describe a detailed protocol to measure ROS in a diagnostic laboratory and revise our previous cutoff value of ROS in seminal ejaculates in a larger cohort of infertile men and controls with proven and unproven fertility.
Methods
A total of 258 infertile men and 92 controls were enrolled in the study. Following initial semen analysis, ROS measurement in whole ejaculates was carried out using luminol-based chemiluminescence assay. Chemiluminescence was measured for 15 min with a Berthold luminometer. Results were expressed as relative light units (RLU/s/106 sperm). The test’s specificity, sensitivity, and cutoff values were calculated using the receiver operating characteristic (ROC) curve.
Results
Significantly higher ROS levels were seen in infertile men compared to controls (p < 0.001). The optimal cutoff value to differentiate between controls and infertile men was 102.2 RLU/s/106 sperm. At this cutoff value, the test was 76.4% sensitive and 53.3% specific. The positive and negative predictive values of the test were 82.1% and 44.5%, respectively. A total of 76.4% infertile population was above this cutoff value compared to 46.7% of controls.
Conclusions
The luminol-based chemiluminescence assay can be used in routine diagnostic screening to test for male infertility diagnosis in a clinical setting. The current ROS cutoff value substantially distinguishes infertile from normal controls. Patients with elevated ROS must be evaluated for the underlying cause of ROS production.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Free oxygen radicals such as superoxide anion (O2−), hydrogen peroxide (H2O2), hypochlorite (OHCl), and hydroxyl radical (OH) are highly reactive oxygen species (ROS) [1, 2]. The production of these radicals occurs during normal metabolism of the cell. In semen, ROS are produced by spermatozoa and play a vital role in many physiologic processes of spermatozoa, such as acrosome reaction, capacitation, mitochondrial sheath stability, and fusion with oocyte [3–6]. Seminal plasma contains several antioxidants and has a strong antioxidant capacity. For the continuity of normal physiological spermatozoa functions, a balance exists between seminal ROS and antioxidant capacity. An overproduction of ROS which exceeds the seminal plasma antioxidant capacity results in oxidative stress (OS), thereby inducing deleterious effects on spermatozoa function and, may consequently, lead to male infertility [5, 7–9].
The harmful effects of higher levels of ROS have been documented on several sperm parameters including sperm concentration, motility, morphology, viability, and DNA fragmentation [1, 4, 10–13]. Elevated ROS levels have also been reported in some clinical conditions (e.g., varicocele and reproductive tract infections) as well as in association with lifestyle choices (e.g., smoking) [14–18]. Similarly, low fertilization and pregnancy rates were achieved in couples in which the male partner had elevated seminal ROS levels [19, 20].
Convincing evidence suggests that ROS levels have a positive impact on sperm function and male fertility if they are within the normal physiological range and effect negatively in uncontrolled production. Therefore, accurate measurement and defined cutoff values of ROS levels are important. The measurement of seminal ROS levels in infertile and fertile populations has been a challenge, requiring a method with high specificity and sensitivity. Previously, we published that elevated levels of ROS are directly related to male factor infertility [21], and 91.9 RLU/s/106 sperm was considered a cutoff to distinguish between ROS negative and positive semen samples [22]. However, questions concerning the accuracy of this predictor of male factor fertility and cutoff values have yet to be fully answered.
The present study describes a detailed protocol for use in clinical laboratory for measurement of seminal ROS via luminol-based chemiluminescence assay. We also established ROS cutoff values in a large population of infertile patients and controls with or without proven fertility.
Material and methods
Selection of subjects
This study was approved by the Institutional Review Board of Cleveland Clinic. A total of 258 infertile men and 92 controls (with or without proven fertility) were enrolled in this study.
Inclusion criteria:
All infertile men were attending the male infertility clinic for confirmed male factor infertility and evaluated by an infertility specialist. The female partner of these patients had gone through gynecological investigations and was declared normal.
