Abstract
Purpose
To investigate the predictive value of the motile sperm organelle morphology examination (MSOME) on embryo morphology.
Methods
The morphologies of 540 embryos obtained from 60 couples undergoing ICSI were evaluated from days 1 to 5 of development and were examined for associations with the percentages of morphologically normal paternal sperm and of the paternal sperm with large nuclear vacuoles (LNVs) as determined by MSOME.
Results
An increased percentage of LNV sperm was associated with increased odds of a zygote presenting with pronuclear abnormalities. It was also associated with decreased odds of (i) normal cleavage on days 2 and 3 of development, (ii) the presence of a high-quality embryo on day 3, (iii) the development of an embryo to the blastocyst stage, and (iv) an embryo possessing a normal trophectoderm and inner cell mass. The calculated areas under the curves differed for the embryos that did and did not develop to the blastocyst stage and for the high- and low-quality blastocysts. The optimal cut-off value for the percentage of LNV sperm that maximised proper blastocyst formation was ≤24.5 %, and the cut-off value that maximised blastocyst quality was ≤19.5 %.
Conclusions
These results suggest a very early onset of paternal influences on embryo development. The evaluation of the incidence of vacuoles by MSOME may significantly improve upon the prognostic information provided by conventional semen analyses.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
The advent of the intracytoplasmic sperm injection (ICSI) [1] revolutionized the treatment of couples with male factor infertility and led to an in depth investigation of potential paternal effects on embryo development and quality. Sperm parameters, such as concentration, motility and morphology have been shown to correlate with embryo morphology and cleavage rhythm [2], blastocyst formation and quality [2–7] and implantation rate after embryo transfer [2, 7].
Since 2001, motile sperm organelle morphology examination (MSOME) has been used to select vacuole-free spermatozoa under high-magnification (DIC/Nomarski) for ICSI [8]. More recently, research on male factor infertility has focused on sperm morphology, particularly that of the sperm head. Vacuolated spermatozoa have been associated with reduced ICSI outcomes [9], increased DNA fragmentation [10] and the failure of chromatin condensation [11].
Despite the well-established correlation between semen parameters and embryonic development, scarce information exists regarding the influences of microscopic morphological characteristics of sperm on embryo quality and development to the blastocyst stage. Therefore, the aim of this study was to examine the associations between the sperm quality visualised under high magnification with non-invasive criteria for the evaluation of pronuclear-stage zygotes, cleavage-stage embryos and blastocysts to investigate the onset of detectable paternal effects on embryonic development.
Materials and methods
Experimental design, patients and inclusion criteria
This prospective non-randomised study analysed semen samples from 60 couples undergoing their first ICSI attempt as a result of male factor infertility (oligo- and/or astheno- and/or teratozoospermia). A total of 200 spermatozoa from each sample were analysed under high magnification (6,600×). The incidences of normal sperm and sperm with LNV in each sample were assessed and associated with embryo quality and development (from fertilisation to the blastocyst stage).
Inclusion criteria were as follows: women aged ≤37 years undergoing ICSI as a result of male factor with regular menstrual cycles of 25–35 days, normal basal FSH and LH levels, a BMI of less than 30 kg/m2, the presence of both ovaries and an intact uterus, the absence of polycystic ovaries, endometriosis, and gynaecological/medical disorders and negative results on a screening for sexually transmitted diseases. No patient received any hormone therapy for at least 60 days prior to the study.
Written informed consent was obtained, in which patients agreed to share their outcomes for research purposes, and the study was approved by the local institutional review board.
Controlled ovarian stimulation
Ovarian stimulation was achieved by the administration of recombinant follicle-stimulating hormone and a gonadotropin-releasing hormone antagonist, as previously described [12].
Semen sample collection and preparation
Semen samples were collected in the laboratory and were evaluated according to the values established by the World Health Organisation (WHO) in 2010 [13]. Sperm samples were prepared using a 2-layered density gradient centrifugation technique (50 % and 90 %; ISolate, Irvine Scientific, Santa Ana, CA, USA).
MSOME
ICSI was performed according to Palermo et al. [1]. Immediately after ICSI, MSOME was performed. For each semen sample, 200 spermatozoa were analysed at 6,600× using an inverted microscope equipped with high-powered differential interference contrast optics (DIC/Nomarski). The same technician performed all MOSME analyses. An aliquot of the sperm cell suspension was transferred to a microdroplet of modified human tubal fluid medium containing 8 % polyvinyl pyrrolidone (PVP; Irvine Scientific, Santa Ana, CA) in a sterile glass dish (FluoroDish; World Precision Instruments, Sarasota, FL). The dish was placed on a microscope stage above an Uplan Apo 100× oil/1.35 objective lens covered by a droplet of immersion oil.
A sperm cell exhibiting a normal nucleus (normal shape and normal chromatin content) as well as a normal acrosome, postacrosomal lamina, neck, tail, and mitochondria and lacking cytoplasmic droplets or cytoplasm surrounding the head was considered to be morphologically normal [14] (Fig. 1a). An LNV sperm was defined by the presence of one or more vacuoles occupying >13 % of the sperm nuclear area [15–17] (Fig. 1b). The incidences of normal and LNV sperm in each sample were recorded.
Fertilisation, embryo quality and embryo transfer
Fertilisation was confirmed by the presence of two pronuclei (PN) and the extrusion of the second polar body approximately 16 h after ICSI. Embryo morphology was assessed on the mornings of days 1, 2, 3 and 5 of embryonic development using an inverted microscope equipped with a Hoffmann modulation contrast system under 400× magnification.
For the PN morphologies, the following characteristics were recorded: the presence of cytoplasmic halos, the sizes and positions of the PN and the numbers and distributions of the nucleolar precursor bodies (NPBs) in the PN. Zygotes presenting abnormalities in any of these characteristics were considered to be of low quality [18, 19].
High-quality embryos were defined as those possessing 4 blastomeres and 8–10 blastomeres on days 2 and 3 of development, respectively, less than 15 % fragmentation, and symmetric and mononucleated blastomeres.
The following morphological characteristics were recorded at the blastocyst stage: the size and compactness of the inner cell mass (ICM), the cohesiveness and number of the trophectoderm (TE) cells and the grade of expansion. The blastocysts were graded according to the Istanbul consensus workshop on embryo assessment [20]. High-quality blastocysts presented with a tightly packed ICM containing a large number of cells and a TE in which many cells formed a cohesive epithelium; any embryo lacking one of these characteristics were considered to be of low quality.
Embryos were placed in a 50-μL drop of culture medium (Global®, LifeGlobal, CT, USA) supplemented with 10 % protein supplement and covered with paraffin oil in a humidified atmosphere under 7.5 % CO2 at 37 °C for 5 days.
Embryo transfer was performed on day 5 of development using a soft catheter with transabdominal ultrasound guidance. One to two embryos were transferred per patient, depending on embryo quality and maternal age.
Clinical follow-up
A pregnancy test was performed 10 days after embryo transfer. All women with a positive test received a transvaginal ultrasound scan after 2 weeks. A clinical pregnancy was diagnosed when the foetal heartbeat was detected. Pregnancy rates were calculated per transfer. Miscarriage was defined as pregnancy loss before 20 weeks.
Data analysis and statistics
Data are expressed as the mean ± standard deviation for numerical variables, while percentages are used for categorical variables.
Binary and linear regression models, which controlled for maternal and paternal age, the number of retrieved oocytes and the number of transferred embryos, were performed to study the influences of morphologically normal and LNV sperm on embryo quality from the pronuclear to blastocyst stages. Cubic regression models were used to investigate the association between the incidences of normal and LNV sperm and the implantation rate, whereas binary regression models were used to investigate the influences of these variables on the odds of pregnancy.
Receiver operating characteristic (ROC) curve analyses were performed to assess the predictive value of morphologically normal and LNV spermatozoa on blastocyst development. The Youden index was used to enable the selection of the optimal threshold value (cut-off point) for the proportion of LNV sperm that maximised blastocyst quality and proper formation.
The results from the binary regression models are expressed as odds ratios (OR) with 95 % confidence intervals (CI) and p values, whereas the results from the linear and cubic regression models are expressed as R2 (adjusted) and p values. The ROC curve results are expressed as the areas under the curve (AUCs) with 95 % CIs. A p < 0.05 was considered to be statistically significant. Data analyses were carried out using the Minitab® version 16 and MedCalc® version 12.2 statistical programs.
Results
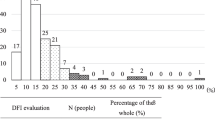
The mean ± SD male age was 43.00 ± 9.73 years. The results for the conventional semen analysis are shown in Table 1. The MSOME showed that the mean + SD incidence rates of morphologically normal and LNV spermatozoa were 1.93 ± 0.88 % (232/12,000) and 25.20 ± 7.53 % (3,024/12,000), respectively. The outcomes of the ICSI cycles are shown in Table 1.