Exclusion criteria
Patients with cryptorchidism, azoospermia, retrograde ejaculate, partial ejaculate, or too low volume of ejaculate were excluded from the study. The controls comprised healthy male volunteers with normal semen analysis according to the WHO, 2010 guidelines [23]. Both men with proven and unproven fertility were included.
Semen collection and processing
Semen samples from patients and controls were collected by masturbation after 2–3 days of abstinence. After complete liquefaction for 20 min, basic semen analysis was performed according to WHO (5th Ed) guidelines [23]. ROS levels were measured using luminol-based chemiluminescence assay. The principle and detailed protocol of the test is described below.
-
I.
Principle
Luminol is extremely sensitive and reacts with a variety of ROS at neutral pH. It can measure both extracellular and intracellular ROS. The free radical combines with luminol to produce a light signal that is converted to an electrical signal (photon) by a luminometer. The number of free radicals produced is measured as relative light units/s/106 sperm.
-
II.
Equipment and materials
-
A.
Disposable polystyrene tubes with caps (15 mL)
-
B.
Eppendorf pipettes (5 μL, 10 μL)
-
C.
Pipettes (1 mL, 2 mL, 10 mL)
-
D.
Centrifuge
-
E.
Computer-assisted semen analyzer (CASA)
-
F.
Disposable microcell slides
-
G.
Dimethyl sulfoxide (DMSO; Catalog #D8779, Sigma Chemical Co., St. Louis, MO)
-
H.
Luminol (5-amino-2,3dehydro-1,4 phthalazinedione; Catalog #A8511, Sigma Chemical Co., St. Louis, MO)
-
I.
Polystyrene round bottom tubes (6 mL)
-
J.
Luminometer (Model: Berthold, AutoLumat Plus LB 953, Oakridge, TN)
-
K.
Dulbecco’s Phosphate buffered saline solution 1X (PBS-1X; Catalog #9235, Irvine Scientific, Santa Ana, CA)
-
A.
-
III.
Reagent preparation
-
A.
Luminol stock solution: 100 mM solution; 177.09 mg of luminol was weighed and added to 10 mL of DMSO solution in a polystyrene tube. The tube was covered with aluminum foil as luminol is light sensitive. This solution can be stored at room temperature until its expiration date.
-
B.
Working Luminol Solution: 5 mM solution; 20 μL of the luminol stock solution was mixed with 380 μL DMSO in a foil-covered polystyrene tube. It was prepared fresh prior to use. It can be stored at room temperature until needed and is stable for 24 h if not exposed to light.
-
C.
DMSO solution: It is provided ready to use and is stored in a dark container at room temperature until expiration date.
-
A.
-
IV.
Specimen preparation
-
A.
The semen sample was allowed to undergo liquefaction in the 37°C incubator for 20 min.
-
B.
Patient’s name, clinic number, period of sexual abstinence, date and time of specimen collection, and age of specimen when the semen analysis was performed was recorded.
-
C.
Initial physical characteristics such as volume, pH, color, etc. were also recorded.
-
D.
Five microliter of the well-mixed semen was loaded onto a counting chamber (MicroCell slide, Vitrolife Inc., San Diego, CA, USA).
-
E.
Semen analysis was performed using manual sperm count and motility. In addition, round cells were counted and the Endtz test was performed when the round cell concentration was greater than 1 × 106/ mL.
-
A.
-
V.
ROS determination
-
A.
Luminometer was set up with a computer attached to it (Fig. 1a–c).
-
B.
11 Falcon tubes (12 × 75 mm) were labeled in triplicate and the reagents were added as indicated in Table 1 (Fig. 2).
Note: All readings were performed in the dark.
-
C.
It is important that the instrument settings are in place before adding reagents to the tube and loading samples.
-
D.
Reagents were added to the bottom of the tubes and not on the side. They were vortexed to ensure that the luminol was mixed with the rest of the reagent/sample.
-
E.
The pipette tip was changed when adding reagent/sample to each tube.
-
F.
Tubes were gently vortexed to mix the aliquots uniformly and to avoid bubbles.
-
G.