The results from the regression analyses assessing the influences of normal and LNV sperm on embryo quality are shown in Tables 2 and 3, respectively. The morphological characteristics and development of the embryos on days 1, 2, 3 and 5 were not influenced by the percentage of morphologically normal sperm. However, an increased percentage of LNV sperm was associated with the elevated odds of a zygote presenting with abnormalities related to PN size (OR: 1.21, CI: 1.02–1.44), position in the cytoplasm (OR: 1.17, CI: 1.02–1.36), and apposition (OR: 1.27, CI: 1.04–1.54), and NPB number (OR: 1.26, CI: 1.16–1.52) and distribution in the PN (OR: 1.29, CI: 1.25–1.62). Increased levels of LNV sperm were also associated with decreased odds of an embryo possessing a normal number of blastomeres on days 2 and 3 of development (OR: 0.78, CI: 0.69–0.87 and OR: 0.75, CI: 0.68–0.88, respectively) and of an embryo exhibiting high quality on day 3 of development (OR: 0.96, CI: 0.92–0.99). The increased percentage of LNV sperm was associated with the decreased chance of an embryo developing to the blastocyst stage (OR: 0.90, CI: 0.87–0.94) and of an embryo possessing a normal TE (OR: 0.84, CI: 0.79–0.89) and ICM (OR: 0.85, CI: 0.79–0.92). The blastocysts’ grades of expansion were not influenced by the percentage of LNV sperm or normal sperm.
Regarding the ICSI outcomes, regression analyses showed that the increased percentage of LNV sperm negatively influenced the implantation rate (R2: 39.7 %, p < 0.001) and was associated with decreased odds of pregnancy (OR: 0.93, CI: 0.84–0.98).
ROC curve analyses were performed to assess the predictive values of the percentage of LNV spermatozoa on blastocyst development and quality on day 5. The calculated areas under the ROC curves (AUCs) were sufficient to allow for the discernment between the embryos that did and did not develop to the blastocyst stage (AUC: 0.673; CI: 0.618–0.725, p < 0.001) in addition to those that were of high and low quality (AUC: 0.766; CI: 0.691–0.83, p < 0.001). According to the Youden index, the optimal cut-off for the percentage of LNV sperm that maximised (sensitivity + specificity) blastocyst formation was ≤ 24.5, and the cut-off value that maximised blastocyst quality was ≤ 19.5.
Discussion
Sperm morphology has been designated as one of the main determinants of male in vitro fertility [21, 22]. However, the standard morphological evaluation of random stained cells from ejaculate is of limited value during ICSI [23]. With the advent of the MSOME [8], researchers have developed renewed interest in the impact of sperm morphology on ICSI outcome. In this study, we observed that the morphological characteristics of sperm visualised at high magnification are associated with the success of embryonic development from the zygote to the blastocyst stage.
There is scarce information available regarding the mechanism by which the sperm cell influences embryonic development. The initial divisions of the zygote are controlled by maternally inherited mRNA, and the embryonic genome is inactive until the 4-cell stage is completed, after which the substantial expression of sperm-derived genes begins. Therefore, the paternal influence on embryonic development should not be obvious until the 8-cell stage [24]. However, sperm cytoplasm deficiencies can be detected as early as the pronuclear stage and then throughout preimplantation development and are referred to as “early” and “late” paternal effects, respectively [25].
Early paternal effects comprise sperm deficiencies associated with oocyte activation and abnormalities of the centrosome-centriole complex [25]. The centrosome, which is contributed by the sperm cell, is necessary for the formation of the mitotic spindle [26] and controls the first mitotic divisions post-fertilisation [27]. Centrosomal defects may lead to disorders in fertilisation and early embryonic development [28, 29]. In this study, we observed that a 1 % increase in LNV sperm increased the chance of zygote abnormalities by 17 %–29 %, suggesting that spermatozoa that are able to achieve fertilisation may not necessarily be able to contribute to further embryonic development. Our findings corroborate those reported by Tesarik et al. [30], who observed high proportions of zygotes with abnormal pronuclear morphologies following fertilisation with sperm derived from poor-quality semen samples. Three possible mechanisms may explain why sperm quality is able to affect embryogenesis at such a premature stage, including (i) weak transcriptional activity in the human male PN [31, 32], (ii) abnormal calcium signalling patterns resulting in abortive oocyte activation and the failure of pronuclear development [33] and (iii) abnormalities in the sperm-derived aster resulting in the improper apposition of PN [28].
Moreover, it has been demonstrated that the sperm centrosome/centriole complex duplicates during each cell cycle and is perpetuated in early human embryos from cleavage until the hatching blastocyst stage [34]. This study substantiates previous reports suggesting that poor-quality semen samples may negatively affect early embryonic development. Our results showed that the incidence of LNV sperm negatively influenced embryonic cleavage rhythms on days 2 and 3 and embryo quality on day 3 of development. Ron-el et al. [35] observed that poor sperm morphology results in reduced blastomere numbers. Parinaud et al. [36] reported an association between embryos with poor morphology and a lower percentage of morphologically normal sperm. Moreover, sperm morphology has been shown to be positively associated with the blastomere cleavage rate [37].
The late paternal effect influences embryonic development after the activation of paternal DNA [38] and may primarily be caused by DNA damage in sperm, which has been previously demonstrated [39]. The results obtained in this study also suggest a late paternal influence on blastocyst development. The incidence of LNV sperm was significantly associated with blastocyst formation and quality. Regression analysis showed that a 1 % increase in LNV sperm levels was associated with (i) a 10 % decrease in the chance of an embryo developing to the blastocyst stage, (ii) a 16 % lower chance of a blastocyst possessing a normal TE and (iii) a 15 % reduced chance of a blastocyst having a normal ICM. This is in agreement with previous studies that reported relationships of sperm quality with the cleavage rate and blastocyst formation [5, 6].
Regarding the ICSI outcomes, our results demonstrated that the increased percentage of LNV sperm negatively influenced the implantation rate and was associated with a decreased likelihood of pregnancy. These findings are in accordance with previous studies from our group [17, 40], which have demonstrated that MSOME can be used as a predictive tool for ICSI success.
One important drawback of MSOME is related to the absence of a consensus regarding the definition of an LNV [17]. In a previous study in which an LNV was defined as a vacuolar area occupying > 13.0 % of the sperm head, the incidence of LNV sperm was shown to be a useful tool for the prediction of ICSI success [17]. Moreover, a threshold has not been established for the incidence of LNV beyond which the outcomes of cycles are compromised. In this study, the results from the ROC curve analyses showed that the differences in the percentages of LNV sperm (occupying > 13 % of the sperm head) were sufficient to distinguish between embryos that did and did not develop to the blastocyst stage (cut-off ≤ 24.5) and to identify blastocysts of high and low quality (cut-off ≤ 19.5).
The limitations of this study included the small number of subjects. In addition, because multiple outcomes were assessed, it is possible that the findings were due to chance. More than one embryo originating from the same patient were included, which has been shown to inflate type I errors. Finally, the high mean paternal age may have influenced the results.
In this study, we observed a mean paternal age of 43 years, and the individuals possessed a wide range of ages (25–48 years). It has been previously demonstrated that increased male age in infertile patients is associated with a decline in semen quality [41]. Male ageing has also been correlated with the presence of sperm vacuoles, which has been demonstrated by morphological evaluation using MSOME [42]. Moreover, a higher percentage of LNV sperm, a lower percentage of those with normal morphologies and a higher DNA fragmentation rate were observed in the older patients (≥41 years) when compared to the younger patients (<40 years). Therefore, despite attempts to control for paternal age in the regression analyses, its influences on the findings must not be disregarded.
In conclusion, the present study reinforces previous studies that point to potential early and late paternal effects, both of which may influence zygote, embryo and blastocyst development and quality. Moreover, our results suggest that the evaluation of the incidence of vacuoles under high magnification may significantly improve upon the prognostic information obtained by conventional semen analysis. Further studies are necessary to confirm the cut-off values for the incidence of LNV proposed in this study over which blastocyst formation and quality are negatively affected.
References
Palermo G, Joris H, Devroey P, Van Steirteghem AC. Pregnancies after intracytoplasmic injection of single spermatozoon into an oocyte. Lancet. 1992;340:17–8.
Shoukir Y, Chardonnens D, Campana A, Sakkas D. Blastocyst development from supernumerary embryos after intracytoplasmic sperm injection: a paternal influence? Hum Reprod. 1998;13:1632–7.