All the labeled tubes were placed in the luminometer in the following order: blank (tubes labeled 1–3), negative control (tubes labeled 4–6), test sample (tubes labeled 7–8), and positive control (tubes labeled 9–11) (Figs. 3 and 4).
Fig. 1 Table 1 Set up for the measurement of ROS Fig. 2 Fig. 3 -
A.
-
VI.
Analyzing the samples
-
A.
After the tubes were loaded, the “Start button was clicked. The luminometer will start scanning for tubes.
-
B.
After scanning, the monitor will show how many tubes are detected by the instrument in each batch, press “Next.”
-
C.
Select the “Assay Type.” Click “Next” and then click ‘Finish.’
-
D.
The “Excel spreadsheet” will open.
-
E.
Measurement of the tubes will start.
-
F.
Do not touch the computer during this time.
-
G.
Wait (3–5 min) to make sure everything is working fine.
-
H.
After finishing the measurements, the computer will prompt to “Save” the Excel spreadsheet. Save it in “My Computer,” in a folder under “Clinical ROS.”
-
I.
Save Berthold measurement “Measurement Files” (*.txr) in the same directory as the Excel spreadsheet using the same name.
-
A.
-
VII.
Printing ROS results
-
A.
Print spreadsheet as well as the “chart 1” and the Berthold sheet (Fig. 5).
-
B.
Close the Excel spreadsheet.
-
C.
Print the “Work Load” sheet (Fig. 6). Make sure all 3 sheets are printed before saving and closing the file.
Fig. 5 A representative display of the readings showing the number of signals generated in each of the above 11 tubes (S1–S11). The measurement is for a total of 900 s. As seen here, blanks have the lowest amount of ROS and the positive control, to which hydrogen peroxide was added, has the highest amount of ROS
-
A.
-
VIII.
Calculating results
-
A.
The “average RLU” for the negative control, samples and positive control was calculated.
-
B.
Sample ROS was calculated by subtracting the negative control average from its average.
-
C.
Sample ROS = average “RLU mean” for sample − average “RLU mean” for negative control.
-
D.
The sample ROS was calculated by dividing it with “sperm concentration/mL.”
Corrected sample ROS: calculated sample ROS/ sperm concentration = XX.X (RLU/s/ × 106 sperm)
A typical example of calculating ROS values is illustrated in Fig. 7.
-
A.
-
IX.
Reference values were calculated and recorded as:
Normal values or ROS negative and critical values or ROS positive as described in the results.
-
X.
Quality control
-
A.
It is important to record the reagent lot numbers and expiration dates on the worksheet and placed in the quality control book.
-
B.
Criteria for rejection: No sperm are present.
-
A.
-
XI.
Factors affecting ROS measurement
-
A.
The luminometer instrumentation, its calibration, determination of sensitivity, dynamic range, and units used.
-
B.
The concentration and type of probe used.
-
C.
The concentration and volume of the semen sample, use of reagent, and temperature of the luminometer.
-
D.
Semen age (i.e., time to analysis) after sample is collected to the time of ROS measurement.
-
E.
Viscous samples and poor liquefaction, which may interfere with chemiluminescent signals.
-
F.
Repeated centrifugation can cause artificial increase in chemiluminescent signal because of the shearing forces generated by centrifugation.
-
G.
Use of media that contains serum albumin can generate spurious signals in the presence of human seminal plasma.
-
H.
Sensitivity of luminol to pH changes.
-
I.
Many compounds can artificially increase (e.g., cysteine or thiol-containing compounds) or decrease (e.g., ascorbate or uric acid) the chemiluminescent signal generated by spermatozoa. Hence, it is necessary to run sperm-free controls as an integral part of the chemiluminescent assay.
-
A.
Statistical analysis
The Wilcoxon rank sum test was used for group comparison with respect to quantitative variables and chi square test with respect to categorical variables. The difference in the distribution of ROS levels between the two groups (control and patient) was assessed. A receiver operating characteristic (ROC) curve was used to assess the ability of ROS as a means of discriminating patients with controls. A cutoff value that maximized the sum of estimated sensitivity and specificity was chosen. A p value of <0.05 was considered significant.