Loutradi KE, Tarlatzis BC, Goulis DG, et al. The effects of sperm quality on embryo development after intracytoplasmic sperm injection. J Assist Reprod Genet. 2006;23:69–74.
Virro MR, Larson-Cook KL, Evenson DP. Sperm chromatin structure assay (SCSA) parameters are related to fertilization, blastocyst development, and ongoing pregnancy in in vitro fertilization and intracytoplasmic sperm injection cycles. Fertil Steril. 2004;81:1289–95.
Janny L, Menezo YJ. Evidence for a strong paternal effect on human preimplantation embryo development and blastocyst formation. Mol Reprod Dev. 1994;38:36–42.
Miller JE, Smith TT. The effect of intracytoplasmic sperm injection and semen parameters on blastocyst development in vitro. Hum Reprod. 2001;16:918–24.
Van der Zwalmen P, Bertin-Segal G, Geerts L, Debauche C, Schoysman R. Sperm morphology and IVF pregnancy rate: comparison between Percoll gradient centrifugation and swim-up procedures. Hum Reprod. 1991;6:581–8.
Bartoov B, Berkovitz A, Eltes F. Selection of spermatozoa with normal nuclei to improve the pregnancy rate with intracytoplasmic sperm injection. N Engl J Med. 2001;345:1067–8.
Berkovitz A, Eltes F, Ellenbogen A, Peer S, Feldberg D, Bartoov B. Does the presence of nuclear vacuoles in human sperm selected for ICSI affect pregnancy outcome? Hum Reprod. 2006;21:1787–90.
Garolla A, Fortini D, Menegazzo M, et al. High-power microscopy for selecting spermatozoa for ICSI by physiological status. Reprod Biomed Online. 2008;17:610–6.
Boitrelle F, Ferfouri F, Petit JM, et al. Large human sperm vacuoles observed in motile spermatozoa under high magnification: nuclear thumbprints linked to failure of chromatin condensation. Hum Reprod. 2011;26:1650–8.
Setti AS, Cortezzi SS, Figueira Rde C, et al. A chromosome 19 locus positively influences the number of retrieved oocytes during stimulated cycles in Brazilian women. J Assist Reprod Genet. 2012;29:443–9.
World Health Organization. WHO laboratory manual for the examination and processing of human semen. 5th ed. Geneva: World Health Organization. xiv; 2010. 271.
Berkovitz A, Eltes F, Lederman H, et al. How to improve IVF-ICSI outcome by sperm selection. Reprod Biomed Online. 2006;12:634–8.
Saidi R, Rives N, Gruel E, Mazurier S, Mousset-Simeon N, Mace B. Nouvelle classification du spermocytogramme a’ fort grossissement. Med Reprod Gyn Endo. 2008;10:315–24.
Perdrix A, Travers A, Chelli MH, et al. Assessment of acrosome and nuclear abnormalities in human spermatozoa with large vacuoles. Hum Reprod. 2011;26:47–58.
Setti AS, de Almeida Ferreira Braga DP, Vingris L, de Cassia Savio Figueira R, Iaconelli Jr A, Borges Jr E. The prevalence of sperm with large nuclear vacuoles is a prognostic tool in the prediction of ICSI success. J Assist Reprod Genet. 2014;31:307–12.
Scott L, Alvero R, Leondires M, Miller B. The morphology of human pronuclear embryos is positively related to blastocyst development and implantation. Hum Reprod. 2000;15:2394–403.
Tesarik J, Greco E. The probability of abnormal preimplantation development can be predicted by a single static observation on pronuclear stage morphology. Hum Reprod. 1999;14:1318–23.
Alpha Scientists in Reproductive M, Embryology ESIGo. The Istanbul consensus workshop on embryo assessment: proceedings of an expert meeting. Hum Reprod. 2011;26:1270–83.
De Vos A, Van De Velde H, Joris H, Verheyen G, Devroey P, Van Steirteghem A. Influence of individual sperm morphology on fertilization, embryo morphology, and pregnancy outcome of intracytoplasmic sperm injection. Fertil Steril. 2003;79:42–8.
Lundin K, Soderlund B, Hamberger L. The relationship between sperm morphology and rates of fertilization, pregnancy and spontaneous abortion in an in-vitro fertilization/intracytoplasmic sperm injection programme. Hum Reprod. 1997;12:2676–81.