Results
ROC curve analysis
Test sensitivity, specificity, and positive and negative predictive values were calculated using ROC curve. Significantly, higher ROS levels were seen in infertile men compared to controls (p < 0.001). The optimal cutoff value to distinguish between controls and infertile men was 102.2 RLU/s/106 sperm. At this cutoff value, the positive and negative predictive values of the test were 82.1% and 44.5%, respectively (Fig. 8). The percentage of patients and controls above and below this cutoff value as well as sensitivity and specificity of the test are given in Table 2.
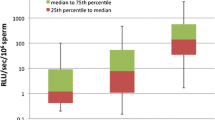
ROS distribution by group
The distribution of the ROS levels in infertile patients and controls is given in Fig. 9.
Discussion
In recent decades, male infertility has increased significantly due to a decline in semen quality and a concurrent increase in male reproductive pathologies. Linked to male infertility is OS, which has become a focus of interest and is implicated in its pathogenesis. A uniform consensus exists on the role of ROS in male fertility and infertility. Previous studies from our center have demonstrated that ROS is an independent factor of male factor infertility [4, 21]. We have reported different cutoff values for ROS in washed [2, 11, 24] and seminal ejaculates [4, 22].
Before a test is recommended for clinical use, its reliability, repeatability, sensitivity, and accuracy must be validated. In this current study, we used liquefied or neat semen to measure ROS in infertile males and controls using the luminol-based chemiluminescence method.
Luminol-based chemiluminescence assay is a reliable technique for measuring seminal ROS [4]. Our goal was to establish a cutoff value of ROS and describe the methodology, which can serve as a guide for other laboratories. Our cutoff for ROS at which the test can effectively differentiate between infertile and control group was 102.2 RLU/s/106 sperm. At this value, the sensitivity and specificity of the test were 76.4 and 53.3%, respectively. Positive and negative predictive values (PPV and NPV) of the test were 82.1% and 44.5%, respectively. Overall, 68% of the population was above the cutoff value, while 31.4 % was below. When intra-group analysis was carried out, 76.4% of infertile population was above this cutoff compared to 46.7% of control. Only 23.6% of the infertile population was below this cutoff compared to 53.3% of control.
In our earlier study by Agarwal et al. [22], the control group comprised only healthy men who had established a pregnancy (within 2 years or more than 2 years). The current study had a larger cohort that included both proven fertile and those who did not establish a pregnancy. This was a study limitation. Therefore, sensitivity and specificity in this study are lower compared to our earlier study. The present cutoff of ROS suggests that any value above this threshold was still able to differentiate between subjects that were fertile from those who were not.
Physiological levels are necessary for the normal functions of sperm in capacitation, acrosome reaction, and zona binding. From our results, ROS levels below 102.2 RLU/s/106 sperm can be regarded as physiological levels, while those above this value should be considered pathological. Measurement of ROS levels can also serve a predictive tool in assisted reproduction [20, 25]. Deleterious effects of OS have been documented on fertilization rates in In vitro fertilization (IVF/ICSI) [19, 26]. In vitro addition of antioxidants into sperm preparation and oral intake of antioxidants have shown to increase the fertilization rates and pregnancy outcome [27–30]. Furthermore, the negative impact of ROS on sperm DNA integrity has been shown in semen samples with high ROS levels. Agarwal et al. demonstrated that even in low levels of leukocytospermia, ROS levels are significantly increased compared to control that screened negative for leukocytes. [31]. This reveals the importance of ROS assessment in such patients, considering the fact that damaged sperm DNA has negative implications on fertilization rates and early embryo development.
Our goal was to provide a detailed protocol for the measurement of ROS using chemiluminescence assay to allow other diagnostic laboratories to establish this test in their labs and offer it to their patients. However, we recommend that controls of only proven fertility should be used so that the sensitivity and specificity can be further increased and the test employed as a screening/diagnostic test.
Taken together, we have demonstrated that the luminol-based chemiluminescence assay is sensitive and reliable in measuring ROS levels. The reproducibility of this test makes it a potential screening tool in male infertility investigations as well as a prognostic test for assisted reproduction outcomes.
References
Aitken RJ, Clarkson JS. Cellular basis of defective sperm function and its association with the genesis of reactive oxygen species by human spermatozoa. J Reprod Fertil. 1987;81(2):459–69.
Shekarriz M, Thomas Jr AJ, Agarwal A. Incidence and level of seminal reactive oxygen species in normal men. Urology. 1995;45(1):103–7.
Du Plessis SS, Agarwal A, Halabi J, Tvrda E. Contemporary evidence on the physiological role of reactive oxygen species in human sperm function. J Assist Reprod Genet. 2015;32(4):509–20.
Desai N, Sharma R, Makker K, Sabanegh E, Agarwal A. Physiologic and pathologic levels of reactive oxygen species in neat semen of infertile men. Fertil Steril. 2009;92(5):1626–31.
Agarwal A, Makker K, Sharma R. Clinical relevance of oxidative stress in male factor infertility: an update. Am J Reprod Immunol. 2008;59(1):2–11.
Aitken RJ, Mclaughlin EA. Molecular mechanisms of sperm capacitation: progesterone-induced secondary calcium oscillations reflect the attainment of a capacitated state. Soc Reprod Fertil Suppl. 2007;63:273–93.
Mahfouz R, Sharma R, Sharma D, Sabanegh E, Agarwal A. Diagnostic value of the total antioxidant capacity (TAC) in human seminal plasma. Fertil Steril. 2009;91(3):805–11.
Sharma RK, Pasqualotto AE, Nelson DR, Thomas Jr AJ, Agarwal A. Relationship between seminal white blood cell counts and oxidative stress in men treated at an infertility clinic. J Androl. 2001;22(4):575–83.
Sharma RK, Agarwal A. Role of reactive oxygen species in male infertility. Urology. 1996;48(6):835–50.
Agarwal A, Mulgund A, Sharma R, Sabanegh E. Mechanisms of oligozoospermia: an oxidative stress perspective. Syst Biol Reprod Med. 2014;60(4):206–16.
Sharma RK, Pasqualotto FF, Nelson DR, Thomas Jr AJ, Agarwal A. The reactive oxygen species-total antioxidant capacity score is a new measure of oxidative stress to predict male infertility. Hum Reprod. 1999;14(11):2801–7.
Sharma RK, Sabanegh E, Mahfouz R, Gupta S, Thiyagarajan A, Agarwal A. TUNEL as a test for sperm DNA damage in the evaluation of male infertility. Urology. 2010;76(6):1380–6.
Khosrowbeygi A, Zarghami N. Levels of oxidative stress biomarkers in seminal plasma and their relationship with seminal parameters. BMC Clin Pathol. 2007;7(6.
Fraczek M, Szumala-Kakol A, Jedrzejczak P, Kamieniczna M, Kurpisz M. Bacteria trigger oxygen radical release and sperm lipid peroxidation in in vitro model of semen inflammation. Fertil Steril. 2007;88(4 Suppl):1076–85.
Henkel R, Kierspel E, Stalf T, Mehnert C, Menkveld R, Tinneberg HR, et al. Effect of reactive oxygen species produced by spermatozoa and leukocytes on sperm functions in non-leukocytospermic patients. Fertil Steril. 2005;83(3):635–42.
Saleh RA, Agarwal A, Kandirali E, Sharma RK, Thomas AJ, Nada EA, et al. Leukocytospermia is associated with increased reactive oxygen species production by human spermatozoa. Fertil Steril. 2002;78(6):1215–24.
Agarwal A, Deepinder F, Cocuzza M, Agarwal R, Short RA, Sabanegh E, et al. Efficacy of varicocelectomy in improving semen parameters: new meta-analytical approach. Urology. 2007;70(3):532–8.