Demir B, Arikan II, Bozdag G, Esinler I, Karakoc Sokmensuer L, Gunalp S. Effect of sperm morphology on clinical outcome parameters in ICSI cycles. Clin Exp Obstet Gynecol. 2012;39:144–6.
Braude P, Bolton V, Moore S. Human gene expression first occurs between the four- and eight-cell stages of preimplantation development. Nature. 1988;332:459–61.
Barroso G, Valdespin C, Vega E, et al. Developmental sperm contributions: fertilization and beyond. Fertil Steril. 2009;92:835–48.
Hinduja I, Baliga NB, Zaveri K. Correlation of human sperm centrosomal proteins with fertility. J Hum Reprod Sci. 2010;3:95–101.
Schatten H. The mammalian centrosome and its functional significance. Histochem Cell Biol. 2008;129:667–86.
Asch R, Simerly C, Ord T, Ord VA, Schatten G. The stages at which human fertilization arrests: microtubule and chromosome configurations in inseminated oocytes which failed to complete fertilization and development in humans. Hum Reprod. 1995;10:1897–906.
Terada Y, Nakamura S, Simerly C, et al. Centrosomal function assessment in human sperm using heterologous ICSI with rabbit eggs: a new male factor infertility assay. Mol Reprod Dev. 2004;67:360–5.
Tesarik J, Mendoza C, Greco E. Paternal effects acting during the first cell cycle of human preimplantation development after ICSI. Hum Reprod. 2002;17:184–9.
Tesarik J, Kopecny V. Nucleic acid synthesis and development of human male pronucleus. J Reprod Fertil. 1989;86:549–58.
Ao A, Erickson RP, Winston RM, Handyside AH. Transcription of paternal Y-linked genes in the human zygote as early as the pronucleate stage. Zygote. 1994;2:281–7.
Tesarik J, Sousa M, Testart J. Human oocyte activation after intracytoplasmic sperm injection. Hum Reprod. 1994;9:511–8.
Sathananthan A, The paternal centrosome: its role in human embryonic development and infertility, in Current issues in Obstetrics and Gynaecology, S. Arulkumaran and S. Ng, editors. Oxford University Press: Singapore, 1996. p. 101–16.
Ron-el R, Nachum H, Herman A, Golan A, Caspi E, Soffer Y. Delayed fertilization and poor embryonic development associated with impaired semen quality. Fertil Steril. 1991;55:338–44.
Parinaud J, Mieusset R, Vieitez G, Labal B, Richoilley G. Influence of sperm parameters on embryo quality. Fertil Steril. 1993;60:888–92.
Salumets A, Suikkari AM, Mols T, Soderstrom-Anttila V, Tuuri T. Influence of oocytes and spermatozoa on early embryonic development. Fertil Steril. 2002;78:1082–7.
Tesarik J. Paternal effects on cell division in the human preimplantation embryo. Reprod Biomed Online. 2005;10:370–5.
Tesarik J, Greco E, Mendoza C. Late, but not early, paternal effect on human embryo development is related to sperm DNA fragmentation. Hum Reprod. 2004;19:611–5.
Vingris L, Setti A, Braga D, Figueira R, Iaconelli Jr A, Borges Jr A. Motile sperm organelle morphology examination predicts blastocyst formation, implantation and miscarriage rates in couples undergoing ICSI. Hum Reprod. 2012;27:ii121–50.
Plastira K, Msaouel P, Angelopoulou R, et al. The effects of age on DNA fragmentation, chromatin packaging and conventional semen parameters in spermatozoa of oligoasthenoteratozoospermic patients. J Assist Reprod Genet. 2007;24:437–43.
de Almeida Ferreira Braga DP, Setti AS, Figueira RC, et al. Sperm organelle morphologic abnormalities: contributing factors and effects on intracytoplasmic sperm injection cycles outcomes. Urology. 2011;78:786–91.
Author information
Authors and Affiliations
Corresponding author
Additional information
Capsule The proportion of sperm with large nuclear vacuoles negatively influences zygote, embryo and blastocyst development and quality.
Rights and permissions
About this article
Cite this article
Setti, A.S., Braga, D.P.A.F., Vingris, L. et al. Sperm morphological abnormalities visualised at high magnification predict embryonic development, from fertilisation to the blastocyst stage, in couples undergoing ICSI. J Assist Reprod Genet 31, 1533–1539 (2014). https://doi.org/10.1007/s10815-014-0326-9
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10815-014-0326-9