Cocuzza M, Athayde KS, Agarwal A, Pagani R, Sikka SC, Lucon AM, et al. Impact of clinical varicocele and testis size on seminal reactive oxygen species levels in a fertile population: a prospective controlled study. Fertil Steril. 2008;90(4):1103–8.
Hammadeh ME, Radwan M, Al-Hasani S, Micu R, Rosenbaum P, Lorenz M, et al. Comparison of reactive oxygen species concentration in seminal plasma and semen parameters in partners of pregnant and non-pregnant patients after IVF/ICSI. Reprod Biomed Online. 2006;13(5):696–706.
Zorn B, Vidmar G, Meden-Vrtovec H. Seminal reactive oxygen species as predictors of fertilization, embryo quality and pregnancy rates after conventional in vitro fertilization and intracytoplasmic sperm injection. Int J Androl. 2003;26(5):279–85.
Agarwal A, Sharma RK, Nallella KP, Thomas Jr AJ, Alvarez JG, Sikka SC. Reactive oxygen species as an independent marker of male factor infertility. Fertil Steril. 2006;86(4):878–85.
Agarwal A, Sharma RK, Sharma R, Assidi M, Abuzenadah AM, Alshahrani S, et al. Characterizing semen parameters and their association with reactive oxygen species in infertile men. Reprod Biol Endocrinol. 2014;12-33.
World Health Organization. WHO laboratory manual for the examination and processing of human semen. World Health Organization. 2010.
Agarwal A, Ikemoto I, Loughlin KR. Effect of sperm washing on levels of reactive oxygen species in semen. Arch Androl. 1994;33(3):157–62.
Moustafa MH, Sharma RK, Thornton J, Mascha E, Abdel-Hafez MA, Thomas Jr AJ, et al. Relationship between ROS production, apoptosis and DNA denaturation in spermatozoa from patients examined for infertility. Hum Reprod. 2004;19(1):129–38.
Agarwal A, Allamaneni SS, Nallella KP, George AT, Mascha E. Correlation of reactive oxygen species levels with the fertilization rate after in vitro fertilization: a qualified meta-analysis. Fertil Steril. 2005;84(1):228–31.
Omu AE, Dashti H, Al-Othman S. Treatment of asthenozoospermia with zinc sulphate: andrological, immunological and obstetric outcome. Eur J Obstet Gynecol Reprod Biol. 1998;79(2):179–84.
Agarwal A, Gupta S, Sikka S. The role of free radicals and antioxidants in reproduction. Curr Opin Obstet Gynecol. 2006;18(3):325–32.
Piomboni P, Gambera L, Serafini F, Campanella G, Morgante G, De Leo V. Sperm quality improvement after natural anti-oxidant treatment of asthenoteratospermic men with leukocytospermia. Asian J Androl. 2008;10(2):201–6.
Comhaire FH, El Garem Y, Mahmoud A, Eertmans F, Schoonjans F. Combined conventional/antioxidant "Astaxanthin" treatment for male infertility: a double blind, randomized trial. Asian J Androl. 2005;7(3):257–62.
Agarwal A, Mulgund A, Alshahrani S, Assidi M, Abuzenadah AM, Sharma R, et al. Reactive oxygen species and sperm DNA damage in infertile men presenting with low level leukocytospermia. Reprod Biol Endocrinol. 2014;12(126.
Acknowledgments
Gulfam Ahmad was supported by a Fulbright Fellowship. The authors are grateful to the Andrology Center technologists for scheduling the study subjects and Jeff Hammel, senior biostatistician, for his contribution to data analysis.
Author information
Authors and Affiliations
Corresponding author
Additional information
Capsule Chemiluminescence based measurement of ROS can distinguish infertile men from normal controls.
Rights and permissions
About this article
Cite this article
Agarwal, A., Ahmad, G. & Sharma, R. Reference values of reactive oxygen species in seminal ejaculates using chemiluminescence assay. J Assist Reprod Genet 32, 1721–1729 (2015). https://doi.org/10.1007/s10815-015-0584-1
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10815-015-0584-1